Andrographolide Inhibits Biofilm and Virulence in Listeria monocytogenes as a Quorum-Sensing Inhibitor
Abstract
1. Introduction
2. Results
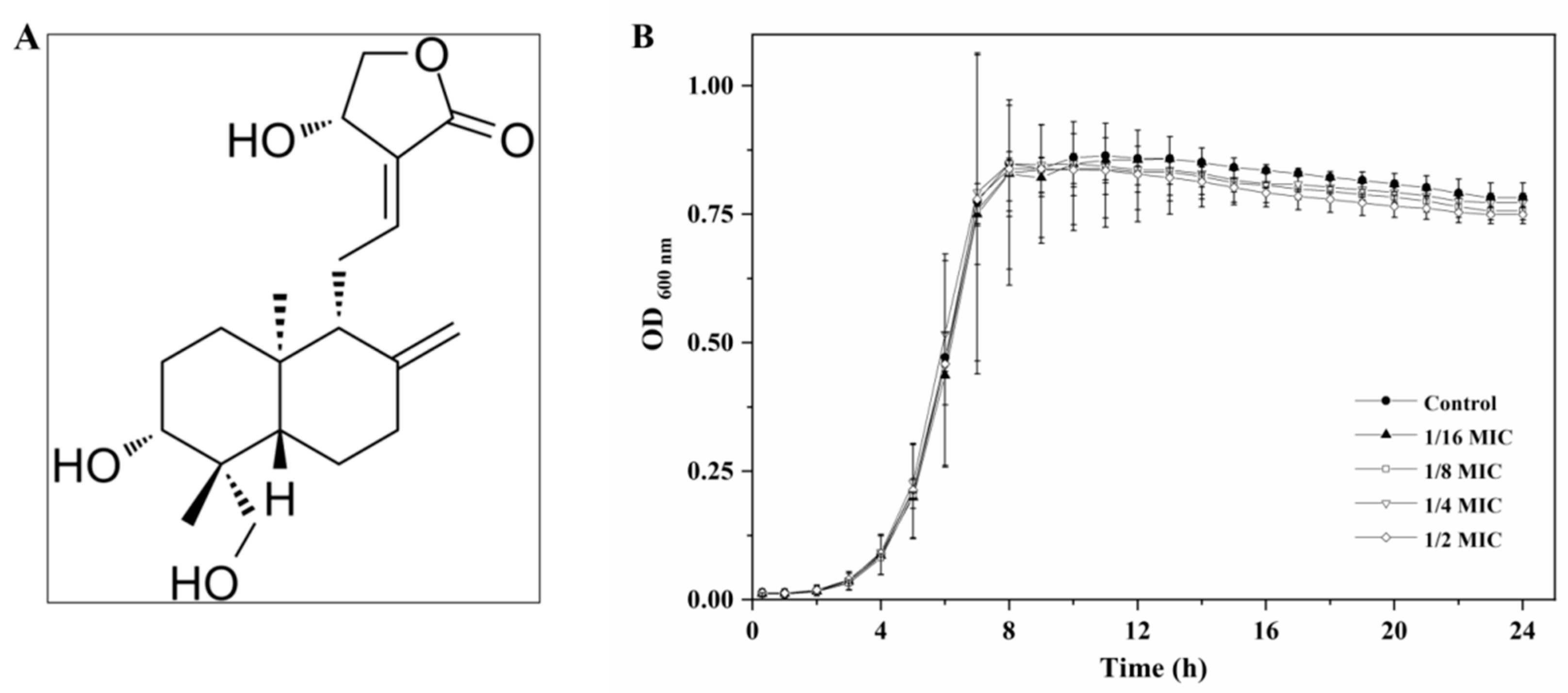
2.1. Determination of Minimum Inhibitory Concentration (MIC) of Andrographolide
2.2. Growth in the Presence of Andrographolide
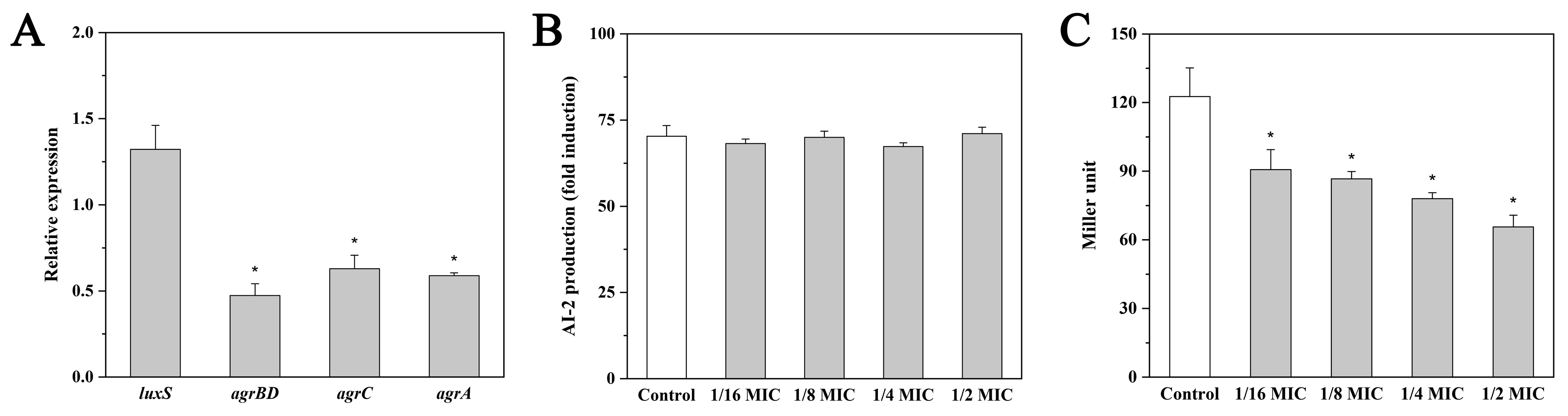
2.3. Effect of Andrographolide on luxS Expression and AI-2 Production
2.4. Effect of Andrographolide on agr Expression and the agr Promoter (P2) Activity
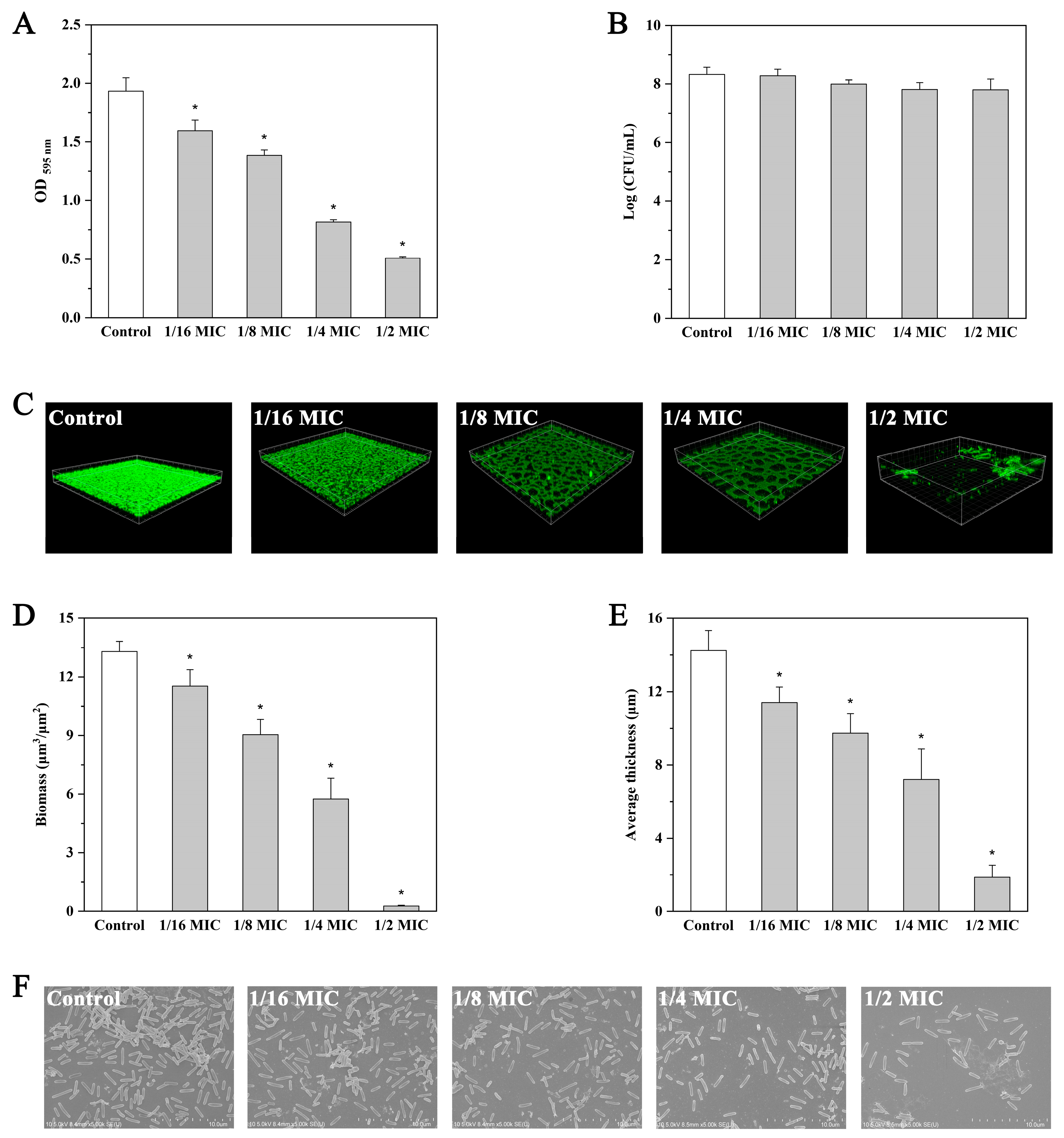
2.5. Inhibition of Biofilm Formation by Andrographolide
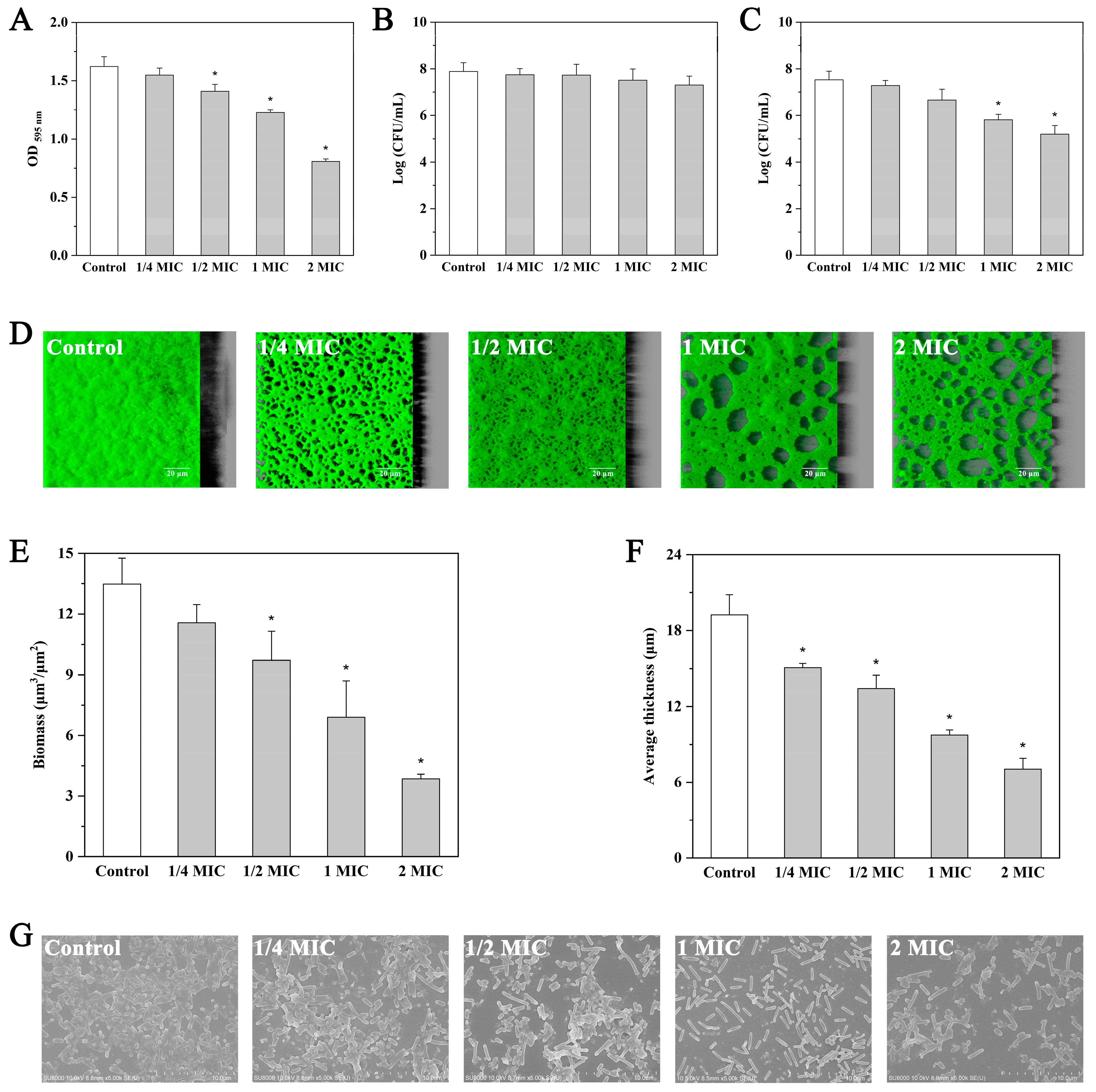
2.6. Effect of Andrographolide on Preformed Biofilms
2.7. Effect of Andrographolide on Motility
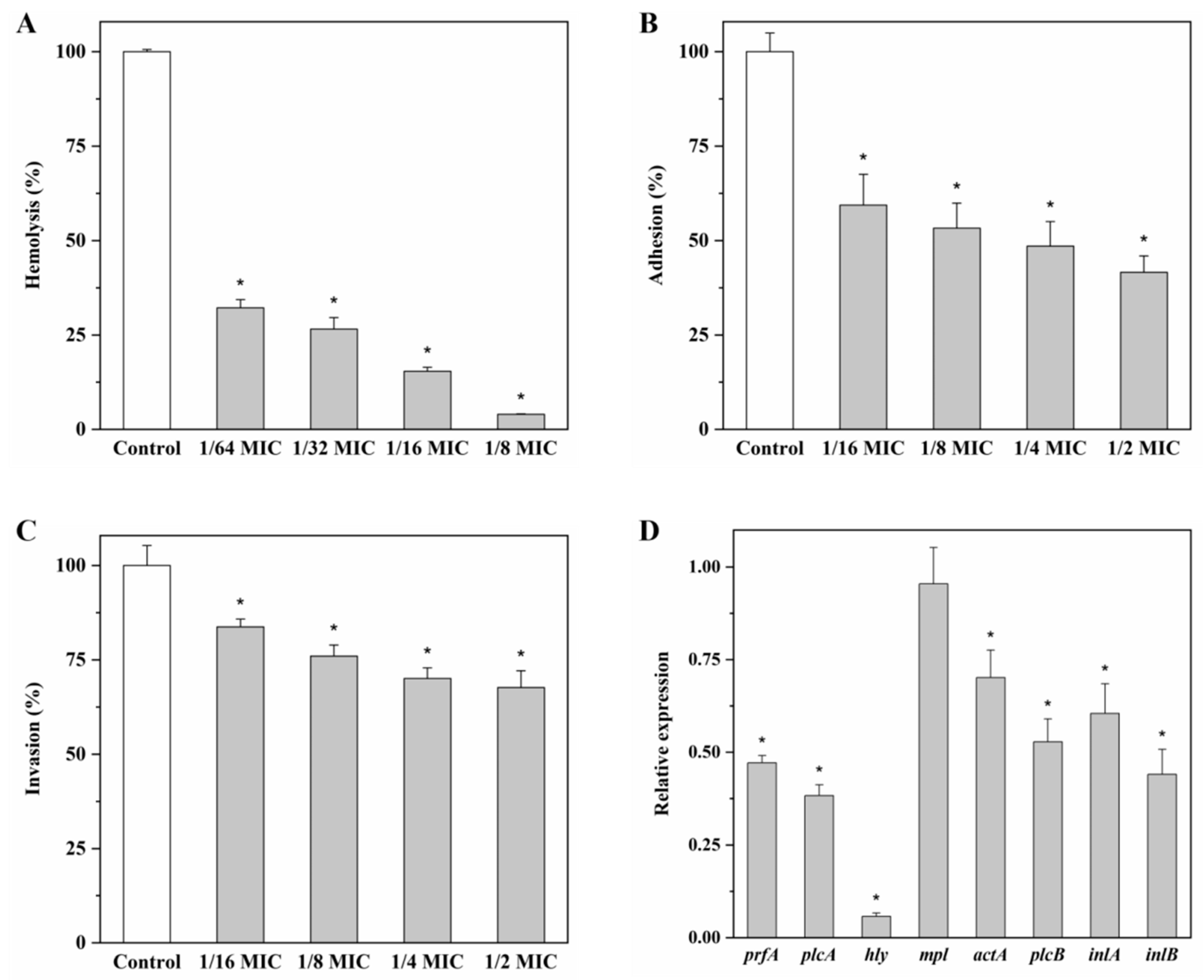
2.8. Effect of Andrographolide on Virulence Factors
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Determination of MIC of Andrographolide
4.3. Growth Curve Analysis
4.4. Gene Expression Analyses
4.5. Detection of AI-2
4.6. Determination of the agr Promoter P2 Activity
4.7. Effect of Andrographolide on Biofilm Formation by L. monocytogenes
4.8. Effect of Andrographolide on Preformed Biofilms by L. monocytogenes
4.9. CLSM Biofilm Formation Assay
4.10. FE-SEM Analysis
4.11. Motility
4.12. Effect of Andrographolide on L. monocytogenes Key Virulence Factors
