Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities
Abstract
:1. Introduction
2. Extraction and Purification Methods for MFHPs
3. Structure–Activity Relationship of MFHPs
| Sources | Purification Methods | Compound Name | Monosaccharide Composition | Analysis Technique | Chemical Structure | References |
|---|---|---|---|---|---|---|
| Chinese yam | Ultrafiltration | HSY-I, HSY-Ⅱ, HSY-Ⅲ | HSY-I: GluA:Gal = 1.86:5.19 HSY-Ⅱ: GluA:Ara:Rha:Glu:Gal = 0.81:1.24: 2.35:66.79:28.81 HSY-Ⅲ: Man:Glu:Gal = 13.20:12.79:74.0 | HPGPC, GC, FT–IR | 1 | [40] |
| Ginger | DEAE-52 | HGP, EGP1, EGP2, UGP1, UGP2 | HGP: Glu EGP1: Man, Ara, Glu, Gal EGP2: Man, Rha, Ara, Gal, Xyl, Glu UGP1: Ara, Gal, Glu, Man UGP2: Man, Rha, Ara, Gal, Glu, Xyl | FT–IR, NMR | ND | [19] |
| Pueraria lobata roots | DEAE-52 | PS-D1, PS-D2, PS-D3 | PS-D1: Glu:Fru = 24.4:1.0 PS-D2: Glu:Fru:Ara = 54.5:1.0:1.0 PS-D3: Glu:Fru:Gal:Ara = 61.0:1.0:2.7:2.4 | FT–IR, UV | ND | [41] |
| Raspberry | Macroporous resin, Sephadex G-100 | RCP-II | GalA:Glu:Ara:Xyl:Rha:Gal = 1.00: 0.44:1.19:0.52:0.55:1.90 | GC, UV, FT–IR, NMR | ND | [24] |
| Angelica dahurice roots | DEAE-52, Sephadex G-100 | ADPs-1a, ADPs-1b, ADPs-2, ADPs-3a, ADPs-3b | ADPs-1a: Glu, Man, Xyl, Gal = 26.1:0.22:0.31:0.11 ADPs-1b: Ara, Glu, Man, Xyl, Gal = 0.10:15.3:0.07:0.26:1.37 ADPs-2: Ara, Glu, Man, Xyl, Gal, Rha = 1.79:15.8:0.40:0.35:5.59:0.34 ADPs-3a: Ara, Glu, Man, Xyl, Gal, Rha = 2.01:1.68:0.41:0.13:4.97:1.06 ADPs-3b: Ara, Glu, Man, Xyl, Gal, Rha = 0.36:13.5:0.09:0.25:1.59:0.18 | HPSEC, GC, FT–IR, NMR | 2 | [42] |
| Imperial Chrysanthemum | Sephadex G-200 | ICP-1 | Rha, Ara, Man, Glu, GluA, GalA = 1:0.70:1.14:1.48:0.81:1.67 | FT–IR, NMR, SEM, HPGPC | 3 | [43] |
| Lotus | Sephadex G-100 | LLWP-1, LLWP-3 | LLWP-1: Rha, Glu, Gal, Ara, GalA = 7.0:6.0:28.0:24.8:26.4 LLWP-3: Rha, Glu, Gal, Ara, Man, GalA = 6.6:8.9:15.0:9.8:6.1:47.2 | HPAEC–PAD, FT–IR, | ND | [44] |
| Turmeric | DEAE-52 | TPS-0, TPS-1, TPS-2, TPS-3 | TPS-0: Ara, Gal, Glu TPS-1: Ara, Gal, Glu TPS-2: Xyl, Glu, Gal, Ara, Rha, GalA, GluA TPS-3: Rha, Glu, Gal, Ara, Xyl, GalA, GluA | HPGPC, FT–IR, GC–MS, NMR, SEM | 4 | [16] |
| Platycodon grandiflorus | Ultrafiltration | LMw-PGP | ND | HPGPC, FT–IR | ND | [45] |
| Ganoderma lucidum | QFF anion-exchange column | GLP-1, GLP-2 | GLP:Man:Glc:Gal:Fuc = 4.9:63.5:26.2:5.4 GLP-2: Man:Glc:Gal = 1.6:90.6:7.8 | Agilent ZORBAX Eclipse XDB-C18 column, HPGPC, FT–IR, NMR | 5 | [33] |
| Longan | DEAE-Sepharose | LPIIa | Rha, Rib, Ara, Xyl, Glu, Gal = 1.05:1:22.88:1.01:2.59:34.58 | GC–MS, APC, FT–IR, NMR, | 6 | [46] |
| Dandelion | DEAE-Sepharose | PD1-1 | Glu, Man | HPGPC, GC–MS, UV, FT–IR, NMR | 7 | [39] |
| Cistanche tubulosa | Ua-ternary ammonium salt precipitation | CTP | Rha, Man, Glu, Gal = 2.18:1:28.29:1.43 | FT–IR, CD, SEM, | ND | [47] |
| Mulberry leaf | Sephadex G-100 | MLP | Ara, Xyl, Glu, Rha, Man = 1:2.13:6.53:1.04:8.73 | HPSEC, HPLC, FT–IR | ND | [48] |
| Sea buckthorn | Sephacryl S-200 column | SP0.1-1 | Ara, Glu, Gal, Man = 11.2:2.3:1.9:1 | HPGPC, GC–MS, NMR, | 8 | [49] |
| Amomum villosum Lour | DEAE-52, Sephadex G-100 | AVPG-1, AVPG-2 | AVPG-1: Glucose, Galactose, Xylose, Arabinose, GluA = 73.11:10.29:6.21:8.83:1.57 AVPG-2:Rha, Glu, Gal, Xyl, Ara, Glu GalA = 3.11:40.23:17.82:12.53:15.81:3.99 | HPSEC, SEM, GC–MS, NMR, FT–IR | 9 | [50] |
| Gastrodia elata | Membrane separation | GEP-1 | Glc | GC–MS, FT–IR, NMR, SEM | 10 | [51] |
| Dendrobium officinale | DEAE-52, Sephacryl S-300 | DOPA-1, DOPA-2 | DOPA-1: Man:Glu = 5.8:1 DOPA-2: Man, Glu = 4.5:1 | HPGPC, FT–IR, GC–MS, NMR | 11 | [52] |
| Phyllanthus emblica | DEAE-52, Sephadex G-100 | PEPW80-1 | Rha, Arab, Gal = 3.02:1.00:4.23 | HPAEC–PAD, GC, FT–IR, NMR, SEM | 12 | [34] |
