Application of Dendrimers in Anticancer Diagnostics and Therapy
Abstract
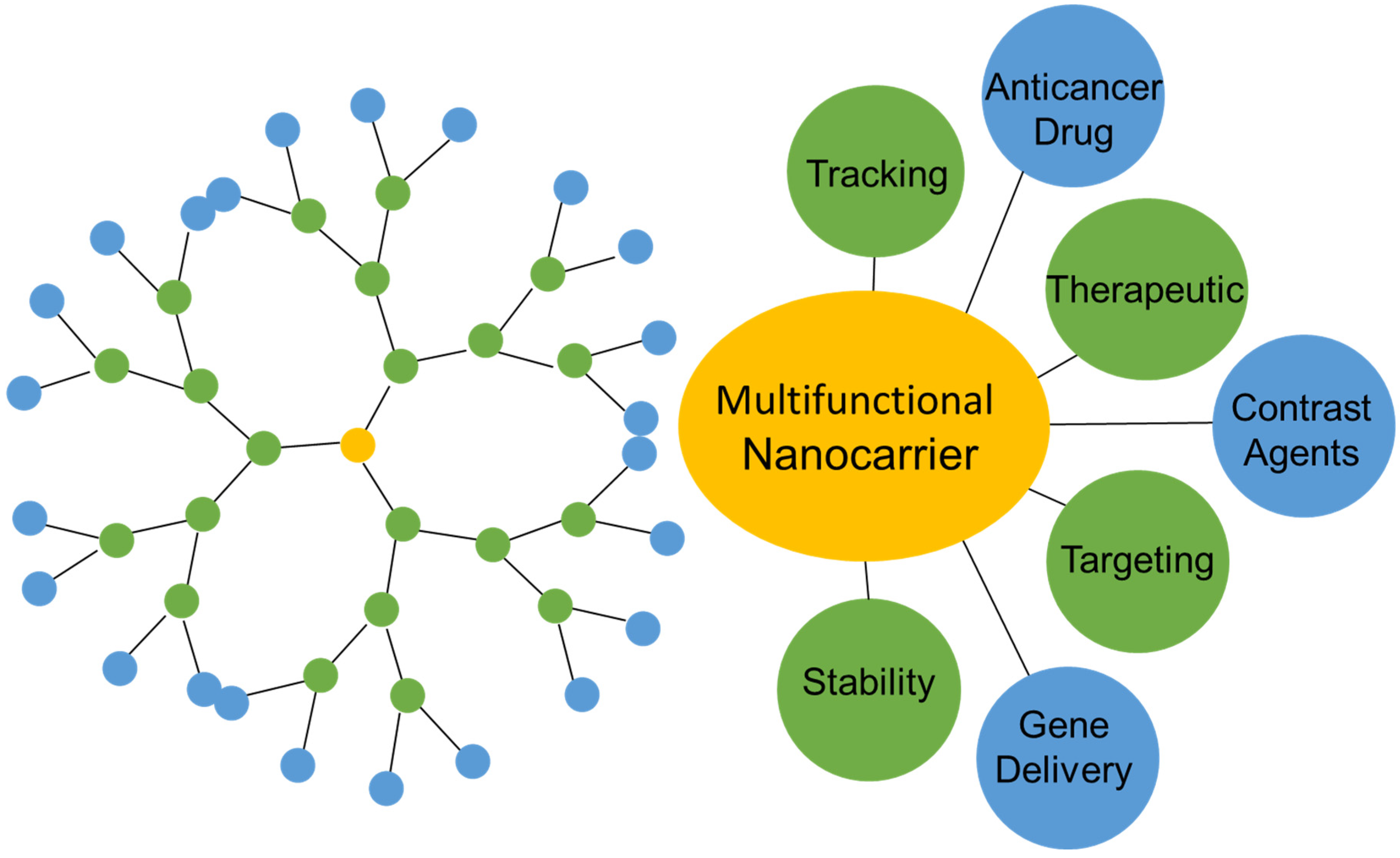
:1. Introduction
2. Different Generations of Polyamidoamine (PAMAM) Dendrimers
3. The Use of Dendrimers in Drug Delivery
3.1. Dendrimer Conjugates with Pharmaceutically Active Substances In Vitro
3.2. Application of Dendrimers in Oncological Therapy
3.3. Use of Dendrimers as Contrast Agents
3.4. In Vitro Study—Use of Dendrimers As Contrast Agents
3.5. In Vivo Study—Use of Dendrimers As Contrast Agents
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Doubrovin, M.; Serganova, I.; Mayer-Kuckuk, P.; Ponomares, V.; Blasberg, R.G. Multimodality in vivo molecular-genetic imaging. Bioconjugate Chem. 2004, 15, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Franano, F.N.; Edwards, W.B.; Welch, M.J.; Brechbiel, M.W.; Gansow, O.A.; Duncan, J.R. Biodistribution and metabolism of targeted and nontargeted protein-chelate-gadolinium complexes: Evidence for gadolinium dissociation in vitro and in vivo. Magn. Reson. Imaging 1995, 13, 201–214. [Google Scholar] [CrossRef]
- Stiriba, S.E.; Frey, H.; Haag, R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew. Chem. Int. Ed. 2002, 9, 1329–1334. [Google Scholar] [CrossRef]
- Srinivasa-Gopalan, S.; Yarema, K.J. Nanotechnologie for the Life Sciences: Dendrimers in Cancer Treatment and Diagnosis; Wiley: New York, NY, USA, 2007. [Google Scholar]
- Tomalia, D.A.; Frechet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A 2002, 9, 2719. [Google Scholar] [CrossRef]
- Camacho, C.; Tomás, H.; Rodrigues, J. Use of Half-Generation PAMAM Dendrimers (G0.5–G3.5) with Carboxylate End-Groups to Improve the DACHPtCl2 and 5-FU Efficacy as Anticancer Drugs. Molecules 2021, 26, 2924. [Google Scholar] [CrossRef]
- Zimmerman, S.C. Dendrimers w rozpoznawaniu molekularnym i samoorganizacji. Curr. Opin. Colloid Interface Sci. 1997, 9, 89. [Google Scholar] [CrossRef]
- Caminade, A.M.; Ching, K.I.M.; Delavaux-Nicot, B. The Usefulness of Trivalent Phosphorus for the Synthesis of Dendrimers. Molecules 2021, 26, 269. [Google Scholar] [CrossRef]
- Cheng, B.; Kaifer, A.E. Reaction of Amino-Terminated PAMAM Dendrimers with Carbon Dioxide in Aqueous and Methanol Solutions. Molecules 2022, 27, 540. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir-Riahi, H.A. Thermodynamic analysis of biogenic and synthetic polyamines conjugation with PAMAM-G4 nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 155, 13–19. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Fang, L.F.; Shen, Y.J.; Yu, W.H.; Zhu, B.K.; Hélix-Nielsen, C.; Zhang, W. Ionic Dendrimer Based Polyamide Membranes for Ion Separation. ACS Nano 2021, 15, 7522–7535. [Google Scholar] [CrossRef]
- Cooper, B.M.; Iegre, J.; O’Donovan, D.H.; Halvarsson, M.Ö.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide-drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Bazyari-Delavar, S.; Badalkhani-Khamseh, F.; Ebrahim-Habibi, A.; Hadipour, N.L. PAMAM and polyester dendrimers as favipiravir nanocarriers: A comparative study using DFT method. J. Nanoparticle Res. 2021, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent Advances in Epsilon-Poly-L-Lysine and L-Lysine-Based Dendrimer Synthesis, Modification, and Biomedical Applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef]
- Gómez-Avilés, A.; Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Functionalized carbon-silicates from caramel-sepiolite nanocomposites. Angew. Chem. Int. Ed. Engl. 2007, 46, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar] [CrossRef]
- Fedeli, E.; Lancelot, A.; Dominguez, J.M.; Serrano, J.L.; Calvo, P.; Sierra, T. Self-Assembling Hybrid Linear-Dendritic Block Copolymers: The Design of Nano-Carriers for Lipophilic Antitumoral Drugs. Nanomaterials 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Li, R.; Shu, W.; Zhao, L.; Wan, J. Self-assembly of peptide dendrimers and their bio-applications in teranostics. Mater. Today Bio. 2022, 14, 100239. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Dias Martins, A.S.; Pallante, L.; Zizzi, E.A.; Miceli, M.; Ba Tal, M.; Reis, C.P.; Deriu, M.A.; Klajnert-Maculewicz, B. Noncovalent Interactions with PAMAM and PPI Dendrimers Promote the Cellular Uptake and Photodynamic Activity of Rose Bengal: The Role of the Dendrimer Structure. J. Med. Chem. 2021, 64, 15758–15771. [Google Scholar] [CrossRef]
- Grayson, S.M.; Frechet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 9, 3819–3868. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.F.; Altalbawy, M.A.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, S.; Rahman, S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, M.D.K.; Chougule, M.B. Recent Developments in Active Tumor Targeted Multifunctional Nanoparticles for Combination Chemotherapy in Cancer Treatment and Imaging. J. Biomed. Nanotechnol. 2015, 11, 1859–1898. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, A.; Malinga-Drozd, M.; Kałas, W.; Kopaczyńska, M.; Wołowiec, S.; Borowska, K. Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System. Int. J. Mol. Sci. 2021, 22, 1982. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310. [Google Scholar] [CrossRef]
- Jurj, A.; Braicu, C.; Pop, L.A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [Green Version]