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Drevets, D.A.; Bronze, M.S. Listeria monocytogenes: Epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 2008, 53, 151–165. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J.; Mitchell, R.T.; Smerdon, W.J.; Jewell, K. Listeria monocytogenes and listeriosis: A review of hazard characterisation for use in microbiological risk assessment of foods. Int. J. Food Microbiol. 2004, 92, 15–33. [Google Scholar] [CrossRef]
- Clark, C.G.; Farber, J.; Pagotto, F.; Ciampa, N.; Dor, K.; Bernard, K.; Ng, L.K. Surveillance for Listeria monocytogenes and listeriosis, 1995–2004. Epidemiol. Infect. 2010, 138, 559–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Todd, E.C.D.; Notermans, S. Surveillance of listeriosis and its causative pathogen, Listeria monocytogenes. Food Control 2011, 22, 1484–1490. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Chong, G.; Kimyon, Ö.; Manefield, M. Quorum sensing signal synthesis may represent a selective advantage independent of its role in regulation of bioluminescence in Vibrio fischeri. PLoS ONE 2013, 8, e67443. [Google Scholar] [CrossRef]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef]
- Chong, Y.M.; How, K.Y.; Yin, W.F.; Yin, W.F.; Chan, K.G. The effects of chinese herbal medicines on the quorum sensing-regulated virulence in Pseudomonas aeruginosa PAO1. Molecules 2018, 23, 972. [Google Scholar] [CrossRef]
- Schauder, S.; Shokat, K.; Surette, M.G.; Bassler, B.L. The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 2001, 41, 463–476. [Google Scholar] [CrossRef]
- Pei, D.; Zhu, J. Mechanism of action of S-ribosylhomocysteinase (LuxS). Curr. Opin. Chem. Biol. 2004, 8, 492–497. [Google Scholar] [CrossRef]
