Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties
Abstract
:1. Introduction
2. Results
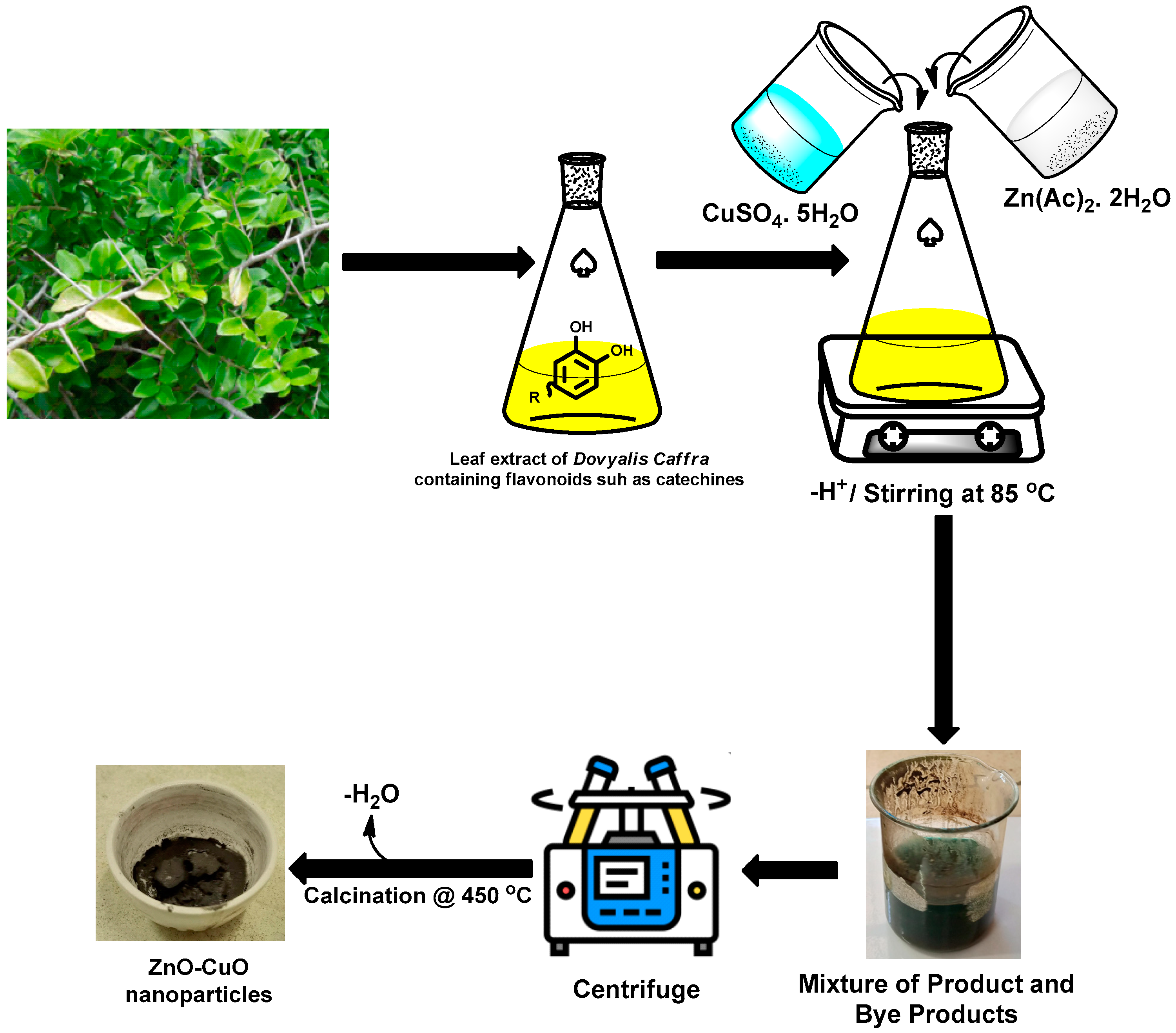
2.1. Synthesis
2.2. Phase Identification and Structural Properties of the Prepared CuO
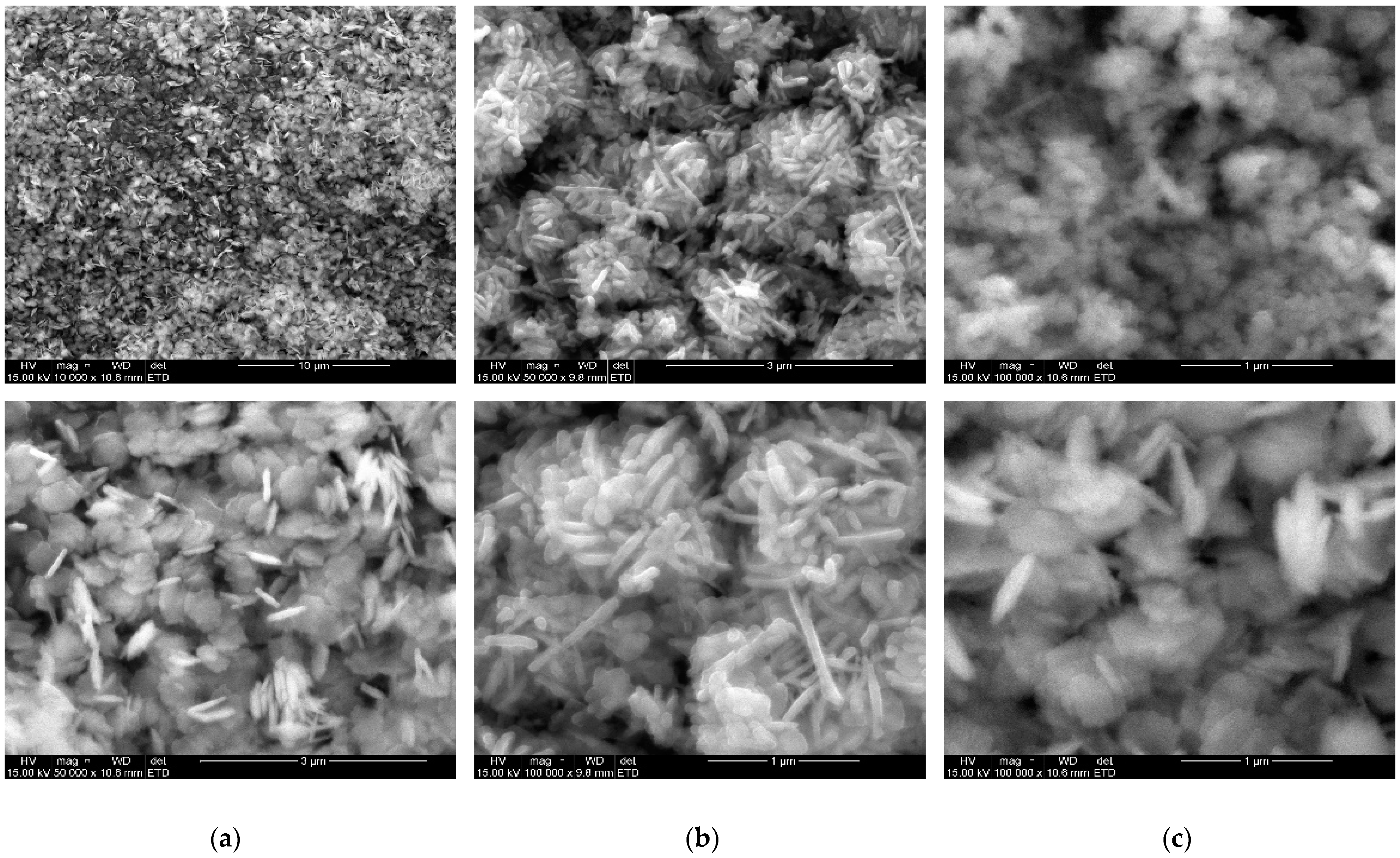
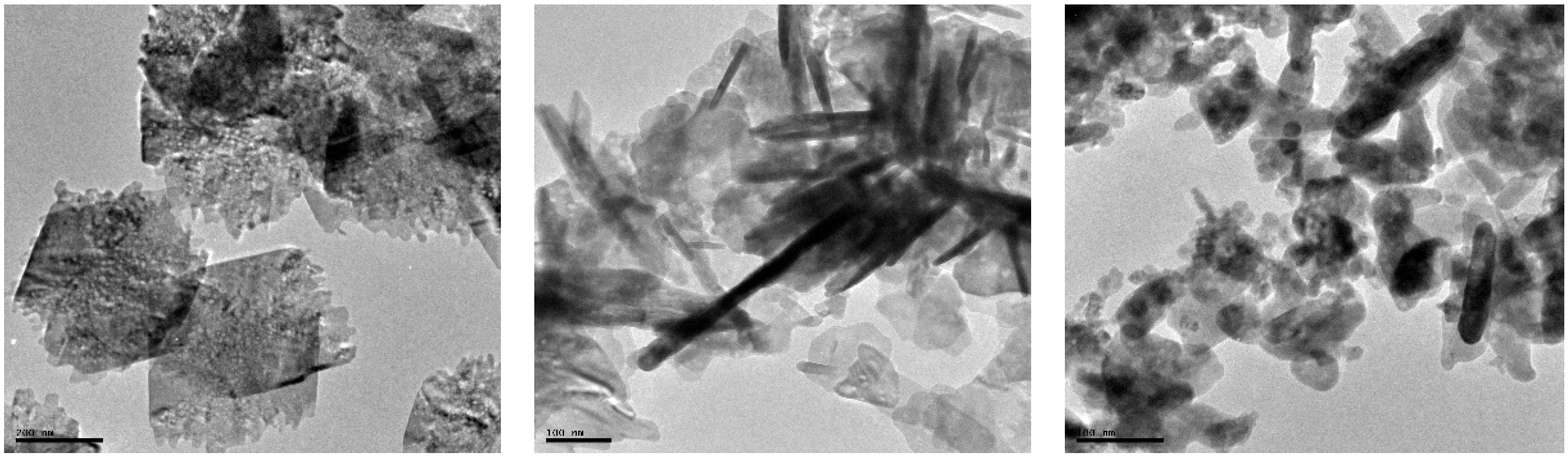
2.3. Surface Morphology and Elemental Analysis of CuO Nanoflakes
2.4. Biological Studies
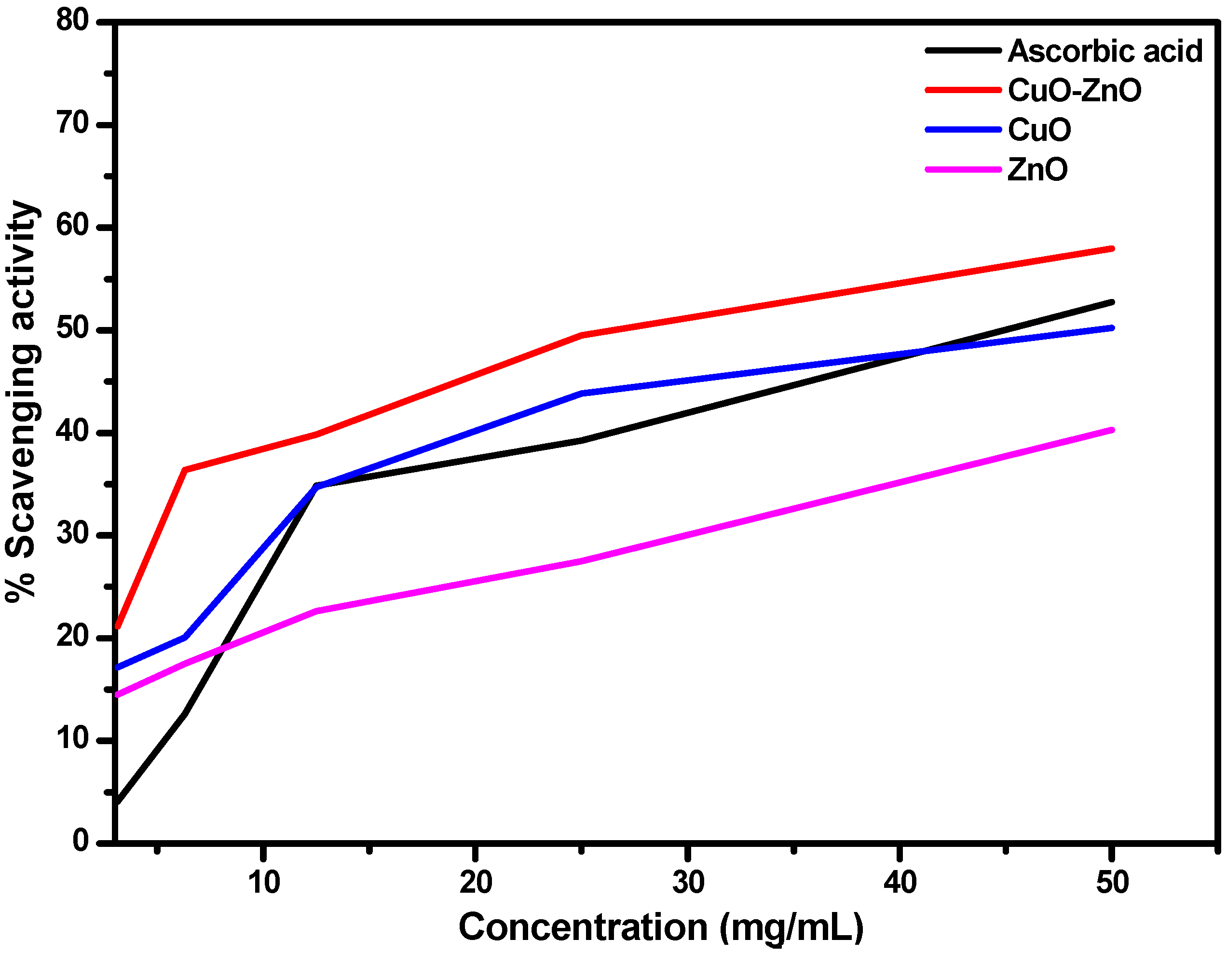
2.4.1. Antioxidant Study
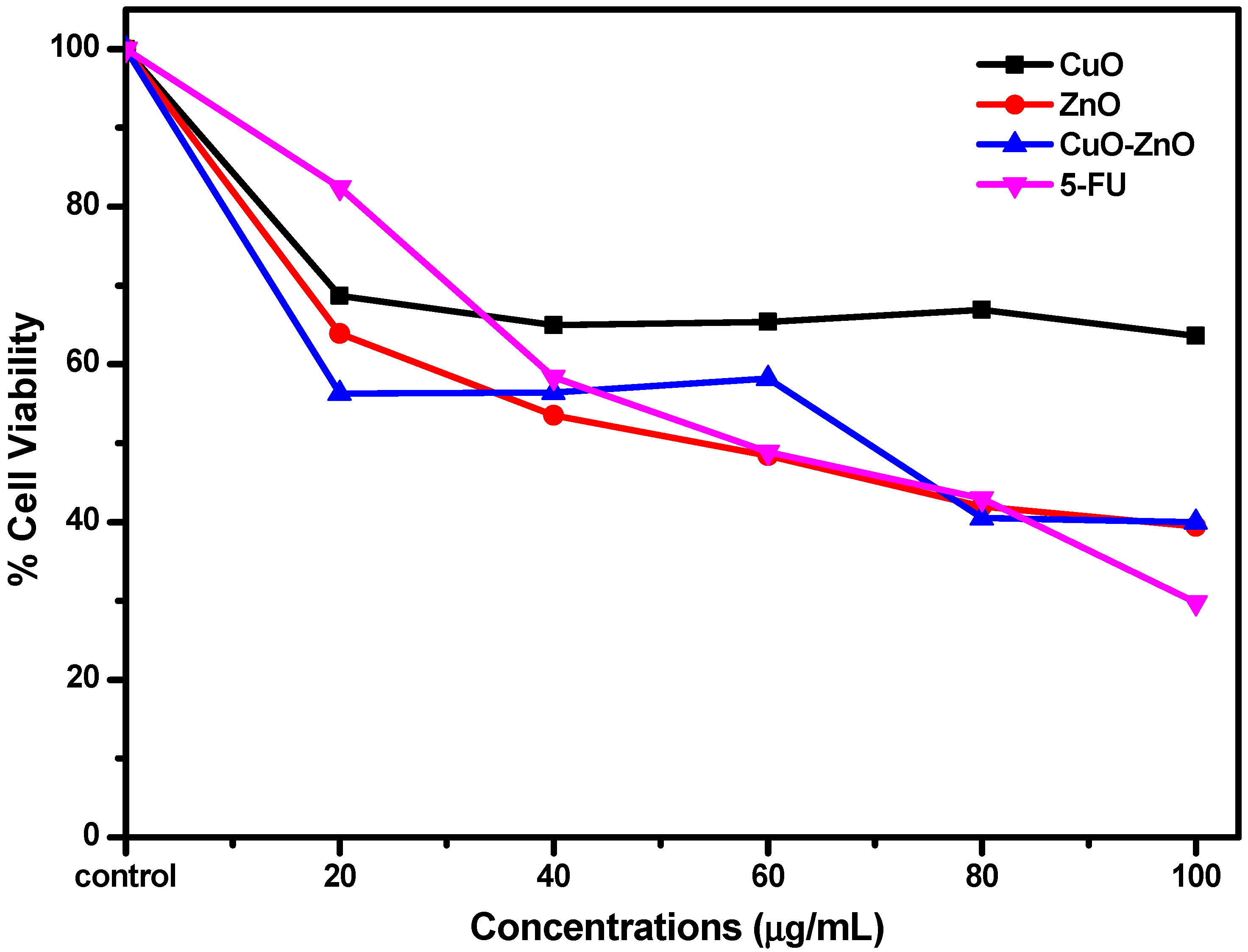
2.4.2. In Vitro Cytotoxicity Studies Using MTT Assay
3. Materials and Methods
3.1. Collection and Preparation of Plant Materials
3.2. Synthesis of CuO Nanoparticle Using Leave Extracts of Dovyalis caffra
3.3. Synthesis of CuO Nanoparticle Using Leave Extracts of Dovyalis caffra
3.4. Synthesis of CuO–ZnO Nanoparticle Using Leaf Extracts of Dovyalis caffra
3.5. Structural and Morphological Studies
3.6. Free Radical Scavenging Activity Using DPPH Assay
3.7. Cytotoxicity Evaluation Using MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Cho, B.-K.; Wood, T.K.; Lee, J.-K. Emerging Applications of Bacteria as Antitumor Agents. Semin. Cancer Biol. 2021, in press. [CrossRef]
- Thun, M.J.; DeLancey, J.O.; Center, M.M.; Jemal, A.; Ward, E.M. The Global Burden of Cancer: Priorities for Prevention. Carcinogenesis 2010, 31, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, Á. Assessment of the Evolution of Cancer Treatment Therapies. Cancers 2011, 3, 3279–3330. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Noren, H.; Jove, R.; Beljanski, V.; Grinnemo, K.H. Differences and Similarities between Cancer and Somatic Stem Cells: Therapeutic Implications. Stem Cell Res. Ther. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 939378. [Google Scholar] [CrossRef] [Green Version]
- Zulkipli, I.N.; David, S.R.; Rajabalaya, R.; Idris, A. Medicinal Plants: A Potential Source of Compounds for Targeting Cell Division. Drug Target Insights 2015, 9, 9. [Google Scholar] [CrossRef]