- Kulhari, H.; Pooja, D.; Shrivastava, S.; Kuncha, M.; Naidu, V.G.M.; Bansal, V.; Sistla, R.; Adams, D.J. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 2016, 6, 23179. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.Y.; Setó, S.; Al Khalil, N.; Aleshaiwi, L.; Alghamdi, M.; Alquadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H.; et al. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. Int. J. Nanomed. 2020, 15, 5433–5443. [Google Scholar] [CrossRef]
- Otis, J.B.; Zong, H.; Kotylar, A.; Yin, A.; Bhattacharjee, S.; Wang, H.; Baker, J.R., Jr.; Wang, S.H. Dendrimer antibody conjugate to target and image HER-2 overexpressing cancer cells. Oncotarget 2016, 7, 36002–36013. [Google Scholar] [CrossRef] [Green Version]
- Matai, I.; Gopinath, P. Hydrophobic myristic acid modified PAMAM dendrimers augment the delivery of tamoxifen to breast cancer cells. RSC Adv. 2016, 6, 24808–24819. [Google Scholar] [CrossRef]
- Sztandera, K.; Działak, P.; Marcinkowska, M.; Stańczyk, M.; Gorzkiewicz, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Sugar Modification Enhances Cytotoxic Activity of PAMAM-Doxorubicin Conjugate in Glucose-Deprived MCF-7 Cells—Possible Role of GLUT1 Transporter. Pharm. Res. 2019, 36, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavan, A.; Ilaiyaraja, P.; Beaula, W.S.; Kumari, V.V.; Lal, J.S.; Arunkumar, C.; Anjana, G.; Srinivas, S.; Ramesh, A.; Rayala, S.K.; et al. Tumor targeting using polyamidoamine dendrimer-cisplatin nanoparticles functionalized with diglycolamic acid and herceptin. Eur. J. Pharm. Biopharm. 2015, 96, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.L.; Zhang, J.; Li, W.; Bian, B.X.; Hong, Y.D.; Song, Z.Y.; Wang, H.Y.; Cui, F.B.; Li, R.T.; Liu, Q.; et al. Enhanced antiproliferative activity of antibody-functionalized polymeric nanoparticles for targeted delivery of anti-miR-21 to HER2 positive gastric cancer. Oncotarget 2017, 8, 67189–67202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narsireddy, A.; Vijayashree, K.; Adimoolam, M.G.; Manorama, S.V.; Rao, N.M. Photosensitizer and peptide-conjugated PAMAM dendrimer for targeted in vivo photodynamic therapy. Int. J. Nanomed. 2015, 10, 6865–6878. [Google Scholar]
- Wang, J.; Li, B.; Huang, D.; Norat, P.; Grannonico, M.; Cooper, R.C.; Gui, Q.; Chow, W.N.; Liu, X.; Yang, H. Nano-in-Nano Dendrimer Gel Particles for Efficient Topical Delivery of Antiglaucoma Drugs into the Eye. Chem. Eng. J. 2021, 425, 130498. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Chrzanowski, G.; Bober, Z.; Aebisher, D. An analytical study of Trastuzumab-dendrimer-fluorine drug delivery system in breast cancer therapy in vitro. Biomed. Pharmacother. 2021, 133, 111053. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Birhan, Y.S.; Andrgie, A.T.; Hanurry, E.Y.; Darge, H.F.; Chou, H.Y.; Lai, J.Y.; Tsai, H.C.; Yang, J.M.; Chang, Y.H. Encapsulation of gadolinium ferrite nanoparticle in generation 4.5 poly(amidoamine) dendrimer for cancer theranostics applications using low frequency alternating magnetic field. Colloids Surf. B Biointerfaces 2019, 184, 110531. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Sobierajska, E.; Stanczyk, M.; Janaszewska, A.; Chworos, A.; Klajnert-Maculewicz, B. Conjugate of PAMAM Dendrimer, Doxorubicin and Monoclonal Antibody—Trastuzumab: The New Approach of a Well-Known Strategy. Polymers 2018, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, M.; Mehravi, B.; Ardestani, M.S.; Ziaee, S.A.; Pourghasem, P. In Vitro Evaluation of Gd(3+)-Anionic Linear Globular Dendrimer-Monoclonal Antibody: Potential Magnetic Resonance Imaging Contrast Agents for Prostate Cancer Cell Imaging. Mol. Imaging Biol. 2015, 17, 770–776. [Google Scholar] [CrossRef]
- Jain, K.; Verma, A.K.; Mishra, P.R.; Jain, N.K. Characterization and evaluation of amphotericin B loaded MDP conjugated poly (propylene imine) dendrimers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 705–713. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.; Zhang, X.; Ni, L.; Li, J.; Zhang, F.; Wang, Z.; Lian, S.; Sun, K. Targeted delivery of polyamidoamine-paclitaxel conjugate functionalized with anti-human epidermal growth factor receptor 2 trastuzumab. Int. J. Nanomed. 2015, 10, 2173–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Z.H.; Zhuang, Q.F.; Li, Y.C.; He, Z.; Chen, Z.; Huang, S.P.; Jia, H.Y.; Zhou, J.W.; Liu, Y.; Du, L.B. Polyamidoamine dendrimer conjugated chitosan nanoparticles for the delivery of methotrexate. Carbohydr. Polym. 2013, 98, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Rouhollah, K.; Pelin, M.; Serap, Y.; Gozde, U.; Ufuk, G. Doxorubicin loading, release, and stability of polyamidoamine dendrimer-coated magnetic nanoparticles. J. Pharmaceut. Sci. 2013, 102, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Mullen, D.G.; Fang, M.; Desai, A.; Baker, J.R., Jr.; Orr, B.G.; Banaszak Holl, M.M. A Quantitative Assessment of Nanoparticle-Ligand Distributions: Implications for Targeted Drug and Imaging Delivery in Dendrimer Conjugates. ACS Nano 2010, 4, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Itoh, Y.; Hosaka, Y.; Kobayashi, I.; Nakano, Y.; Maeda, I.; Umeda, F.; Yamakawa, J.; Kawase, M.; Yag, K. Novel transdermal drug delivery system with polyhydroxyalkanoate and starburst polyamidoamine dendrimer. J. Biosci. Bioeng. 2003, 95, 541–543. [Google Scholar] [CrossRef]
- Xiong, Z.; Shen, M.; Shi, X. Dendrimer-based strategies for cancer therapy: Recent advances and future perspectives. Sci. China Mater. 2018, 61, 1387–1403. [Google Scholar] [CrossRef]
- Das, M.; Mohnanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304. [Google Scholar] [CrossRef]