| Houttuynia cordata | Sephacryl S-300, DEAE Cellulose | HCA4S1 | Rha, GalA, Gal, Ara = 15.6:17.5:41.2:25.7 | HPGPC, UV, GC, FT–IR, NMR, | 13 | [53] |
| Polygonatum sibiricum | DEAE-Sepharose | PSP1, PSP2, PSP3, PSP4 | PSP1: Man:Glu:Gal = 14.96:2.13:82.91 PSP2: Rha:Glu:Gal:Xyl = 20.54:2.06:74.37:3.03 PSP3: Man:Rha:Glu:Gal:Xyl = 1.38:57.69:2.02:37.17:1.74 PSP4: Man:Rha:Gal:Xyl = 2.00:72.63:20.74:4.63 | HPSEC, FT–IR, NMR | ND | [54] |
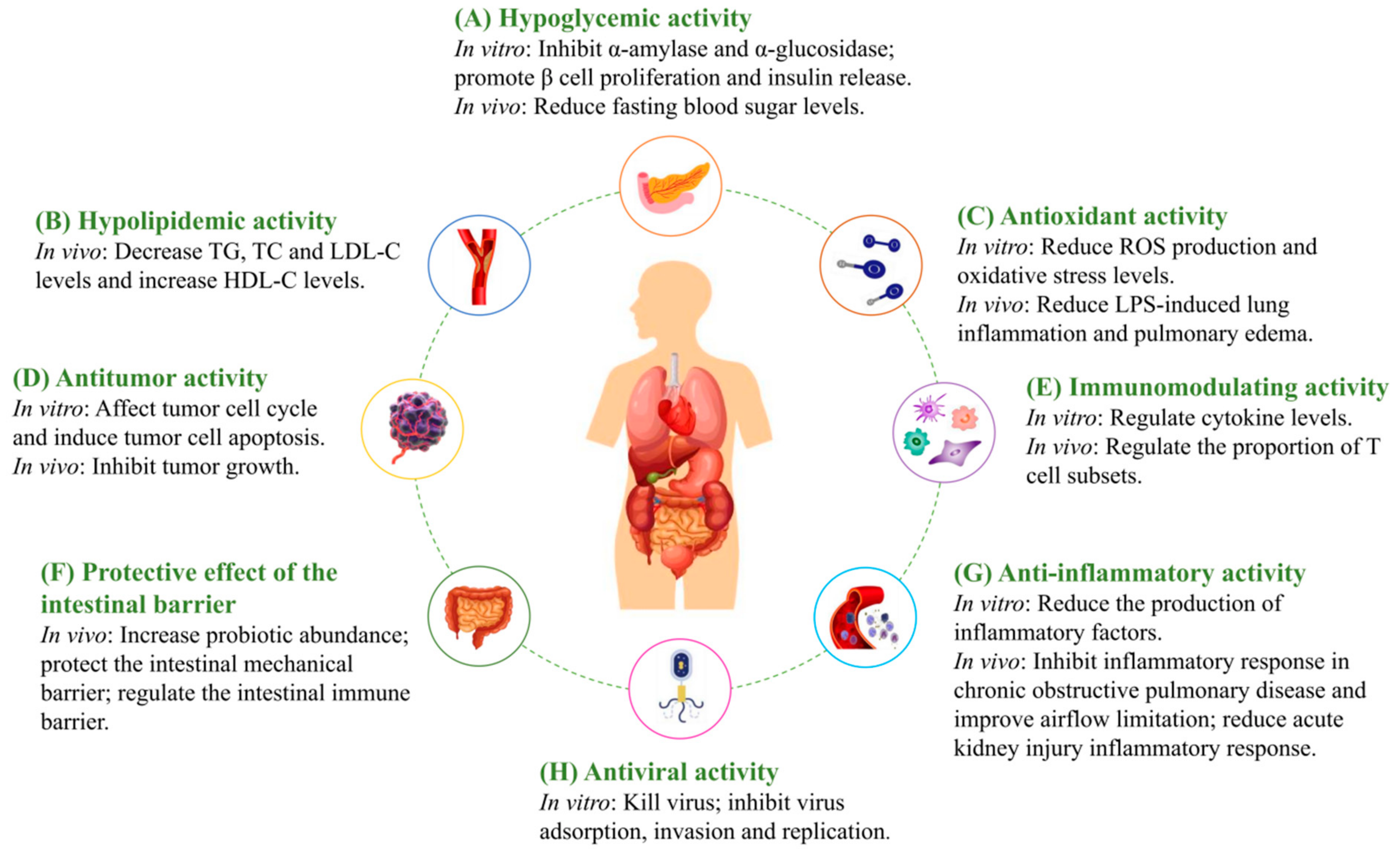
4. Biological Activities of MFHPs
4.1. Hypoglycemic Activity
4.2. Hypolipidemic Activity
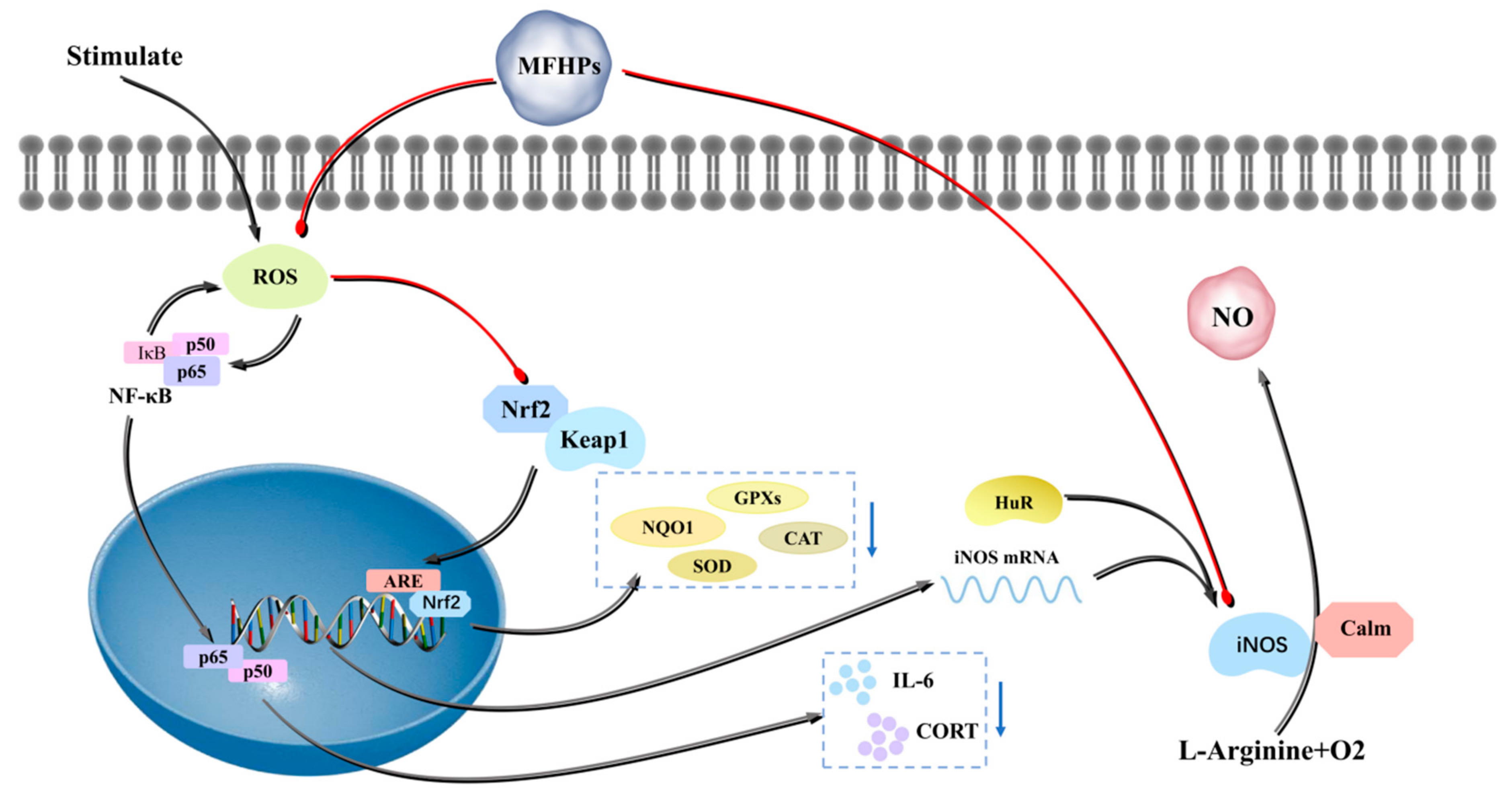
4.3. Antioxidant Activity
4.4. Anti-Tumor Activity
4.5. Immunomodulating Activity
4.6. Protective Effect of the Intestinal Barrier
4.7. Anti-Inflammatory Activity
4.8. Antiviral Activity
4.9. Other Activities
5. Concluding Remarks and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Zhao, D. Research on the medicine food homology piants. Plant Physiol. J. 2021, 57, 1383–1384. [Google Scholar]
- Hou, Y.; Jiang, J. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Jiang, M.; Ding, K. Application of Polysaccharide in Food Industry. Food Sci. 2013, 34, 431–438. [Google Scholar]
- Seweryn, E.; Ziała, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Huang, X.; Li, J.; Jiang, G.; Zhang, Z. Extraction Optimization and Evaluation of the Antioxidant and α-Glucosidase Inhibitory Activity of Polysaccharides from Chrysanthemum morifolium cv. Hangju. Antioxid. 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Gao, R.; Wu, S. Preparation, characterization and hypolipidaemic activity of Astragalus membranaceus polysaccharide. J. Funct. Foods 2017, 39, 264–267. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, M.; Yao, X.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydr. Polym. 2020, 236, 116020. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Fu, Y.; Luo, S.; Luo, X.; Wang, Q.; Hu, M.; Ma, F.; Ma, C.; Zhou, L. Polysaccharide from Radix Codonopsis has beneficial effects on the maintenance of T-cell balance in mice. Biomed. Pharmacother. 2019, 112, 108682. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, X.; Chen, Y.; Yan, J.; Zhang, S.; Wu, B.; Jia, J. Enrichment, purification and in vitro antioxidant activities of polysaccharides from Umbilicaria esculenta macrolichen. Biochem. Eng. J. 2018, 130, 10–20. [Google Scholar] [CrossRef]
- Cai, M.; Zhong, H.; Chu, H.; Zhu, H.; Sun, P.; Liao, X. Forward osmosis concentration of high viscous polysaccharides of Dendrobium officinale: Process optimisation and membrane fouling analysis. Int. J. Food Sci. Technol. 2021, 56, 4871–4882. [Google Scholar] [CrossRef]
- Hammi, K.M.; Hammami, M.; Rihouey, C.; Le Cerf, D.; Ksouri, R.; Majdoub, H. Ultrasonication of Polysaccharides from Tunisian Zizyphus lotus Fruit: Emulsifying Capacities, Rheological Properties and Antioxidant activities. Chem. Afr. 2020, 3, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Shao, X.; Ling, P.; Liu, F.; Han, G.; Wang, F. Recent advances in polysaccharides for osteoarthritis therapy. Eur. J. Med. Chem. 2017, 139, 926–935. [Google Scholar] [CrossRef]
- He, J.-Y.; Zhang, Y.-H.; Ma, N.; Zhang, X.-L.; Liu, M.-H.; Fu, W.-M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J.; Chen, Y.; Ma, Y.; Yang, Q.; Fan, Y.; Fu, C.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides—ScienceDirect. LWT 2022, 154, 112805. [Google Scholar] [CrossRef]
- Ai, S.; Fan, X.; Fan, L.; Sun, Q.; Liu, Y.; Tao, X.; Dai, K. Extraction and chemical characterization of Angelica sinensis polysaccharides and its antioxidant activity. Carbohydr. Polym. 2013, 94, 731–736. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Chen, G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef]
