Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Primary O/W Nanoemulsion
2.2.2. Final O/W/O Multiple Nanoemulsion
2.3. Characterization of the Multiple Nanoemulsions
2.3.1. Globule Size and Zeta Potential
2.3.2. Surface Morphology
2.3.3. Drug Content Determination
2.3.4. Entrapment Efficiency (%) Determination
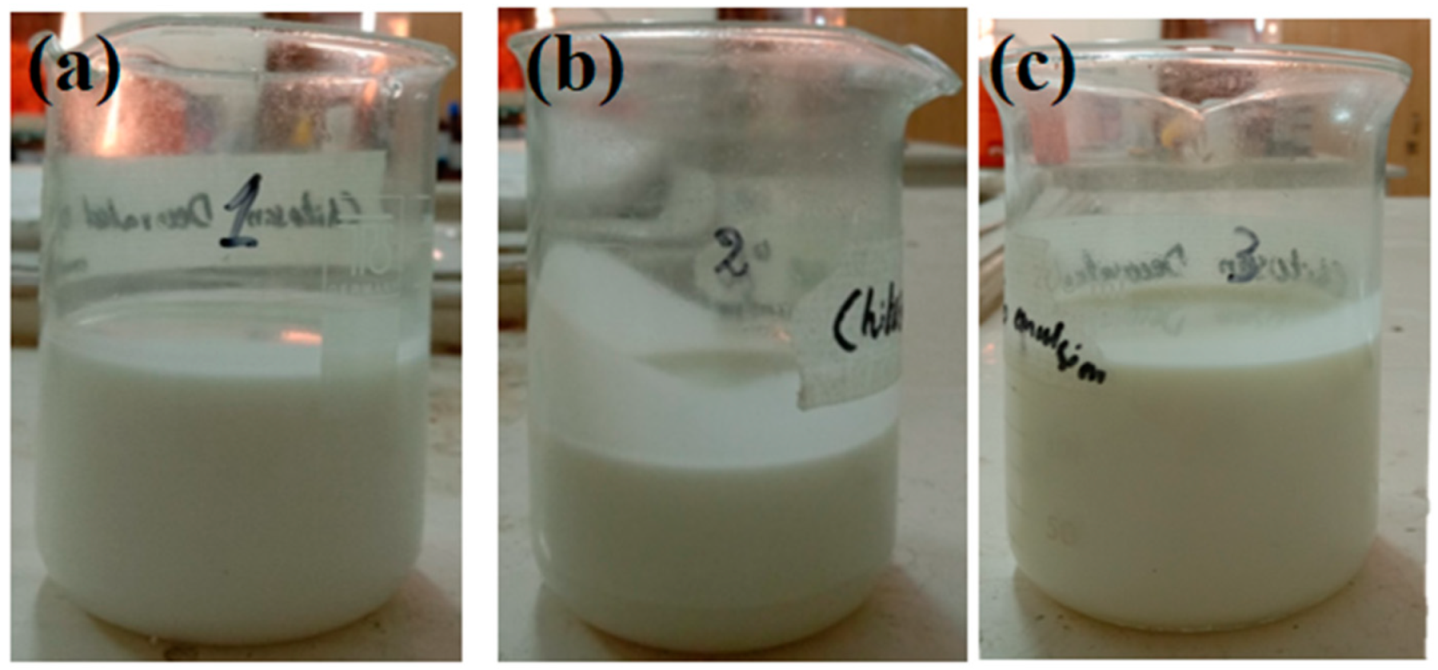
2.3.5. Creaming and Cracking
2.3.6. pH of the Multiple Nanoemulsions
2.3.7. Viscosity Measurement
2.4. In Vitro Release
2.5. In Vitro Permeation
2.6. Preparation of Rabbit Skin
2.7. Skin Retention
2.8. ATR-FTIR Analysis of Skin
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Multiple Nanoemulsions
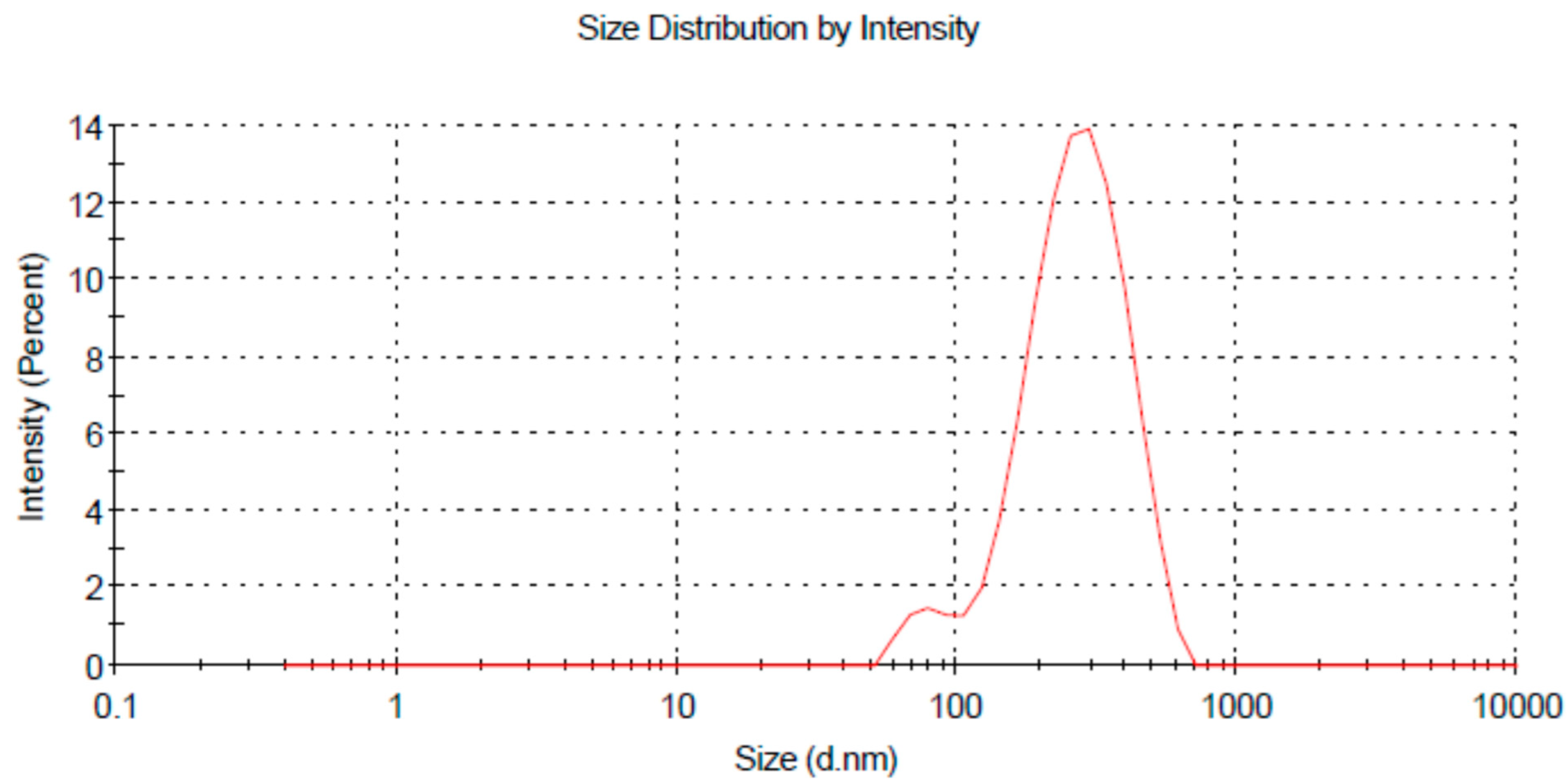
3.1.1. Globule Size and Zeta Potential
3.1.2. pH of the Multiple Nanoemulsions
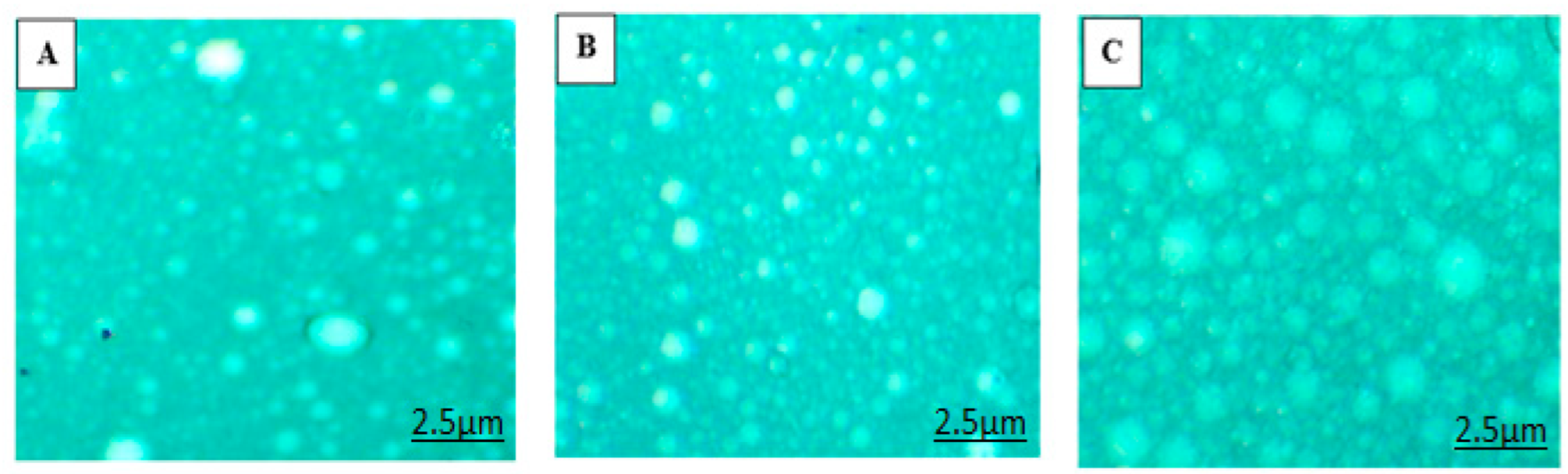
3.1.3. Surface Morphology
3.1.4. Viscosity Measurement
3.2. Drug Content (%) Determination
3.3. Percent Entrapment Efficiency Determination
3.4. In Vitro Drug Release Study
3.5. In Vitro Drug Permeation Study
3.6. Skin Drug Retention
3.7. ATR-FTIR Analysis of Skin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sandeepthi, N.; Satyanarayana, L. Transdermal Drug Delivery: An Overview. J. Glob. Trends Pharm. Sci. 2017, 8, 4537–4541. [Google Scholar]
- Patel, D.; Chaudhary, S.A.; Parmar, B.; Bhura, N. Transdermal drug delivery system: A review. Pharma Innov. 2012, 1, 66. [Google Scholar] [CrossRef][Green Version]
- Sezer, A.D.; Cevher, E.J. Topical drug delivery using chitosan nano-and microparticles. Expert Opin. Drug Deliv. 2012, 9, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Dwivedi, S.; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2011, 52, 103–112. [Google Scholar] [CrossRef]