- Ketab, B.; Chiu, K.; Black, K.L.; Yamamoto, V.; Khalsa, B.; Ljubimova, J.Y.; Ding, H.; Patil, R.; Portilla-Arias, J.A.; Modo, M.; et al. Nanoplatforms for constructing New approaches to cancer treatment, imaging, and drug delivery. What should by the policy? NeuroImage 2011, 54, 106–124. [Google Scholar]
- Tekade, R.K.; Kumar, P.V.; Jain, N.K. Dendrimers in oncology: An expanding horizon. Chem. Rev. 2009, 109, 49–87. [Google Scholar] [CrossRef]
- Larsen, A.K.; Escargueil, A.E.; Skladanowski, A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 2000, 85, 217–229. [Google Scholar] [CrossRef]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Li, S.; Zhan, Q.; Li, X.; Xing, H.; Chen, N.; Long, L.; Hou, X.; Zhao, J.; Yuan, X. PMPC Modified PAMAM Dendrimer Enhances Brain Tumor-Targeted Drug Delivery. Macromol. Biosci. 2021, 21, 2000392. [Google Scholar] [CrossRef]
- Bai, S.B.; Cheng, Y.; Liu, D.Z.; Ji, Q.F.; Liu, M.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Bone-targeted PAMAM nanoparticle to treat bone metastases of lung cancer. Nanomedicine 2020, 15, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Surekha, B.; Kommana, N.S.; Dubey, S.K.; Kumar, A.V.P.; Shukla, R.; Kesharwani, P. PAMAM dendrimer as a talented multifunctional biomimetic nanocarrier for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces 2021, 204, 111837. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ren, X.; Wang, W.; Ke, L.; Ning, E.; Du, L.; Bradshaw, J. A 5-fluorouracil-loaded pH-responsive dendrimer nanocarrier for tumor targeting. Int. J. Pharm. 2011, 420, 378–384. [Google Scholar] [CrossRef]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, J.; Wang, H.; Lv, J.; Cheng, Y. A Combination of Guanidyl and Phenyl Groups on a Dendrimer Enables Efficient siRNA and DNA Delivery. Biomacromolecules 2017, 18, 2371–2378. [Google Scholar] [CrossRef]
- Thakur, S.; Tekade, R.K.; Kesharwani, P.; Jain, N.K. The effect of polyethylene glycol spacer chain length on the tumor-targeting potential of folate-modified PPI dendrimers. J. Nanoparticle Res. 2013, 15, 1625. [Google Scholar] [CrossRef]
- Bhargava, M.; Bhargava, S.; Bhargava, V. Mannosylated Poly (Propylene Imine) Dendrimer Mediated Lung Delivery of Anticancer Bioactive. J. Thorac. Oncol. 2017, 12, 1272. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.; Gajbhiye, V.; Jain, N.K. Synthesis, characterization and brain targeting potential of paclitaxel loaded thiamine-PPI nanoconjugates. J. Drug Target. 2012, 20, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Blatchford, D.R.; Millington, O.; Stevenson, M.L.; Dufès, C. Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J. Control. Release 2014, 188, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studzian, M.; Szulc, A.; Janaszewska, A.; Appelhans, D.; Pułaski, Ł.; Klajnert-Maculewicz, B. Mechanisms of Internalization of Maltose-Modified Poly(propyleneimine) Glycodendrimers into Leukemic Cell Lines. Biomacromolecules 2017, 18, 1509–1520. [Google Scholar] [CrossRef]
- Franiak-Pietryga, I.; Ziemba, B.; Sikorska, H.; Jander, M.; Kuncman, W.; Danilewicz, M.; Appelhans, D.; Lewkowicz, P.; Ostrowska, K.; Bryszewska, M.; et al. Maltotriose-modified poly(propylene imine) Glycodendrimers as a potential novel platform in the treatment of chronic lymphocytic Leukemia. A proof-of-concept pilot study in the animal model of CLL. Toxicol. Appl. Pharmacol. 2020, 403, 115139. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- McMahon, M.T.; Bulte, J.W.M. Two decades of dendrimers as versatile MRI agents: A tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1496. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Oskolkov, N.; Song, X.; Lal, B.; Yang, X.; Pomper, M.; Laterra, J.; Nimmagadda, S.; McMahon, M.T. Salicylic Acid Conjugated Dendrimers Are a Tunable, High Performance CEST MRI NanoPlatform. Nano Lett. 2016, 16, 2248–2253. [Google Scholar] [CrossRef] [Green Version]
- Snoussi, K.; Bulte, J.W.M.; Guéron, M.; van Zijl, P.C.M. Sensitive CEST agents based on nucleic acid imino proton exchange: Detection of poly(rU) and of a dendrimer-poly(rU) model for nucleic acid delivery and pharmacology. Magn. Reason. Med. 2003, 49, 998–1005. [Google Scholar] [CrossRef]
- Shen, J.M.; Li, X.X.; Fan, L.L.; Zhou, X.; Han, J.M.; Jia, M.K.; Wu, L.F.; Zhang, X.X.; Chen, J. Heterogeneous dimer peptide-conjugated polylysine dendrimer-Fe3O4 composite as a novel nanoscale molecular probe for early diagnosis and therapy in hepatocellular carcinoma. Int. J. Nanomed. 2017, 12, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Gale, E.M.; Atanasova, I.; Rietz, T.A.; Caravan, P. Hexameric Mn(II) Dendrimer as MRI Contrast Agent. Chemistry 2014, 20, 14507–14513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Ouyang, Z.; Guo, H.; Qu, J.; Gao, Y.; Xia, J.; Shen, M.; Shi, X. Core-Shell Tecto Dendrimers Enable Enhanced Tumor MR Imaging through an Amplified EPR Effect. Biomacromolecules 2021, 22, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lloveras, V.; Pulido, D.; Liko, F.; Pinto, L.F.; Albericio, F.; Royo, M.; Vidal-Gancedo, J. Radical Dendrimers Based on Biocompatible Oligoethylene Glycol Dendrimers as Contrast Agents for MRI. Pharmaceutics 2020, 12, 772. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Teesdale-Spittle, P.H.; Lewis, A.R.; Rendle, P.M. Gadolinium Complexes Attached to Poly Ethoxy Ethyl Glycinamide (PEE-G) Dendrons: Magnetic Resonance Imaging Contrast Agents with Increased Relaxivity. ChemPlusChem 2020, 85, 1881–1892. [Google Scholar] [CrossRef]