- Liao, D.; Cheng, C.; Liu, J.; Zhao, L.; Huang, D.; Chen, G. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods—ScienceDirect. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Sun, J.; Jiang, B.; Yan, J. Extraction of water-soluble polysaccharide and the antioxidant activity from Semen cassiae. J. Food Drug Anal. 2014, 22, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Li, H. The antioxidant activities of six (1→3)-β-d-glucan derivatives prepared from yeast cell wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzyme Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhao, Y. Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohydr. Polym. 2013, 98, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Teng, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Niu, J.; Mai, B.; Shi, F.; Liu, Q. Effects of extraction methods on antioxidant and immunomodulatory activities of polysaccharides from superfine powder Gynostemma pentaphyllum Makino. Glycoconj. J. 2020, 37, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, W.; Han, Q.; Wang, P.; Xiang, X.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, X.; Liu, S. Study on Microwave-assisted Extraction of Polysaccharide from Angelica sinensis. Anhui Nongye Kexue 2012, 40, 16573–16574. [Google Scholar]
- Zhu, J.; Chen, Z.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.; Hu, X.; Wei, X.; Wang, Y. Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef]
- Hu, X.; Xu, F.; Li, J.; Li, J.; Mo, C.; Zhao, M.; Wang, L. Ultrasonic-assisted extraction of polysaccharides from coix seeds: Optimization, purification, and in vitro digestibility. Food Chem. 2022, 374, 131636. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Wang, Z.; Zhang, G. Ultrasonic-assisted extraction of polysaccharide from Dendrobium officinale: Kinetics, thermodynamics and optimization. Biochem. Eng. J. 2022, 177, 108227. [Google Scholar] [CrossRef]
- Song, J.; Shi, D.; Su, H.; Feng, Y.; Tian, W. Optimization of ultrasonic extraction of Lycium barbarum polysaccharides using response surface methodology. Int. J. Food Eng. 2020, 16, 20200153. [Google Scholar] [CrossRef]
- Nomura, K.; Sakai, M.; Ohboshi, H.; Nakamura, A. Extraction of a water-soluble polysaccharide fraction from lentils and is potential application in acidified protein dispersions. Food Hydrocoll. 2021, 117, 106740. [Google Scholar] [CrossRef]
- Li, J.; Gu, F.; Cai, C.; Hu, M.; Fan, L.; Hao, J.; Yu, G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum—ScienceDirect. Int. J. Biol. Macromol. 2020, 143, 806–813. [Google Scholar] [CrossRef]
- Zeng, Z.; Lv, W.; Jing, Y.; Chen, Z.; Song, L.; Liu, T.; Yu, R. Structural characterization and biological activities of a novel polysaccharide from Phyllanthus emblica. Drug Discov. Ther. 2017, 11, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, X.; Zhang, L.; Zeng, F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, K.; Ren, L.; Sui, Y.; Chen, J.; Zhang, T.; Li, X.; Cao, W. Leukemia cells apoptosis by a newly discovered heterogeneous polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2020, 241, 116279. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Ma, Y.; Bai, R.; Hu, F. Monosaccharide compositions of Codonopsis pilosula polysaccharides and their correlation analysis on cytotoxic activities against HepG2 cells. Zhongcaoyao 2016, 47, 2684–2692. [Google Scholar]
- Wang, Y.; Wang, S.; Song, R.; Cai, J.; Xu, J.; Tang, X.; Li, N. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2019, 123, 81–90. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, W.; Jiang, Y.; Wang, H.; Chen, G.; Guo, M. Physicochemical, Structural, and Biological Properties of Polysaccharides from Dandelion. Molecules 2019, 24, 1485. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, W.; Gao, Q.; Zou, Y. Hypoglycemic Effect of Chinese Yam (Dioscorea opposita rhizoma) Polysaccharide in Different Structure and Molecular Weight. J. Food Sci. 2017, 82, 2487–2494. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Luo, L.; Yan, J. Fractionation, physicochemical characteristics and biological activities of polysaccharides from Pueraria lobata roots. J. Taiwan Inst. Chem. Eng. 2016, 67, 54–60. [Google Scholar] [CrossRef]