- Nastiti, C.M.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.; Roberts, M. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Thacharodi, D.; Rao, K.P. Transdermal absorption of nifedipine from microemulsions of lipophilic skin penetration enhancers. Int. J. Pharm. 1994, 111, 235–240. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernandez-Lauzardo, A.N.; Velazquez-Del Valle, M.G.; Hernández-López, M.; Barka, E.A.; Bosquez-Molina, E.; Wilson, C. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. J. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Hamidi, M.; Rafiei, P.; Azadi, A.; Mohammadi-Samani, S.J. Encapsulation of valproate-loaded hydrogel nanoparticles in intact human erythrocytes: A novel nano-cell composite for drug delivery. J. Pharm. Sci. 2011, 100, 1702–1711. [Google Scholar] [CrossRef]
- Peng, C.C.; Yang, M.H.; Chiu, W.T.; Chiu, C.H.; Yang, C.S.; Chen, Y.W.; Peng, R.Y.J.M. Composite Nano-Titanium Oxide–Chitosan Artificial Skin Exhibits Strong Wound-Healing Effect—An Approach with Anti-Inflammatory and Bactericidal Kinetics. Macromol. Biosci. 2008, 8, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Ortaggi, G.J. Chitosan micro-and nanospheres: Fabrication and applications for drug and DNA delivery. Mini Rev. Med. Chem. 2009, 9, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Hatipoglu, F.; Cevher, E.; Oğurtan, Z.; Bas, A.L.; Akbuğa, J.J. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS Pharmscitech 2007, 8, E94–E101. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Kim, J.K.; Song, Y.K.; Park, J.S.; Kim, C.K. A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J. Control. Release 2006, 110, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Pandit, J.; Kumar, A.; Swaroop, P.; Gupta, S.J. Pharmaceutical nanotechnology novel nanoemulsion-high energy emulsification preparation, evaluation and application. Pharm. Res. 2010, 3, 117–138. [Google Scholar]