- Belval, S.C.; Gal, L.; Margiewes, S.; Garmyn, D.; Piveteau, P.; Guzzo, J. Assessment of the roles of LuxS, S-ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 2006, 72, 2644–2650. [Google Scholar] [CrossRef] [PubMed]
- Sela, S.; Frank, S.; Belausov, E.; Pinto, R. A Mutation in the luxS gene influences Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2006, 72, 5653–5658. [Google Scholar] [CrossRef] [PubMed]
- Autret, N.; Raynaud, C.; Dubail, I.; Berche, P.; Charbit, A. Identification of the agr locus of Listeria monocytogenes: Role in bacterial virulence. Infect. Immun. 2003, 71, 4463–4471. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.U.; Monk, I.R.; Casey, P.G.; Waidmann, M.S.; Gahan, C.G.M.; Hill, C. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 2009, 71, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Rieu, A.; Weidmann, S.; Garmyn, D.; Piveteau, P.; Guzzo, J. agr system of Listeria monocytogenes EGD-e: Role in adherence and differential expression pattern. Appl. Environ. Microbiol. 2007, 73, 6125–6133. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Zhang, Y.; Fang, Z.; Sun, L. Interferce effect of 4-hydroxy-2,5-dimethyl-3(2H)-furanone on AI-2 quorum sensing of Listeria monocytogenes. Sci. Technol. Food Ind. 2018, 39, 87–92. (In Chinese) [Google Scholar] [CrossRef]