- Wang, J.; Lian, P.; Yu, Q.; Wei, J.; Kang, W. Purification, characterization and procoagulant activity of polysaccharides from Angelica dahurice roots. Chem. Cent. J. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Meng, J.; Qiu, J.; Geng, X.; Sun, H.-Q.; Zhu, Z. Structural characterization and prebiotic potential of an acidic polysaccharide from Imperial Chrysanthemum. Nat. Prod. Res. 2020, 36, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Han, A.; Lim, T.; Lee, E.; Hong, H. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Y.; Yu, S.; Ji, H.; Feng, Y.; Liu, A.; Yu, J. Extraction, optimization, and biological activities of a low molecular weight polysaccharide from Platycodon grandiflorus. Ind. Crops Prod. 2021, 165, 113427. [Google Scholar] [CrossRef]
- Bai, Y.; Jia, X.; Huang, F.; Zhang, R.; Dong, L.; Liu, L.; Zhang, M. Structural elucidation, anti-inflammatory activity and intestinal barrier protection of longan pulp polysaccharide LPIIa. Carbohydr. Polym. 2020, 246, 116532. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, J.; Wang, W.; Li, Q.; Chen, Y.; Feng, W.; Zheng, D.; Zhao, T.; Mao, G.; Yang, L.; et al. Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 2016, 93, 448–458. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, C.; Lu, G.; Cui, W.; Mu, Z.; Gao, H.; Wang, Y. Purification, characterization and anti-diabetic activity of a polysaccharide from mulberry leaf. Regul. Toxicol. Pharmacol. 2014, 70, 687–695. [Google Scholar] [CrossRef]
- Shen, C.; Wang, T.; Guo, F.; Sun, K.; Wang, B.; Wang, J.; Zhang, Z.; Zhang, X.; Zhao, Y.; Chen, Y. Structural characterization and intestinal protection activity of polysaccharides from Sea buckthorn (Hippophae rhamnoides L.) berries. Carbohydr. Polym. 2021, 274, 118648. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, C.; Yang, D.; Tang, C.; Zhao, Z. Purification, Structural Characterization and Immunomodulatory Effects of Polysaccharides from Amomum villosum Lour. on RAW 264.7 Macrophages. Molecules 2021, 26, 2672. [Google Scholar] [CrossRef]
- Huo, J.; Lei, M.; Li, F.; Hou, J.; Zhang, Z.; Long, H.; Zhong, X.; Liu, Y.; Xie, C.; Wu, W. Structural Characterization of a Polysaccharide from Gastrodia elata and Its Bioactivity on Gut Microbiota. Molecules 2021, 26, 4443. [Google Scholar] [CrossRef]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Jin, C.; Chen, H.; Wang, P.; Yu, M.; Ding, K. Structural characterization and anti-A549 lung cancer cells bioactivity of a polysaccharide from Houttuynia cordata. Int. J. Biol. Macromol. 2018, 120, 288–296. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, N.; Xue, X.; Li, Q.; Sun, D.; Zhao, Z. Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 160, 688–694. [Google Scholar] [CrossRef]
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.P.; Huang, Q.; Chen, C.; You, L.J.; Liu, R.; Luo, Z.G.; Zhao, M.M.; Fu, X. The chemical structure and biological activities of a novel polysaccharide obtained fromFructus Mori and its zinc derivative. J. Funct. Foods 2019, 54, 64–73. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Y.; Cui, W.; Lu, G.; Wang, Y.; Gao, H.; Huang, L.; Mu, Z. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int. J. Biol. Macromol. 2015, 72, 951–959. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, Y.; Sun, C.; Adu-Frimpong, M.; Yu, J.; Deng, W.; Xu, X. Physicochemical properties and antidiabetic effects of a polysaccharide obtained from Polygonatum odoratum. Int. J. Food Sci. Technol. 2018, 53, 2810–2822. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Luo, Y.; Dong, G.L.; Ren, Y.Y.; Chen, L.J.; Guo, M.Z.; Wang, X.T.; Yang, X.Y.; Zhang, Y. Effects of the ultra-high pressure on structure and α-glucosidase inhibition of polysaccharide from Astragalus. Int. J. Biol. Macromol. 2016, 87, 570–576. [Google Scholar] [CrossRef]