- Cox, P.A.; King, S. Bioprospecting. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2013; pp. 588–599. ISBN 9780123847195. [Google Scholar]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200. [Google Scholar] [CrossRef]
- Otari, S.V.; Pawar, S.H.; Patel, S.K.S.; Singh, R.K.; Kim, S.Y.; Lee, J.H.; Zhang, L.; Lee, J.K. Canna Edulis Leaf Extract-Mediated Preparation of Stabilized Silver Nanoparticles: Characterization, Antimicrobial Activity, and Toxicity Studies. J. Microbiol. Biotechnol. 2017, 27, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, J.M.; Ansari, M.A.; Fatma, S.; Abdullah, S.M.S.; Iqbal, J.; Madkhali, A.; Hamali, A.H.; Ahmad, S.; Jerah, A.; Echeverria, V.; et al. Inhibiting Effect of Zinc Oxide Nanoparticles on Advanced Glycation Products and Oxidative Modifications: A Potential Tool to Counteract Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2018, 55, 7438–7452. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green Synthesis of ZnO Nanoparticles Using Orange Fruit Peel Extract for Antibacterial Activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Drummer, S.; Madzimbamuto, T.; Chowdhury, M. Green Synthesis of Transition-Metal Nanoparticles and Their Oxides: A Review. Materials 2021, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yao, J.; Zhu, X.; Qi, Y. Nanomedicines: Redefining Traditional Medicine. Biomed. Pharmacother. 2021, 134, 111103. [Google Scholar] [CrossRef]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green Synthesis of Copper Oxide Nanoparticles Using Abutilon Indicum Leaf Extract: Antimicrobial, Antioxidant and Photocatalytic Dye Degradation Activities. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Aziz, A.; Yangjeh, H.; Bayrami, A.; Latifi, S.; Asadollah, N. Green Synthesis of ZnO and ZnO / CuO Nanocomposites in Mentha Longifolia Leaf Extract: Characterization and Their Application as Anti-Bacterial Agents. J. Mater. Sci. Mater. Electron. 