- Todd, D.A.; Parlet, C.P.; Crosby, H.A.; Malone, C.L.; Heilmann, K.P.; Horswill, A.R.; Cech, N.B. Signal biosynthesis inhibition with ambuic acid as a strategy to target antibiotic-resistant infections. Antimicrob. Agents Chemother. 2017, 61, e00263-17. [Google Scholar] [CrossRef]
- Melian, C.; Segli, F.; Gonzalez, R.; Vignolo, G.; Castellano, P. Lactocin AL705 as quorum sensing inhibitor to control Listeria monocytogenes biofilm formation. J. Appl. Microbiol. 2019, 127, 911–920. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lii, C.K.; Tsai, I.T.; Li, C.C.; Liu, K.L.; Tsai, C.W.; Chen, H.W. Andrographolide inhibits ICAM-1 expression and NF-κB activation in TNF-α-treated EA.hy926 cells. J. Agric. Food Chem. 2011, 59, 5263–5271. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Liang, H.; Che, Y.; Chen, C.; Dai, H.; Yu, K.; Liu, M.; Ma, L.; Yang, C.H.; et al. Effects of 14-alpha-lipoyl andrographolide on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2012, 56, 6088–6094. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.Y.; Wu, S.C.; Xia, F.; Fu, Y.X.; Wu, Y.L.; Leng, C.Q.; Yi, P.F.; Shen, H.Q.; Wei, X.B.; et al. Andrographolide interferes quorum sensing to reduce cell damage caused by avian pathogenic Escherichia coli. Vet. Microbiol. 2014, 174, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’Orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- In Lee, S.H.; Barancelli, G.V.; de Camargo, T.M.; Corassin, C.H.; Rosim, R.E.; da Cruz, A.G.; Cappato, L.P.; de Oliveira, C.A.F. Biofilm-producing ability of Listeria monocytogenes isolates from Brazilian cheese processing plants. Food Res. Int. 2017, 91, 88–91. [Google Scholar] [CrossRef]