- Xu, C.; Qin, N.; Yan, C.; Wang, S. Isolation, purification, characterization and bioactivities of a glucan from the root of Pueraria lobata. Food Funct. 2018, 9, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.P.; Huang, Q.; You, L.J.; Liu, R.H.; Zhao, M.M.; Fu, X.; Luo, Z.G. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of polysaccharides extraction from Pacific oyster (Crassostrea gigas) using subcritical water: Structural characterization and biological activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, S.S.; Schmiegelow, K.; Grell, K.; Albertsen, B.K.; Wehner, P.S.; Kampmann, P.; Frandsen, T.L. Hyperlipidemia is a risk factor for osteonecrosis in children and young adults with acute lymphoblastic leukemia. Haematologica 2017, 102, e175–e178. [Google Scholar] [CrossRef] [Green Version]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Harangi, M.; Zsíros, N.; Juhász, L.; Paragh, G. Statin-induced adverse effects—Facts and genes. Orv. Hetil. 2013, 154, 83–92. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Yan, X.; Zhang, J.; Wang, L.; Xue, H.; Jiang, G.; Ma, X.; Liu, X. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, Y.; Zhang, B. Antidiabetic Activity of a Lotus Leaf Selenium (Se)-Polysaccharide in Rats with Gestational Diabetes Mellitus. Biol. Trace Elem. Res. 2017, 176, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-F.; Zhang, Y.-Y.; Paulsen, B.S.; Rise, F.; Chen, Z.; Jia, R.; Li, L.; Song, X.; Feng, B.; Tang, H.; et al. Structural features of pectic polysaccharides from stems of two species of Radix Codonopsis and their antioxidant activities. Int. J. Biol. Macromol. 2020, 159, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S. Regulation of Nrf2-Mediated Phase II Detoxification and Anti-oxidant Genes. Biomol. Ther. 2012, 20, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Ren, Z.; Liu, X.; Luo, Y.; Long, Y.; Peng, S.; Chen, S.; Zhang, J.; Ma, Y.; Li, J.; et al. Study of the selenizing Codonopsis pilosula polysaccharides protects RAW264.7 cells from hydrogen peroxide-induced injury. Int. J. Biol. Macromol. 2019, 125, 534–543. [Google Scholar] [CrossRef]
- Gong, G.; Dang, T.; Deng, Y.; Han, J.; Zou, Z.; Jing, S.; Zhang, Y.; Liu, Q.; Huang, L.; Wang, Z. Physicochemical properties and biological activities of polysaccharides from Lycium barbarum prepared by fractional precipitation. Int. J. Biol. Macromol. 2018, 109, 611–618. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Qi, D.; Wang, D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress and inflammation in pulmonary endothelial cells. Free Radic. Res. 2018, 52, 480–490. [Google Scholar] [CrossRef]
- Liu, L.; Lao, W.; Ji, Q.; Yang, Z.; Yu, G.; Zhong, J. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015, 8, 11–16. [Google Scholar]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281–286. [Google Scholar] [CrossRef]
- Wang, A. Study on antioxidant and immune regulation effects of Codonopsis polysaccharides on deficiency of kidney-yin type rats. C. J. Tradit. Chin. Med. 2018, 30, 287–290. [Google Scholar]
- Zhang, D.; Li, S.; Xiong, Q.; Jiang, C.; Lai, X. Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr. Polym. 2013, 95, 114–122. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA-Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, M.; Wang, C.; Yang, Y.; Chen, J.; Ding, J.; Guo, W. Characterization and in vitro antitumor activity of polysaccharides from the mycelium of Sarcodon aspratus. Int. J. Biol. Macromol. 2013, 52, 52–58. [Google Scholar] [CrossRef]