2018, 29, 13596–13605. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carrageenan-Based Antimicrobial Bionanocomposite Films Incorporated with ZnO Nanoparticles Stabilized by Melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic Activity of Biochar-Supported ZnO Nanorods in Degradation of Gemifloxacin: Synergy Study, Effect of Parameters and Phytotoxicity Evaluation. Ultrason. Sonochem. 2019, 55, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Antioxidant and Catalytic Activity of Biosynthesized CuO Nanoparticles Using Extract of Galeopsidis Herba. J. Inorg. Organomet. Polym. Mater. 2018, 28, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef] [Green Version]
- Mandal, A. Copper Nanomaterials as Drug Delivery System against Infectious Agents and Cancerous Cells. J. Appl. Life Sci. Int. 2017, 15, 38444. [Google Scholar] [CrossRef]
- Ilves, M.; Kinaret, P.A.S.; Ndika, J.; Karisola, P.; Marwah, V.; Fortino, V.; Fedutik, Y.; Correia, M.; Ehrlich, N.; Loeschner, K.; et al. Surface PEGylation Suppresses Pulmonary Effects of CuO in Allergen-Induced Lung Inflammation. Part. Fibre Toxicol. 2019, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Parwaz Khan, A.A.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.-H. Engineering Nanostructures of CuO-Based Photocatalysts for Water Treatment: Current Progress and Future Challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Khataee, A.; Kalderis, D.; Gholami, P.; Fazli, A.; Moschogiannaki, M.; Binas, V.; Lykaki, M.; Konsolakis, M. Cu2O-CuO@biochar Composite: Synthesis, Characterization and Its Efficient Photocatalytic Performance. Appl. Surf. Sci. 2019, 498, 143846. [Google Scholar] [CrossRef]
- Asemani, M.; Anarjan, N. Self-Dual Leonard Pairs Green Synthesis of Copper Oxide Nanoparticles Extract Assessment and Biological Properties. Green Process Synth 2019, 8, 557–567. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Venckatesh, R. Aloe Barbadensis Miller Mediated Green Synthesis of Mono-Disperse Copper Oxide Nanoparticles: Optical Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.Z.; Zhu, J.J.; Chen, H.Y. Preparation of CuO Nanoparticles by Microwave Irradiation. J. Cryst. Growth 2002, 244, 88–94. [Google Scholar] [CrossRef]