- Hectors, S.J.; Jacobs, I.; Lok, J.; Peters, J.; Bussink, J.; Hoeben, F.J.; Keizer, H.M.; Janssen, H.M.; Nicolay, K.; Schabel, M.C.; et al. Improved Evaluation of Antivascular Cancer Therapy Using Constrained Tracer-Kinetic Modeling for Multiagent Dynamic Contrast-Enhanced MRI. Cancer Res. 2018, 78, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Kondo, T.; Kimura, Y.; Yamada, H.; Aoyama, Y. Polymeric 1 H MRI Probes for Visualizing Tumor In Vivo. Chem. Rec. 2017, 17, 555–568. [Google Scholar] [CrossRef]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent Folate-Dendrimer-Coated Iron Oxide Theranostic Nanoparticles for Simultaneous Magnetic Resonance Imaging and Precise Cancer Cell Targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Gündüz, S.; Savić, T.; Toljić, Đ.; Angelovski, G. Preparation and In Vitro Characterization of Dendrimer-based Contrast Agents for Magnetic Resonance Imaging. J. Vis. Exp. 2016, 118, 54776. [Google Scholar] [CrossRef] [Green Version]
- Gündüz, S.; Savić, T.; Pohmann, R.; Logothetis, N.K.; Scheffler, K.; Angelovski, G. Ratiometric Method for Rapid Monitoring of Biological Processes Using Bioresponsive MRI Contrast Agents. ACS Sens. 2016, 1, 483–487. [Google Scholar] [CrossRef]
- Haribabu, V.; Farook, A.S.; Goswami, N.; Murugesan, R.; Girigoswami, A. Optimized Mn-doped iron oxide nanoparticles entrapped in dendrimer for dual contrasting role in MRI. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 817–824. [Google Scholar] [CrossRef]
- Miyake, Y.; Ishikawa, S.; Kimura, Y.; Son, A.; Imai, H.; Matsuda, T.; Yamada, H.; Toshimitsu, A.; Kondo, T. Pharmacokinetics of Chiral Dendrimer-Triamine-Coordinated Gd-MRI Contrast Agents Evaluated by in Vivo MRI and Estimated by in Vitro QCM. Sensors 2015, 15, 31973–31986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Coman, D.; Hyder, F.; Ali, M.M. Dendrimer-Based Responsive MRI Contrast Agents (G1-G4) for Biosensor Imaging of Redundant Deviation in Shifts (BIRDS). Bioconjug. Chem. 2015, 26, 2315–2323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, C.D.; Olson, E.S.; Mattrey, R.F.; Jiang, T.; Tsien, R.Y.; Nguyen, Q.T. Tumor Detection at 3 Tesla with an Activatable Cell Penetrating Peptide Dendrimer (ACPPD-Gd), a T1 Magnetic Resonance (MR) Molecular Imaging Agent. PLoS ONE 2015, 10, 0137104. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.P.; Aryal, M.P.; Janic, B.; Karki, K.; Varma, N.R.; Ewing, J.R.; Arbab, A.S.; Ali, M.M. Concentration-independent MRI of pH with a dendrimer-based pH-responsive nanoprobe. Contrast Media Mol. Imaging 2015, 10, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Li, K.; Li, J.; Wen, S.; Chen, Q.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-Assisted Formation of Fe3O4/Au Nanocomposite Particles for Targeted Dual Mode CT/MR Imaging of Tumors. Small 2015, 11, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, Y.; Bo, S.; Li, Y.; Chen, S.; Yang, Z.; Zheng, X.; Jiang, Z.X.; Zhou, X. Design and synthesis of fluorinated dendrimers for sensitive (19)F MRI. J. Org. Chem. 2015, 80, 4443–4449. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, X.; Wang, Y.; Qian, C.; Huang, P.; Lizak, M.; Niu, G.; Wang, F.; Rong, P.; Kiesewetter, D.O.; et al. A symmetrical fluorous dendron-cyanine dye-conjugated bimodal nanoprobe for quantitative 19F MRI and NIR fluorescence bioimaging. Adv. Healthc. Mater. 2014, 3, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Filippi, M.; Martinelli, J.; Mulas, G.; Ferraretto, M.; Teirlinck, E.; Botta, M.; Tei, L.; Terreno, E. Dendrimersomes: A new vesicular nano-platform for MR-molecular imaging applications. Chem. Commun. 2014, 50, 3453–3456. [Google Scholar] [CrossRef]
- Ghalandarlaki, N.; Mohammadi, T.D.; Babaei, R.A.; Tabasi, M.A.; Keyhanvar, P.; Mehravi, B.; Yaghmaei, P.; Cohan, R.A.; Ardestani, M.S. Gd3+-DTPA-bis (N-methylamine)—Anionic linear globular Dendrimer-G1—A more efficient MRI contrast media. Drug Res. 2014, 64, 57–65. [Google Scholar] [CrossRef]
- Lee, H.; Ooya, T. 19F-NMR, 1H-NMR, and fluorescence studies of interaction between 5-fluorouracil and polyglycerol dendrimers. J. Phys. Chem. B. 2012, 116, 12263–12267. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Unique properties of amphiphilic POSS and their applications. Polym. J. 2013, 45, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Klemm, P.J.; Floyd, W.C., 3rd; Andolina, C.M.; Fréchet, J.M.; Raymond, K.N. Conjugation to Biocompatible Dendrimers Increases Lanthanide T2 Relaxivity of Hydroxypyridinone (HOPO) Complexes for Magnetic Resonance Imaging (MRI). Eur. J. Inorg. Chem. 2012, 2012, 2108–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.T.; Shih, T.T.; Chen, R.C.; Tu, S.Y.; Hsieh, W.Y.; Yang, P.C. Integrin αvβ3-targeted dynamic contrast-enhanced magnetic resonance imaging using a gadolinium-loaded polyethylene gycol-dendrimer-cyclic RGD conjugate to evaluate tumor angiogenesis and to assess early antiangiogenic treatment response in a mouse xenograft tumor model. Mol. Imaging 2012, 11, 286–300. [Google Scholar] [PubMed]