- Bai, R.; Li, W.; Li, Y.; Ma, M.; Wang, Y.; Zhang, J.; Hu, F. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. Int. J. Biol. Macromol. 2018, 120, 1544–1550. [Google Scholar] [CrossRef]
- Mao, F.; Xiao, B.; Jiang, Z.; Zhao, J.; Huang, X.; Guo, J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med. Oncol. 2011, 28, 121–126. [Google Scholar] [CrossRef]
- Miao, Y.; Xiao, B.; Jiang, Z.; Guo, Y.; Mao, F.; Zhao, J.; Huang, X.; Guo, J. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med. Oncol. 2010, 27, 785–790. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Huang, J.; Li, Z.; Zhu, C.; Zhang, S. Effect of lycium barbarum polysaccharide on human hepatoma QGY7703 cells: Inhibition of proliferation and induction of apoptosis. Life Sci. 2005, 76, 2115–2124. [Google Scholar] [CrossRef]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.; Liu, A. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef]
- Thangam, R.; Sathuvan, M.; Poongodi, A.; Suresh, V.; Pazhanichamy, K.; Sivasubramanian, S.; Kanipandian, N.; Ganesan, N.; Rengasamy, R.; Thirumurugan, R.; et al. Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohydr. Polym. 2014, 107, 138–150. [Google Scholar] [CrossRef]
- Jiang, J.; Meng, F.; He, Z.; Ning, Y.; Li, X.; Song, H.; Wang, J.; Zhou, R. Sulfated modification of longan polysaccharide and its immunomodulatory and antitumor activity in vitro. Int. J. Biol. Macromol. 2014, 67, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, S.; Song, X.; Jia, J.; Zhang, Z.; Zhou, H.; Fu, H.; Cui, H.; Hu, S.; Fang, M.; et al. Inhibition effect of glycyrrhiza polysaccharide (GCP) on tumor growth through regulation of the gut microbiota composition. J. Pharmacol. Sci. 2018, 137, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, L.; Bai, R.; Zheng, X.; Ma, Y.; Gao, X.; Sun, B.; Hu, F. Structural characterization of a pectic polysaccharide from Codonopsis pilosula and its immunomodulatory activities in vivo and in vitro. Int. J. Biol. Macromol. 2017, 104, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Feng, B.; Zhu, Z.K.; Feng, X.; Chen, S.F.; Li, L.X.; Yin, Z.Q.; Huang, C.; Chen, X.F.; Zhang, B.Z.; et al. The Polysaccharides from Codonopsis pilosula Modulates the Immunity and Intestinal Microbiota of Cyclophosphamide-Treated Immunosuppressed Mice. Molecules 2018, 23, 1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ge, B.; Li, Z.; Guan, F.; Li, F. Structural analysis and immunoregulation activity comparison of five polysaccharides from Angelica sinensis. Carbohydr. Polym. 2016, 140, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Qian, L.; Gong, C.; Shen, X. Immune-Enhancing Activity of Polysaccharides from Gastrodia elata. J. Food Process. Preserv. 2016, 41, e13016. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Wu, X.; Liu, L.; Fu, W.; Song, D. Isolation, purification, characterization and immunostimulatory activity of polysaccharides derived from American ginseng. Carbohydr. Polym. 2016, 156, 9–18. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Li, J.; Wang, Z.; Cao, J.; Li, T.; Chen, J.; Chen, Y. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Wang, X.; Chen, K.; Zhang, K.; Zhang, L.; Fei, C.; Wang, C.; Liu, Y.; Xue, F.; et al. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. Int. J. Biol. Macromol. 2020, 158, 740–749. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Fu, X.; Liu, R. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Funct. 2016, 7, 4637–4643. [Google Scholar] [CrossRef]