- De Doncker, P.; Pande, S.; Richarz, U.; Garodia, N.J. Itraconazole: What clinicians should know? Indian J. Drugs Dermatol. 2017, 3, 4. [Google Scholar]
- Debruyne, D.; Coquerel, A. Pharmacokinetics of antifungal agents in onychomycoses. Clin. Pharmacokinet. 2001, 40, 441–472. [Google Scholar] [CrossRef]
- Thiim, M.; Friedman, L. Hepatotoxicity of antibiotics and antifungals. Clin. Liver Dis. 2003, 7, 381–399. [Google Scholar] [CrossRef]
- Smith, J.D. Cutaneous fungal infections. US Pharm. 2015, 40, 35–39. [Google Scholar]
- Berben, P.; Mols, R.; Brouwers, J.; Tack, J.; Augustijns, P. Gastrointestinal behavior of itraconazole in humans–Part 2: The effect of intraluminal dilution on the performance of a cyclodextrin-based solution. Int. J. Pharm. 2017, 526, 235–243. [Google Scholar] [CrossRef]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N.J. Biopharmaceutics. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Pople, P.V.; Singh, K. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS Pharmscitech 2006, 7, E63–E69. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Deore, S.K.; Surawase, R.K.; Maru, A.J. Formulation and Evaluation of O/W Nanoemulsion of Ketoconazole. Res. J. Pharm. Technol. 2019, 11, 269–274. [Google Scholar] [CrossRef]
- Teixeira, M.; Severino, P.; Andreani, T.; Boonme, P.; Santini, A.; Silva, A.; Souto, E.J.S. D-α-tocopherol nanoemulsions: Size properties, rheological behavior, surface tension, osmolarity and cytotoxicity. Saudi Pharm. J. 2017, 25, 231–235. [Google Scholar] [CrossRef]
- de Almeida Borges, V.R.; Simon, A.; Sena, A.R.C.; Cabral, L.M.; de Sousa, V.P. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int. J. Nanomed. 2013, 8, 535. [Google Scholar]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Formulation consideration and skin retention study of microemulsion containing tazarotene for targeted therapy of acne. J. Pharm. Investig. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mukherjee, P.K.; Biswas, S. Nanoemulsion as a novel carrier system for improvement of betulinic acid oral bioavailability and hepatoprotective activity. J. Mol. Liq. 2017, 237, 361–371. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, B.A.; Uzair, B.; Niaz, S.I.; Khan, H.; Hosny, K.M.; Menaa, F. Development of Chitosan-Based Nanoemulsion Gel Containing Microbial Secondary Metabolite with Effective Antifungal Activity: In vitro and in vivo Characterizations. Int. J. Nanomed. 2021, 16, 8203–8219. [Google Scholar] [CrossRef]
- Morsi, N.; Mohamed, M.; Refai, H.; El Sorogy, H. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int. J. Pharm. Pharm. Sci. 2014, 6, 227–236. [Google Scholar]
- Chiesa, M.; Garg, J.; Kang, Y.T.; Chen, G.J.C. Thermal conductivity and viscosity of water-in-oil nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 67–72. [Google Scholar] [CrossRef]
- Shinde, S.; Yadav, V.; Jadhav, P.; Jadhav, A. Formulation and evaluation of microemulsion containing anti-hypertensive drug. Res. J. Pharm. Technol. 2020, 13, 5726–5732. [Google Scholar] [CrossRef]
- Che Marzuki, N.H.; Wahab, R.A.; Abdul, H.M. An overview of nanoemulsion: Concepts of development and cosmeceutical applications. Biotechnol. Biotechnol. Equip. 2019, 33, 779–797. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50. [Google Scholar] [CrossRef]
- Burapapadh, K.; Takeuchi, H.; Sriamornsak, P. Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole. Asian J. Pharm. Sci. 2016, 11, 365–375. [Google Scholar] [CrossRef]
- Nawaz, A.; Wong, T. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef]




| Phase | Components | Concentrations (%) |
|---|---|---|
| Inner oil phase | Olive oil | 29.5 |
| Span 20 | 1 | |
| ITZ | 1 | |