- Klemm, P.J.; Floyd, W.C., 3rd; Smiles, D.E.; Fréchet, J.M.; Raymond, K.N. Improving T1 and T2 magnetic resonance imaging contrast agents through the conjugation of an esteramide dendrimer to high-water-coordination Gd(III) hydroxypyridinone complexes. Contrast Media Mol. Imaging 2012, 7, 95–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Kitamura, N.; Chujo, Y. Bimodal quantitative monitoring for enzymatic activity with simultaneous signal increases in 19F NMR and fluorescence using silica nanoparticle-based molecular probes. Bioconjug. Chem. 2011, 22, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Nwe, K.; Bernardo, M.; Regino, C.A.; Williams, M.; Brechbiel, M.W. Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates. Bioorganic Med. Chem. 2010, 18, 5925–5931. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Wu, X.; Jeong, E.K.; Chen, Q.; Lu, Z.R. Peptide-targeted Nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging. Biomacromolecules 2010, 11, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Bryant, H.; Shapsa, A.; Street, H.; Mani, V.; Fayad, Z.A.; Frank, J.A.; Tsimikas, S.; Briley-Saebo, K.C. Manganese G8 dendrimers targeted to oxidation-specific epitopes: In vivo MR imaging of atherosclerosis. J. Magn. Reason. Imaging 2015, 41, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Chen, J.; Bhattacharjee, S.; Cao, Z.; Wang, H.; Swanson, S.D.; Zong, H.; Baker, J.R., Jr.; Wang, S.H. Functionalized nanoparticles with targeted antibody to enhance imaging of breast cancer in vivo. J. Nanobiotechnol. 2020, 18, 135. [Google Scholar] [CrossRef]
- Gonawala, S.; Ali, M.M. Application of Dendrimer-based Nanoparticles in Glioma Imaging. J. Nanomed. Nanotechnol. 2017, 8, 444. [Google Scholar]
- Zhou, X.; Ye, M.; Han, Y.; Tang, J.; Qian, Y.; Hu, H.; Shen, Y. Enhancing MRI of liver metastases with a zwitterionized biodegradable dendritic contrast agent. Biomater. Sci. 2017, 5, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Shafeie-Ardestani, M.; Bitarafan-Rajabi, A.; Khalaj, A.; Sabzevari, O. Synthesis, radiolabelling, and biological assessment of folic acid-conjugated G-3 99mTc-dendrimer as the breast cancer molecular imaging agent. IET Nanobiotechnol. 2020, 14, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, T.D.; Amanlou, M.; Ghalandarlaki, N.; Mehravi, B.; Ardestani, M.S.; Yaghmaei, P. Gd3+-DTPA-Meglumine-Anionic Linear Globular Dendrimer G1: Novel Nanosized Low Toxic Tumor Molecular MR Imaging Agent. ISRN Pharm. 2013, 2013, 378452. [Google Scholar]
- Mekuria, S.L.; Addisu, K.D.; Chou, H.Y.; Hailemeskel, B.Z.; Tsai, H.C. Potential fluorescence and magnetic resonance imaging modality using mixed lanthanide oxide nanoparticles. Colloids Surf. B Biointerfaces 2018, 167, 54–62. [Google Scholar] [CrossRef]
- Zhang, G.; Du, R.; Qian, J.; Zheng, X.; Tian, X.; Cai, D.; He, J.; Wu, Y.; Huang, W.; Wang, Y.; et al. A tailored nanosheet decorated with a metallized dendrimer for angiography and magnetic resonance imaging-guided combined chemotherapy. Nanoscale 2017, 10, 488–498. [Google Scholar] [CrossRef]
- Mekuria, S.L.; Debele, T.A.; Tsai, H.C. Encapsulation of Gadolinium Oxide Nanoparticle (Gd2O3) Contrasting Agents in PAMAM Dendrimer Templates for Enhanced Magnetic Resonance Imaging in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 6782–6795. [Google Scholar] [CrossRef]
- Filippi, M.; Catanzaro, V.; Patrucco, D.; Botta, M.; Tei, L.; Terreno, E. First in vivo MRI study on theranostic dendrimersomes. J. Control. Release 2017, 248, 45–52. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Y.; Zhu, J.; He, Y.; Qu, J.; Effenberg, C.; Xia, J.; Appelhans, D.; Shi, X. Gd-Chelated poly(propylene imine) dendrimers with densely organized maltose shells for enhanced MR imaging applications. Biomater. Sci. 2016, 4, 1622–1629. [Google Scholar] [CrossRef]
- Li, F.; Yan, H.; Wang, J.; Li, C.; Wu, J.; Wu, S.; Rao, S.; Gao, X.; Jin, Q. Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αvβ3 expression with an MRI modality in mice. Biomaterials 2016, 102, 162–174. [Google Scholar] [CrossRef]
- Filippi, M.; Remotti, D.; Botta, M.; Terreno, E.; Tei, L. GdDOTAGA(C18)2: An efficient amphiphilic Gd(iii) chelate for the preparation of self-assembled high relaxivity MRI nanoprobes. Chem. Commun. 2015, 51, 17455–17458. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wang, H.; Liu, H.; Wen, S.; Peng, C.; Shen, M.; Zhang, G.; Shi, X. Multifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumors. Anal. Chem. 2015, 87, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, Y.; Xu, Y.; Li, J.; Zhang, Z.; Wang, H.; Shen, M.; Shi, X.; Zhang, G. Conjugation of iron oxide nanoparticles with RGD-modified dendrimers for targeted tumor MR imaging. ACS Appl. Mater. Interfaces 2015, 7, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wen, S.; Larson, A.C.; Shen, M.; Zhang, Z.; Chen, Q.; Shi, X.; Zhang, G. Multifunctional dendrimer-based nanoparticles for in vivo MR/CT dual-modal molecular imaging of breast cancer. Int. J. Nanomed. 2013, 8, 2589–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Li, K.; Wen, S.; Liu, H.; Peng, C.; Cai, H.; Shen, M.; Zhang, G.; Shi, X. Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2013, 34, 5200–5209. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Qian, Y.; Tang, J.; Hu, H.; Sui, M.; Shen, Y. Targeted biodegradable dendritic MRI contrast agent for enhanced tumor imaging. J. Control. Release 2013, 169, 239–245. [Google Scholar] [CrossRef]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef]
- Andolina, C.M.; Klemm, P.J.; Floyd, W.C., 3rd; Fréchet, J.M.; Raymond, K.N. Analysis of Lanthanide Complex Dendrimer Conjugates for Bimodal NIR and MRI Imaging. Macromolecules 2012, 45, 8982–8990. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Nwe, K.; Al Zaki, A.; Brechbiel, M.W.; Tsourkas, A. Biodegradable polydisulfide dendrimer nanoclusters as MRI contrast agents. ACS Nano 2012, 6, 9416–9424. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Turkbey, B.; Bernardo, M.; Bryant, L.H., Jr.; Garzoni, M.; Pavan, G.M.; Nakajima, T.; Choyke, P.L.; Simanek, E.E.; Kobayashi, H. Gadolinium MRI contrast agents based on triazine dendrimers: Relaxivity and in vivo pharmacokinetics. Bioconjug. Chem. 2012, 23, 2291–2299. [Google Scholar] [CrossRef] [Green Version]