- Cai, G.; Wu, Y.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; Feng, Z.; Liu, Z.; Wang, D. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice. Food Funct. 2021, 12, 6863–6877. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Han, Q.; Lan, J.; Chen, G.; Cao, G.; Yang, C. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1096–1105. [Google Scholar] [CrossRef]
- Jin, M.; Zhu, Y.; Shao, D.; Zhao, K.; Xu, C.; Li, Q.; Yang, H.; Huang, Q.; Shi, J. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int. J. Biol. Macromol. 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Chu, X.; Liu, X.; Qiu, J.; Zeng, X.; Bao, H.; Shu, J. Protection of Astragalus Polysaccharides and Codonopsis pilosula Polysaccharides on Alveolar Macrophage Phagocytosis of Chronic Obstructive Pulmonary Disease Mice with PM2.5 Inhalation. Chest 2016, 149, A382. [Google Scholar] [CrossRef]
- Chu, X.; Liu, X.; Qiu, J.; Zeng, X.; Bao, H.; Shu, J. Effects of Astragalus and Codonopsis pilosula polysaccharides on alveolar macrophage phagocytosis and inflammation in chronic obstructive pulmonary disease mice exposed to PM2.5. Environ. Toxicol. Pharmacol. 2016, 48, 76–84. [Google Scholar] [CrossRef]
- Han, C.; Sun, T.; Liu, Y.; Fan, G.; Zhang, W.; Liu, C. Protective effect of Polygonatum sibiricum polysaccharides on gentamicin-induced acute kidney injury in rats via inhibiting p38 MAPK/ATF2 pathway. Int. J. Biol. Macromol. 2020, 151, 595–601. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhao, X.; Tang, Q.; Dernedde, J.; Zhang, J.; Fan, H. Anti-inflammatory properties of GLPss58, a sulfated polysaccharide from Ganoderma lucidum. Int. J. Biol. Macromol. 2017, 107, 486–493. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.; Zhang, C.; Li, P.; Li, X.; Chen, X.; Qiu, S.; et al. Solomonseal polysaccharide and sulfated Codonopsis pilosula polysaccharide synergistically resist Newcastle disease virus. PLoS ONE 2015, 10, e0117916. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, L.; Xu, G.; Guo, S.; Zhang, M.; Cheng, G.; Liu, Y.; Liu, J. Platycodon grandiflorus polysaccharides inhibit Pseudorabies virus replication via downregulating virus-induced autophagy. Res. Vet. Sci. 2021, 140, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.; Lee, S.; Hwang, S.; Young, B.; Choi, H. Porcine epidemic diarrhea virus infection: Inhibition by polysaccharide from Ginkgo biloba exocarp and mode of its action. Virus Res. 2015, 195, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, L.; Zhao, J.; Cui, K. Cardioprotection activity and mechanism of Astragalus polysaccharide in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Zhang, H.; Yang, J.; Zhao, J.; Du, D.; Meng, J.; Yang, F.; Zhao, Y.; Sun, J. Protective effect of a polysaccharide from stem of Codonopsis pilosula against renal ischemia/reperfusion injury in rats. Carbohydr. Polym. 2012, 90, 1739–1743. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, Q.; Chen, H.; Hu, E.; Zhao, C.; Gong, X. Characterizations and hepatoprotective effect of polysaccharides from Mori Fructus in rats with alcoholic-induced liver injury. Int. J. Biol. Macromol. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, X.; Chen, H. Optimization of enzyme assisted extraction of Fructus Mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase. Carbohydr. Polym. 2014, 111, 775–782. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, X.; Chen, H.; Zhao, C.; Gong, X.; Zhou, X. Structural characterization, modification and hepatoprotective effects of polysaccharide from Mori Fructus. Int. J. Biol. Macromol. 2020, 153, 357–363. [Google Scholar] [CrossRef]