- Rezzonico, F.; Duffy, B. Lack of genomic evidence of AI-2 receptors suggests a non-quorum sensing role for luxS in most bacteria. BMC Microbiol. 2008, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Zetzmann, M.; Sánchez-Kopper, A.; Waidmann, M.S.; Blombach, B.; Riedel, C.U. Identification of the agr peptide of Listeria monocytogenes. Front. Microbiol. 2016, 7, 989. [Google Scholar] [CrossRef] [PubMed]
- Dogsa, I.; Spacapan, M.; Dragoš, A.; Danevčič, T.; Pandur, Ž.; Mandic-Mulec, I. Peptide signaling without feedback in signal production operates as a true quorum sensing communication system in Bacillus subtilis. Commun. Biol. 2021, 4, 58. [Google Scholar] [CrossRef]
- Guttenplan, S.B.; Kearns, D.B. Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 2013, 37, 849–871. [Google Scholar] [CrossRef]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Young, G.M. Loss of flagellum-based motility by Listeria monocytogenes results in formation of hyperbiofilms. J. Bacteriol. 2008, 190, 6030–6034. [Google Scholar] [CrossRef]
- Camejo, A.; Carvalho, F.; Reis, O.; Leitão, E.; Sousa, S.; Cabanes, D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2011, 2, 379–394. [Google Scholar] [CrossRef]
- Sheehan, B.; Klarsfeld, A.; Msadek, T.; Cossart, P. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 1995, 177, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, T.; Xu, Y.; Wang, H.; Korkeala, H.; Shi, L. MdrL, a major facilitator superfamily efflux pump of Listeria monocytogenes involved in tolerance to benzalkonium chloride. Appl. Microbiol. Biotechnol. 