- Nwe, K.; Milenic, D.E.; Ray, G.L.; Kim, Y.S.; Brechbiel, M.W. Preparation of cystamine core dendrimer and antibody-dendrimer conjugates for MRI angiography. Mol. Pharm. 2012, 9, 374–381. [Google Scholar] [CrossRef]
- Luo, K.; Liu, G.; She, W.; Wang, Q.; Wang, G.; He, B.; Ai, H.; Gong, Q.; Song, B.; Gu, Z. Gadolinium-labeled peptide dendrimers with controlled structures as potential magnetic resonance imaging contrast agents. Biomaterials 2011, 32, 7951–7960. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Turkbey, B.; Ogawa, M.; Bernardo, M.; Regino, C.A.; Bryant, L.H., Jr.; Choyke, P.L.; Kono, K.; Kobayashi, H. Dendrimer-based MRI contrast agents: The effects of PEGylation on relaxivity and pharmacokinetics. Nanomedicine 2011, 7, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwe, K.; Bryant, L.H., Jr.; Brechbiel, M.W. Poly(amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques. Bioconjugate Chem. 2010, 21, 1014–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Surname, Year | Type of Dendrimer/Fluorination | Application and Results | Ref. |
|---|---|---|---|
| Wang et al., 2021 | Nano-in-Nano Dendrimer Gel Particles with brimonidine tartrate (BT) and timolol maleate (TM) | Efficient topical delivery of antiglaucoma drugs into the eye. | [36] |
| Bartusik-Aebisher et al., 2021 | Trastuzumab-dendrimer-fluorine | To treat breast cancer cells in vitro, monitored by MRI measurements. | [37] |
| Mekonnen et al., 2019 | Gadolinium ferrite nanoparticle in generation 4.5 poly(amidoamine) dendrimer | G4.5-GdIO is a promising alternative non-invasive MRI-tracked anti-cancer drug delivery system. | [38] |
| Marcinkowska et al., 2018 | Conjugate of PAMAM Dendrimer, Doxorubicin, and Monoclonal Antibody—Trastuzumab: | Use in HER-2 positive (SKBR-3) and negative (MCF-7) human breast cancer cell lines. | [39] |
| Mirzaei et al., 2015 | Anionic linear globular dendrimer (ALGDG2) | Use of nanoconjugate in 1H-NMR imaging and 17 O-NMR in in vitro studies. | [40] |
| Jain et al., 2015 | G5 conjugated with Muramyl dipeptide and with Amphotericin B | There was a significant reduction in toxicity in the haemolytic toxicity and cytotoxicity studies in R774A.1 erythrocytes and macrophage cells. | [41] |
| Marcinkowska et al., 2015 | PAMAM dendrimer-trastuzumab conjugates that contain docetaxel or paclitaxel | It was shown to be highly toxic to SKBR-3 HER-2 positive cells and lowly toxic to MCF-7 HER-2 negative cells. | [42] |
| Ma et al., 2015 | TMAB-poliamidoamina (PAMAM) with paklitaksel (PTX) | PAMAM conjugated to TMAB was taken up by BT474 cells overexpressing HER-2 more efficiently than MCF-7 cells, which expressed lower levels of HER-2. | [43] |
| Leng et al., 2013 | G4 conjugated with chitosan I methotrexate nanoparticle | Improvement of the cytotoxicity of free methotrexate on cells. | [44] |
| Rouhollah et al., 2013 | G2, G3, G4, and G7 dendrimers conjugated with magnetic nanoparticles (Fe3O4) and DOX | G4 Fe3O4 dendrimer releases most of the drug at a lower pH, proving to be the most acceptable generation for effective DOX delivery. | [45] |
| Mullen et al., 2010 | G5 PAMAM dendrimer | In this study, the amide coupling methods commonly used to conjugate ligands to poly(amidoamine) (PAMAM) dendrimers were examined. | [46] |
| Wang et al., 2003 | G3-Polihydroksyalkanian/Tamsulosin | Drug solubility is purported to be improved by amine-terminated dendrimers. | [47] |
| Surname, Year | Type of Dendrimer/Fluorination | Application and Results | Ref |
|---|---|---|---|
| Song et al., 2021 | Core-shell tecto dendrimers | MRI through an amplified passive EPR effect and also further extended for different cancer theranostic applications. | [73] |
| Zhang et al., 2020 | OEG Gn-PROXYL radical dendrimers | Used as contrast agents in MRI | [74] |
| Shrestha et al., 2020 | Gadolinium complexes attached to poly ethoxy ethyl glycinamide (PEE-G) dendrons | As contrast agents with enhanced relaxation in MRI | [75] |
| Hectors et al., 2018 | G5 dendrimer, G2 dendrimer | Multiagent DCE-MRI (combination of contrast agents and low and high molecular weight) to improve the accuracy of the assessment of tumor hemodynamic parameters and vascular permeability; sequential injection of G5 dendrimer, G2 dendrimer, and Gd-DOTA | [76] |
| Kondo et al., 2017 | Chiral dendrimer (S-isomeric dendrimer) | Chiral dendrimer Gd-MRI CAs, which showed high r1 values; association constant values (Ka) of S-isomeric dendrimer CAs to bovine serum albumin (BSA) were higher than those of R-isomeric dendrimer CAs (contrast agents) | [77] |
| Luong et al., 2017 | Folic acid-polyamidoamine dendrimers surface (FA-PAMAM) | Polyvalent theranostic nanocarrier consisting of superparamagnetic iron oxide nanoparticle core (SPIONs) decorated with folic acid-polyamidoamine dendrimers surface (FA-PAMAM); research on the overexpression of ovarian (SKOV3) and cervical (HeLa) cells | [78] |
| Gündüz et al., 2016 | Poly(amidoamine) (PAMAM) dendrimers | MRI contrast agents; CA provides a longer tissue retention time due to its high molecular weight and size | [79] |