- Rangel, W.M.; Boca Santa, R.A.A.; Riella, H.G. A Facile Method for Synthesis of Nanostructured Copper (II) Oxide by Coprecipitation. J. Mater. Res. Technol. 2020, 9, 994–1004. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Maham, M.; Mohammad Sajadi, S. Green Synthesis of CuO Nanoparticles by Aqueous Extract of Gundelia Tournefortii and Evaluation of Their Catalytic Activity for the Synthesis of N-Monosubstituted Ureas and Reduction of 4-Nitrophenol. J. Colloid Interface Sci. 2015, 455, 245–253. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green Synthesis of Bimetallic ZnO–CuO Nanoparticles and Their Cytotoxicity Properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO Nanoparticles Induce Cytotoxicity and Apoptosis in Human K562 Cancer Cell Line via Mitochondrial Pathway, through Reactive Oxygen Species and P53. Iran. J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar] [CrossRef]
- Duan, X.; Liao, Y.; Liu, T.; Yang, H.; Liu, Y.; Chen, Y.; Ullah, R.; Wu, T. Zinc Oxide Nanoparticles Synthesized from Cardiospermum Halicacabum and Its Anticancer Activity in Human Melanoma Cells (A375) through the Modulation of Apoptosis Pathway. J. Photochem. Photobiol. B Biol. 2020, 202, 111718. [Google Scholar] [CrossRef] [PubMed]
- Das, D. Multicomponent Reactions in Organic Synthesis Using Copper-Based Nanocatalysts. ChemistrySelect 2016, 1, 1959–1980. [Google Scholar] [CrossRef]
- Hekmati, M. Application of Biosynthesized CuO Nanoparticles Using Rosa Canina Fruit Extract as a Recyclable and Heterogeneous Nanocatalyst for Alkyne/Aldehyde/Amine-A 3 Coupling Reactions. Catal. Lett. 2019, 149, 2325–2331. [Google Scholar] [CrossRef]