| Aqueous phase | Distilled water | 58.5 |
| PEG 400 | 14 | |
| Tween 80 | 1 | |
| Chitosan | 250 mg | |
| Outer oil phase | Olive oil | 44 |
| Span 20 | 1 |
| F. Code | Droplet Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| SNE | 174 ± 3.12 | 0.221 ± 2.21 | −1.32 ± 0.41 |
| CNE | 191 ± 4.36 | 0.292 ± 2.38 | 4.32 ± 1.01 |
| MNE | 242.5 ± 5.22 | 0.225 ± 5.01 | 3.61 ± 0.79 |
| F. Code | pH | % Drug Content | % Entrapment Efficiency |
|---|---|---|---|
| SNE | 5.5 ± 0.91 | 71 ± 2.02% | 35.5 ± 1.52% |
| CNE | 5.2 ± 0.45 | 82 ± 3.12% | 41.2 ± 2.03% |
| MNE | 5.1 ± 0.34 | 90 ± 2.31% | 45 ± 2.09% |
| Scheme | Chitosan-Decorated Simple o/w Nanoemulsion | Chitosan-Decorated o/w/o Multiple Nanoemulsion | ||
|---|---|---|---|---|
| 1 | Spindle 1 | 3.31cp | Spindle 1 | 17.65cp |
| 2 | Spindle 2 | 1.44cp | Spindle 2 | 23.76cp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules 2022, 27, 3183. https://doi.org/10.3390/molecules27103183
Malik MR, Al-Harbi FF, Nawaz A, Amin A, Farid A, Mohaini MA, Alsalman AJ, Hawaj MAA, Alhashem YN. Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules. 2022; 27(10):3183. https://doi.org/10.3390/molecules27103183
Chicago/Turabian StyleMalik, Muhammad Rehan, Fatemah Farraj Al-Harbi, Asif Nawaz, Adnan Amin, Arshad Farid, Mohammed Al Mohaini, Abdulkhaliq J. Alsalman, Maitham A. Al Hawaj, and Yousef N. Alhashem. 2022. "Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo" Molecules 27, no. 10: 3183. https://doi.org/10.3390/molecules27103183
APA StyleMalik, M. R., Al-Harbi, F. F., Nawaz, A., Amin, A., Farid, A., Mohaini, M. A., Alsalman, A. J., Hawaj, M. A. A., & Alhashem, Y. N. (2022). Formulation and Characterization of Chitosan-Decorated Multiple Nanoemulsion for Topical Delivery In Vitro and Ex Vivo. Molecules, 27(10), 3183. https://doi.org/10.3390/molecules27103183