| Gündüz et al., 2016 | Generation 4 (G4) poly-(amidoamine)(PAMAM) dendrimer | They developed a nanosized, calcium-sensitive dendrimeric probe that changes longitudinal and transverse relaxation times | [80] |
| Haribabu et al., 2016 | 3G polyamidoamide (PAMAM) dendrimers | MRI contrast agents, dual mode (T1 and T2) contrast agent based on folic acid functionalized manganese ferrite nanoparticles (MNP) entrapped in 3G polyamidoamide (PAMAM) dendrimers; | [81] |
| Miyake et al., 2015 | 1st, 2nd, and 3rd-generation chiral dendrimer-triamine- coordinated CAs | MRI contrast agents (Gd-MRI CAs), which showed longitudinal relaxivity (r1) values | [82] |
| Huang et al., 2015 | Paramagnetic dendrimers up to the fourth generation (i.e., G1-G4); poly(amido amine) (PAMAM) | Create a dual-modality nanosized contrast agent | [83] |
| Malone et al., 2015 | 5th-generation PAMAM dendrimer | Cell-penetrating peptides and their Gd-loaded dendrimeric form (ACPPD-Gd) have been shown to selectively accumulate in tumors | [84] |
| Bhuiyan et al., 2015 | G5-PAMAM dendrimer | The MRI contrast agent nanoprobe (GdDOTA-4AmP)44-G5, at 3T and 7T magnetic field strengths, shows pH response in the range commonly found in the microenvironment of solid tumors | [85] |
| Cai et al., 2015 | Multilayers of poly(γ-glutamic acid) (PGA)/poly(L-lysine)/PGA/folic acid (FA)-modified dendrimer | Efficient nanoprobe for the targeted dual mode CT/MR imaging of a xenografted tumor model | [86] |
| Yu et al., 2015 | 1st-generation dendron (G1-OH); dendrons 3, 10, 12, 14 | The dendrimer is characterized by a strong 19F NMR peak and short relaxation times | [87] |
| Wang et al., 2014 | Bimodal nanoprobe | Quantitative 19F MRI and NIR fluorescence bioimaging and cell tracking | [88] |
| Filippi et al., 2014 | Amphiphilic Janus dendrimers (dendrimersomes) | Efficient and versatile nanoplatform for biomedical imaging | [89] |
| Ghalandarlaki et al., 2014 | Dendrimer-G1 | New nano contrast medium increases its effectiveness | [90] |
| Lee and Ooya, 2012 | Polyglycerol dendrimers (PGDs) | Attenuation of 19F NMR signals with perfluorinated dendrimers | [91] |
| Tanaka et al., 2012 | Water-soluble perfluorinated dendrimers | Evaluation of glutathione reductase (GR) activity by 19F NMR spectroscopy; GR enzymatic activity was determined from the increase in the size of the 19F NMR signals | [92] |
| Klemm et al., 2012 | Esteramide (EA) dendrimer; PLLG2[Asp(COOH)PEO]8 Polylysine Dendrimer; Yb-TREN-Dendrimer; Dy-TREN-Dendrimer; | MRI contrast agents; these conjugates have relaxivities up to 374 mM−1 s−1 per dendrimer, high bioavailability, and low in vitro toxicity. | [93] |
| Chen et al., 2012 | 3rd-generation (G3) dendrimer | The integrin αvβ3 targeting ability of PEG-G3-(Gd-DTPA)6-(cRGD-DTPA)2 in vitro and in vivo was demonstrated | [94] |
| Klemm et al., 2012 | Esteramide dendrimer (EA) | When covalently conjugated to a highly biocompatible esteramide dendrimer, T2 relaxation rates up to 52 mM−1 s−1 and T1 relaxation rates up to 31 mM−1 s−1 per gadolinium were observed under clinically relevant conditions | [95] |
| Tanaka et al., 2011 | Perfluorinated dendrimers tethered on silica nanoparticles | Bimodal quantitative assay of enzymatic activity in (19) F NMR spectroscopy and fluorescence spectroscopy using a nanoparticle based molecular probe; | [96] |
| Nwe et al., 2010 | 4, 5, and 6 PAMAM dendrimer | This report presents the preparation and characterization of three [Gd-C-DOTA](-1)-dendrimer assemblies by way of analysis, NMRD spectroscopy, and photon correlation spectroscopy (PCS). Molar relaxivity measured at pH 7.4, 22 degrees C, and 3T (29.6, 49.8, and 89.1 mM−1 s−1 indicated the viability of conjugates as MRI contrast agents. | [97] |
| Tan et al., 2010 | G2, G3 | The peptide-targeted nanoglobular contrast agents showed greater contrast enhancement than the corresponding nontargeted agents in tumor at a dose of 0.03 mmol Gd/kg in female athymic mice bearing MDA-MB-231 human breast carcinoma xenografts. | [98] |
| Surname, Year | Type of Dendrimer/ Fluorination | Application and Results | Ref |
|---|---|---|---|
| Chen et al., 2020 | 5 poly(amidoamine) dendrimers, encapsulated gold nanoparticles, chelated gadolinium, and anti-human HER-2 | Intravenous injection of this nanoparticle into mice with HER-2 positive breast tumors significantly increases the MRI signal intensity by ~20% and improves CT resolution and contrast by a factor of 2. | [100] |
| Zamani et al., 2020 | Folic acid-conjugated G-3 99 m Tc-dendrimer | Breast cancer molecular imaging agent | [103] |
| Mekuria et al., 2018 | G4.5 polyamidoamine (PAMAM) dendrimers | For the detection of a dual-channel carcinoma cell line (fluorescence/MR imaging) both in vitro | [105] |
| Zhang et al., 2017 | Gadolinium-labeled dendrimer (FA-GCGLD) | Increasing the T1 contrast capacity in in vivo magnetic resonance angiography | [106] |
| Gonawala and Ali, 2017 | G5 PAMAM dendrimer | For in vivo MRI studies in a preclinical animal model of glioma | [101] |
| Zhou et al., 2017 | 4th-generation zwitterionized biodegradable dendritic contrast agent (DCA) | As a deoditrinated biodegradable dendritic contrast agent to enhance the MRI of liver metastases | [102] |
| Mekuria et al., 2017 | G4.5 dendrimers | As double (T1 and T2) contrast agents in magnetic resonance imaging | [107] |
| Filippi et al., 2017 | Amphiphilic Janus-dendrimers (dendrimersomes); 3,5-C12-EG-(OH)4 dendrimer | As a contrast agent, T1 weighted enhancement in the tumor area | [108] |