- Qiu, F.; He, T.; Zhang, Y. The isolation and the characterization of two polysaccharides from the branch bark of mulberry (Morus alba L.). Arch. Pharmacal Res. 2016, 39, 887–896. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.; Zhang, Y.; Zhang, F.; Wei, T.; Yang, M.; Wang, K.; Wang, Y.; Liu, N.; Cheng, H.; et al. Hepatoprotective effects of polysaccharides extracted from Zizyphus jujube cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Li, P.; Zhao, J.; Duan, J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120, 1696–1704. [Google Scholar] [CrossRef]
- Wang, C.; He, Y.; Tang, X.; Li, N. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J. Food Sci. 2020, 85, 2427–2434. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Radioprotective effects and mechanisms of animal, plant and microbial polysaccharides. Int. J. Biol. Macromol. 2020, 153, 373–384. [Google Scholar] [CrossRef]
- Yu, C.; Fu, J.; Guo, L.; Lian, L.; Yu, D. UPLC-MS-based serum metabolomics reveals protective effect of Ganoderma lucidum polysaccharide on ionizing radiation injury. J. Ethnopharmacol. 2020, 258, 112814. [Google Scholar] [CrossRef]


| Source | Extraction Methods | Extraction Conditions | Yield /% | Reference |
|---|---|---|---|---|
| Turmeric | HWE | Extraction temperature of 60–100 °C, liquid-to-material ratio of 5–25 mL/g, extraction time of 1–3 h, and extraction times of 1–3 | 2.23 | [16] |
| Angelica sinensis | HWE | Liquid-to-material ratio of 5 mL/g, extraction time of 130 min, and extraction times of 5 | 5.60 | [17] |
| Chinese yam | HWE | Extraction temperature of 100 °C, liquid-to-material ratio of 5 mL/g, extraction time of 3 h | 5.71 | [18] |
| Ginger | HWE | Extraction temperature of 100 °C, extraction time of 4 h, and liquid-to-material ratio of 20 mL/g | 11.74 | [19] |
| Cassia | HWE | Extraction temperature of 80 °C, extraction time of 3.5 h | 5.46 | [20] |
| Lentils | ACE | pH 4, extraction temperature 100 °C, extraction time 90 min | 23.30 | [32] |
| Ginger | EAE | 20,000 U/g pectinase, 63,000 U/g cellulase, and 62.5 U/g papain, extraction temperature 40 °C, pH 7.0, and extraction time 2 h | 7.00 | [19] |
| Raspberry | EAE | Pectinase:cellulase:papain of 2.5:1.7:2.1 (g/g/g), pH 4.0, liquid-to-solid ratio of 10:1 mL/g, extraction temperature of 55 °C, and extraction time of 2.6 h | 4.09 | [24] |
| Angelica sinensis | MAE | Microwave power of 500 W, liquid-to-solid ratio of 51 mL/g, and extraction time of 20 min | 7.82 | [27] |
| Cassia seed | MAE | Microwave power of 415 W, liquid-to-solid ratio of 51 mL/g, and extraction time of 7 min | 8.02 | [26] |
| Coix seeds | UAE | Ultrasonic power 480 W, extraction temperature 80 °C, liquid-to-solid ratio 21 mL/g, and extraction time 16 min | 8.34 | [29] |
| Dendrobium officinale | UAE | Ultrasonic power 144 W, extraction temperature 80 °C, liquid-to-material ratio 60 mL/g, and extraction time 3 h | 10.29 | [30] |
| Lycium barbarum | UAE | Ultrasonic power 185 W, extraction temperature 73 °C, liquid-to-material ratio 38 mL/g, and extraction time 80 min | 12.54 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhang, J.; Sang, Y.; Wei, Y.; Chen, X.; Wang, Y.; Xue, H. Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities. Molecules 2022, 27, 3215. https://doi.org/10.3390/molecules27103215
Xu J, Zhang J, Sang Y, Wei Y, Chen X, Wang Y, Xue H. Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities. Molecules. 2022; 27(10):3215. https://doi.org/10.3390/molecules27103215
Chicago/Turabian StyleXu, Jiaqi, Jinling Zhang, Yumei Sang, Yaning Wei, Xingyue Chen, Yuanxin Wang, and Hongkun Xue. 2022. "Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities" Molecules 27, no. 10: 3215. https://doi.org/10.3390/molecules27103215
APA StyleXu, J., Zhang, J., Sang, Y., Wei, Y., Chen, X., Wang, Y., & Xue, H. (2022). Polysaccharides from Medicine and Food Homology Materials: A Review on Their Extraction, Purification, Structure, and Biological Activities. Molecules, 27(10), 3215. https://doi.org/10.3390/molecules27103215