- Aremu, A.O.; Ncama, K.; Omotayo, A.O. Ethnobotanical Uses, Biological Activities and Chemical Properties of Kei-Apple [Dovyalis Caffra (Hook.f. & Harv.) Sim]: An Indigenous Fruit Tree of Southern Africa. J. Ethnopharmacol. 2019, 241, 111963. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Aqueous Extract of Broccoli Mediated Synthesis of CaO Nanoparticles and Its Application in the Photocatalytic Degradation of Bromocrescol Green. IET Nanobiotechnol. 2018, 12, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Chaka, B.; Osano, A.M. Analysis of Selected Nutrient Levels at Different Growth Stages of Dovyalis Caffra (Kei-Apple ) Fruits. Int. J. Res. Innov. Appl. Sci. 2019, IV, 11–18. [Google Scholar]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C. ZnO Nanoparticles Mediated by Aqueous Extracts of Dovyalis Caffra Fruits and the Photocatalytic Evaluations. Mater. Res. Express 2019, 6, 125091. [Google Scholar] [CrossRef]
- Veisi, H.; Karmakar, B.; Tamoradi, T.; Hemmati, S.; Hekmati, M.; Hamelian, M. Biosynthesis of CuO Nanoparticles Using Aqueous Extract of Herbal Tea (Stachys Lavandulifolia) Flowers and Evaluation of Its Catalytic Activity. Sci. Rep. 2021, 11, 1983. [Google Scholar] [CrossRef]
- Jamil, S.; Tariq, T.; Khan, S.R.; Ehsan, M.A.; Rehman, A.; Janjua, M.R.S.A. Structural Characterization, Synthesis and Application of Zincite Nanoparticles as Fuel Additive. J. Clust. Sci. 2021, 32, 1–12. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Eco-Friendly Synthesis of Copper Oxide, Zinc Oxide and Copper Oxide–Zinc Oxide Nanocomposites, and Their Anticancer Applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 400–409. [Google Scholar] [CrossRef]
- Wasim, M.; Khan, M.R.; Mushtaq, M.; Naeem, A.; Han, M.; Wei, Q. Surface Modification of Bacterial Cellulose by Copper and Zinc Oxide Sputter Coating for UV-Resistance/Antistatic/Antibacterial Characteristics. Coatings 2020, 10, 364. [Google Scholar] [CrossRef] [Green