| Xiong et al., 2016 | Fourth-generation poly(propylene imine) (PPI) glycodendrimers | As a contrast agent for imaging the animal aorta, renal artery, kidneys, and bladder in in vivo studies | [109] |
| Li et al., 2016 | Dendrimer nanoprobe labeled with cyclic arginine-glycine-aspartic acid pentapeptide (cRGDyK) | A contrast agent to differentiate the degree of liver fibrosis; the MR T1 signal weighted value increased in parallel with the severity of the liver fibrosis. | [110] |
| Filippi et al., 2015 | Amphiphilic Janus-dendrimers (dendrimersomes) | Performance improvement in in vivo MRI studies in mice | [111] |
| Chen et al., 2015 | Amine-terminated generation 5 poly(amidoamine) dendrimers | For targeted dual-mode computed tomography (CT)/magnetic resonance (MR) imaging of small tumors. | [112] |
| Yang et al., 2015 | G5 dendrimer | Targeted magnetic resonance (MR) imaging of C6 glioma cells. | [113] |
| Nguyen et al., 2015 | Manganese (Mn) G8 dendrimers | For imaging atherosclerotic lesions with 3 Tesla MRI. | [99] |
| Li et al., 2013 | Amine-terminated generation 5 poly(amidoamine) dendrimers | A contrast agent for magnetic resonance (MR)/computed tomography (CT) imaging of breast cancer cells in vitro | [114] |
| Chen et al., 2013 | Amine-terminated generation 5 poly(amidoamine) dendrimers (G5.NH2) | For imaging tumors in CT and MRI, it shows a high intensity of radiation suppression and improved MRI contrast. | [115] |
| Mohamadi et al., 2013 | Dendrimer G1 | The uptake of the drug into the liver hepatocellular cell line and the drug cytotoxicity were evaluated. It also increases the relaxivity of the tissue and enhances the MR images contrast. | [104] |
| Ye et al., 2013 | 2nd-generation dendrimer (G2) | Biodegradable dendritic contrast agent (DCA) (FA-PEG-G2-DTPA-Gd) was prepared from a polyester dendrimer conjugated with gadolinium (Gd) chelates and PEG chains with distal folic acid. The MRI contrasted by FA-PEG-G2-DTPA-Gd outlined the inoculated tumor more clearly and had much higher contrast enhancement for a much longer time than Magnevist. | [116] |
| Wen et al., 2013 | Amine-terminated generation five poly(amidoamine) dendrimers (G5.NH2) | Were used as templates to synthesize gold nanoparticles (AuNPs). With the coexistence of the two radiodense imaging elements of AuNPs and Gd(III) within one NP system, the formed Gd-Au DENPs display both r1 relaxivity for the MR imaging mode and the X-ray attenuation property for CT imaging mode, which enables CT/MR dual mode imaging of the heart, liver, kidneys, and bladder of rats or mice. | [117] |
| Andolina et al., 2012 | Esteramide dendrimer (EA) | DyN1-EA had the largest ionic T(1) relaxivity, 7.60 mM−1 s−1, while YbN3-EA had the largest ionic T(2) relaxivity, with an NIR quantum yield of 0.17 % when evaluated in mouse serum. This is the first Yb(III) bimodal NIR/T(2) MRI contrast agent of its kind that has been evaluated. | [118] |
| Huang et al., 2012 | Individual dendrimers | Biodegradable DNCs were prepared with polydisulfide linkages between the individual dendrimers. DNCs possessed a circulation half-life of >1.6 h in mice and produced significant contrast enhancement in the abdominal aorta and kidneys for as long as 4 h. | [119] |
| Lim et al., 2012 | Dendrimers generation 5 and 3 (G3 and G5) and four gadolinium (Gd)-based macromolecular contrast agents, G3-(Gd-DOTA)(24), G5-(Gd-DOTA)(96), G3-(Gd-DTPA)(24), and G5-(Gd-DTPA)(96), | These triazine dendrimer-based MRI contrast agents exhibit several promising features such as high in vivo r1 relaxivity, desirable pharmacokinetics, and well-defined structure. | [120] |
| Nwe et al., 2012 | Dendrimer G4 and G5, Gd-DOTA (G4SS30, G5SS58), respectively | The in vitro molar relaxivity of the Ab-(G4S15)(4) conjugate measured at pH 7.4, 22 °C, and 3T showed a moderate increase in relaxivity as compared to Magnevist (6.7 vs. 4.0 mM−1 s−1, while the Ab-(G5S29)(4) conjugate was two-fold higher (9.1 vs. 4.0 mM−1 s−1. | [121] |
| Luo et al., 2011 | Third generation (G3) peptide dendrimers; L-lysine-based dendrimer | In vivo studies have shown that the mPEGylated Gd(III)-based dendrimer provided much higher signal intensity enhancement (SI) in mouse kidneys, especially at 60 min post-injection, with 54.8% relatively enhanced SI. | [122] |
| Kojima et al., 2011 | PAMAM dendrimers (generations 4 and 5; G4 and G5) | Surface-PEGylated Gd-PAMAM dendrimers showed decreased plasma clearance and prolonged retention in the blood pool. Shorter PEG, higher generation conjugates led to higher relaxivity. | [123] |
| Nwe et al., 2010 | PAMAM dendrimer generation 4 (G4 dendrimer), gadolinium-dendrimer conjugates of derivatized acyclic Diethylenetriamine-N,N′,N′,N″, N″-pentaacetic acid (1B4M-DTPA) and macrocyclic 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid (C-DOTA). | The macrocyclic-based agent is the more suitable agent for in vivo use for these reasons combined with kinetics. Inertness is associated with the Gd(III) DOTA complex stability properties. | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules 2022, 27, 3237. https://doi.org/10.3390/molecules27103237
Bober Z, Bartusik-Aebisher D, Aebisher D. Application of Dendrimers in Anticancer Diagnostics and Therapy. Molecules. 2022; 27(10):3237. https://doi.org/10.3390/molecules27103237
Chicago/Turabian StyleBober, Zuzanna, Dorota Bartusik-Aebisher, and David Aebisher. 2022. "Application of Dendrimers in Anticancer Diagnostics and Therapy" Molecules 27, no. 10: 3237. https://doi.org/10.3390/molecules27103237