2019, 103, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ren, S.; Geng, Y.; Jiang, C.; Liu, G.; Wang, H.; Yu, T.; Liang, Y. Role of the VirSR-VirAB system in biofilm formation of Listeria monocytogenes EGD-e. Food Res. Int. 2021, 145, 110394. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.E.; Xavier, K.B. Methods for analysis of bacterial autoinducer-2 production. Curr. Protoc. Microbiol. 2011, 1, 1–15. [Google Scholar] [CrossRef]
- Collins, B.; Guinane, C.M.; Cotter, P.D.; Hill, C.; Paul Ross, R. Assessing the contributions of the lias histidine kinase to the innate resistance of Listeria monocytogenes to nisin, cephalosporins, and disinfectants. Appl. Environ. Microbiol. 2012, 78, 2923–2929. [Google Scholar] [CrossRef]
- O’Driscoll, J.; Glynn, F.; Cahalane, O.; O’Connell-Motherway, M.; Fitzgerald, G.F.; Van Sinderen, D. Lactococcal plasmid pNP40 encodes a novel, temperature-sensitive restriction-modification system. Appl. Environ. Microbiol. 2004, 70, 5546–5556. [Google Scholar] [CrossRef]
- Deng, Z.; Shan, Y.; Pan, Q.; Gao, X.; Yan, A. Anaerobic expression of the gadE-mdtEF multidrug efflux operon is primarily regulated by the two-component system ArcBA through antagonizing the H-NS mediated repression. Front. Microbiol. 2013, 4, 194. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Guilbaud, M.; Piveteau, P.; Desvaux, M.; Brisse, S.; Briandet, R. Exploring the diversity of Listeria monocytogenes biofilm architecture by high-throughput confocal laser scanning microscopy and the predominance of the honeycomb-like morphotype. Appl. Environ. Microbiol. 2015, 81, 1813–1819. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, R.; Zhao, X.; Shi, C.; Zhang, X.; Zhang, Y.; Guo, N. Inhibition effect of tea tree oil on Listeria monocytogenes growth and exotoxin proteins listeriolysin O and p60 secretion. Lett. Appl. Microbiol. 2016, 63, 450–457. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Jiang, X.; Xu, X.; Jiang, C.; Kang, R.; Jiang, X. Andrographolide Inhibits Biofilm and Virulence in Listeria monocytogenes as a Quorum-Sensing Inhibitor. Molecules 2022, 27, 3234. https://doi.org/10.3390/molecules27103234
Yu T, Jiang X, Xu X, Jiang C, Kang R, Jiang X. Andrographolide Inhibits Biofilm and Virulence in Listeria monocytogenes as a Quorum-Sensing Inhibitor. Molecules. 2022; 27(10):3234. https://doi.org/10.3390/molecules27103234
Chicago/Turabian StyleYu, Tao, Xiaojie Jiang, Xiaobo Xu, Congyi Jiang, Rui Kang, and Xiaobing Jiang. 2022. "Andrographolide Inhibits Biofilm and Virulence in Listeria monocytogenes as a Quorum-Sensing Inhibitor" Molecules 27, no. 10: 3234. https://doi.org/10.3390/molecules27103234
APA StyleYu, T., Jiang, X., Xu, X., Jiang, C., Kang, R., & Jiang, X. (2022). Andrographolide Inhibits Biofilm and Virulence in Listeria monocytogenes as a Quorum-Sensing Inhibitor. Molecules, 27(10), 3234. https://doi.org/10.3390/molecules27103234