Version]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles-An Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Buledi, J.A.; Pato, A.H.; Kanhar, A.H.; Solangi, A.R.; Batool, M.; Ameen, S.; Palabiyik, I.M. Heterogeneous Kinetics of CuO Nanoflakes in Simultaneous Decolorization of Eosin Y and Rhodamine B in Aqueous Media. Appl. Nanosci. 2021, 11, 1241–1256. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced Photocatalytic Activity of ZnO/CuO Nanocomposite for the Degradation of Textile Dye on Visible Light Illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.L.U.; Van Nguyen, T.T.; Nguyen, T.; Nguyen, P.A.; Nguyen, V.M.; Nguyen, N.H.; Tran, V.L.; Phan, N.A.; Huynh, K.P.H. Antibacterial Shoe Insole-Coated CuO-ZnO Nanocomposite Synthesized by the Sol-Gel Technique. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Soomro, R.A.; Tunesi, M.M.; Karakus, S.; Kalwar, N. Highly Sensitive Electrochemical Determination of Captopril Using CuO Modified ITO Electrode: The Effect of in Situ Grown Nanostructures over Signal Sensitivity. RSC Adv. 2017, 7, 19353–19362. [Google Scholar] [CrossRef] [Green Version]
- Mbenga, Y.; Mthiyane, M.N.; Botha, T.L.; Horn, S.; Pieters, R.; Wepener, V.; Onwudiwe, D.C. Nanoarchitectonics of ZnO Nanoparticles Mediated by Extract of Tulbaghia Violacea and Their Cytotoxicity Evaluation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of Copper Oxide Nanoparticles by Chemical and Biogenic Methods: Photocatalytic Degradation and in Vitro Antioxidant Activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Vibitha, B.V.; Anitha, B.; Krishna, P.G.A.; Tharayil, J.N. Plant Extracts Assisted Synthesis, Characterization and Antioxidant Properties of ZnO: CuO Nanocomposites. In AIP Conference Proceedings; 2020; Volume 2244, p. 070037. [Google Scholar]
- Mittal, A.K.; Kaler, A.; Banerjee, U.C. Free Radical Scavenging and Antioxidant Activity of Silver Nanoparticles Synthesized from Flower Extract of Rhododendron Dauricum. Nano Biomed. Eng. 2012, 4, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, M.S.; Kulkarni, S.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U.S. Green Biosynthesis of CuO & Ag–CuO Nanoparticles from Malus Domestica Leaf Extract and Evaluation of Antibacterial, Antioxidant and DNA Cleavage Activities. New J. Chem. 2018, 42, 204–213. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, Antibacterial, Antioxidant, and Cytotoxic Activities of ZnO Nanoparticles Using Coptidis Rhizoma. Bioorg. Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-Based Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Aqueous Leaf Extract of Aquilegia Pubiflora: Their Antiproliferative Activity against HepG2 Cells Inducing Reactive Oxygen Species and Other in Vitro Properties. Oxid. Med. Cell. Longev. 2021, 2021, 4786227. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green Synthesis of Multifunctional Zinc Oxide (ZnO) Nanoparticles Using Cassia Fistula Plant Extract and Their Photodegradative, Antioxidant and Antibacterial Activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Bio-Inspired Synthesis and Cytotoxic Evaluation of Silver-Gold Bimetallic Nanoparticles Using Kei-Apple (Dovyalis Caffra) Fruits. Inorg. Chem. Commun. 2019, 109, 107569. [Google Scholar] [CrossRef]
- Zayyoun, N.; Bahmad, L.; Laânab, L.; Jaber, B. The Effect of PH on the Synthesis of Stable Cu2O/CuO Nanoparticles by Sol–Gel Method in a Glycolic Medium. Appl. Phys. A Mater. Sci. Process. 2016, 122, 3–8. [Google Scholar] [CrossRef]
- Iftikhar, M.; Zahoor, M.; Naz, S.; Nazir, N.; Batiha, G.E.-S.; Ullah, R.; Bari, A.; Hanif, M.; Mahmood, H.M. Green Synthesis of Silver Nanoparticles Using Grewia Optiva Leaf Aqueous Extract and Isolated Compounds as Reducing Agent and Their Biological Activities. J. Nanomater. 2020, 2020, 8949674. [Google Scholar] [CrossRef]



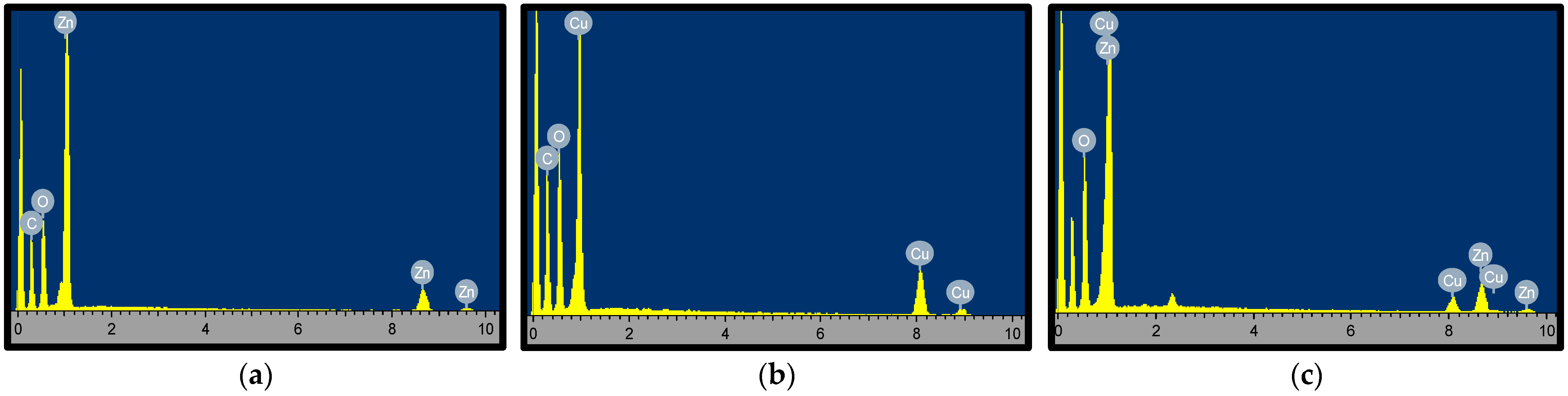
| Samples | Elemental Constituent of the Nanoparticles in Weight % | ||
|---|---|---|---|
| Zn | Cu | O | |
| CuO | - | 72.17 | 27.83 |
| ZnO | 75.03 | - | 24.97 |
| CuO–ZnO | 55.55 | 17.45 | 27 |
| Concentration in mg/mL | |||||||
|---|---|---|---|---|---|---|---|
| 3.13 | 6.3 | 12.5 | 25 | 50 | IC50 | ||
| Ascorbic acid | Mean ± SD | 0.189 ± 0.02 | 0.243 ± 0.01 | 0.261 ± 0.02 | 0.349 ± 0.01 | 0.384 ± 0.01 | |
| % Scavenging activity | 4.082 | 12.625 | 34.875 | 39.25 | 52.75 | 4.96 | |
| CuO–ZnO | Mean ± SD | 0.523 ± 0.02 | 0.422 ± 0.01 | 0.399 ± 0.01 | 0.335 ± 0.03 | 0.279 ± 0.03 | |
| % Scavenging activity | 21.16 | 36.38 | 39.85 | 49.50 | 57.94 | 3.92 | |
| CuO | Mean ± SD | 0.397 ± 0.04 | 0.447 ± 0.02 | 0.520 ± 0.05 | 0.637 ± 0.03 | 0.661 ± 0.05 | |
| % Scavenging activity | 17.15 | 20.083 | 34.728 | 43.849 | 50.209 | 6.76 | |
| ZnO | Mean ± SD | 0.567 ± 0.02 | 0.647 ± 0.03 | 0.513 ± 0.01 | 0.481 ± 0.03 | 0.396 ± 0.02 | |
| % Scavenging activity | 14.52 | 17.54 | 22.66 | 27.49 | 40.30 | 8.99 | |
| NPs | Used Plants | IC50 Values in µg/mL | Ref. |
|---|---|---|---|
| CuO | Galeopsidis herba | 4.12 µg/mL | [20] |
| Abutilon indicum (leaf extract) | 40 ± 0.23 µg/mL | [16] | |
| Solanum nigrum (leaf extract) | 131.54 µg/mL | [51] | |
| Malus domestica leaf extract | 45.90 µg/mL | [54] | |
| ZnO | Coptidis rhizoma | No IC50 estimation | [55] |
| Aquilegia pubiflora | 178.45 ± 2.64 µg/mL | [56] | |
| Cassia fistula | 2853 µg/mL | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties. Molecules 2022, 27, 3206. https://doi.org/10.3390/molecules27103206
Adeyemi JO, Onwudiwe DC, Oyedeji AO. Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties. Molecules. 2022; 27(10):3206. https://doi.org/10.3390/molecules27103206
Chicago/Turabian StyleAdeyemi, Jerry O., Damian C. Onwudiwe, and Adebola O. Oyedeji. 2022. "Biogenic Synthesis of CuO, ZnO, and CuO–ZnO Nanoparticles Using Leaf Extracts of Dovyalis caffra and Their Biological Properties" Molecules 27, no. 10: 3206. https://doi.org/10.3390/molecules27103206









