The Role of Bile Acids in the Human Body and in the Development of Diseases
Abstract
:1. Introduction
2. The Role of Bile Acids in the Regulation of Inflammatory Reactions
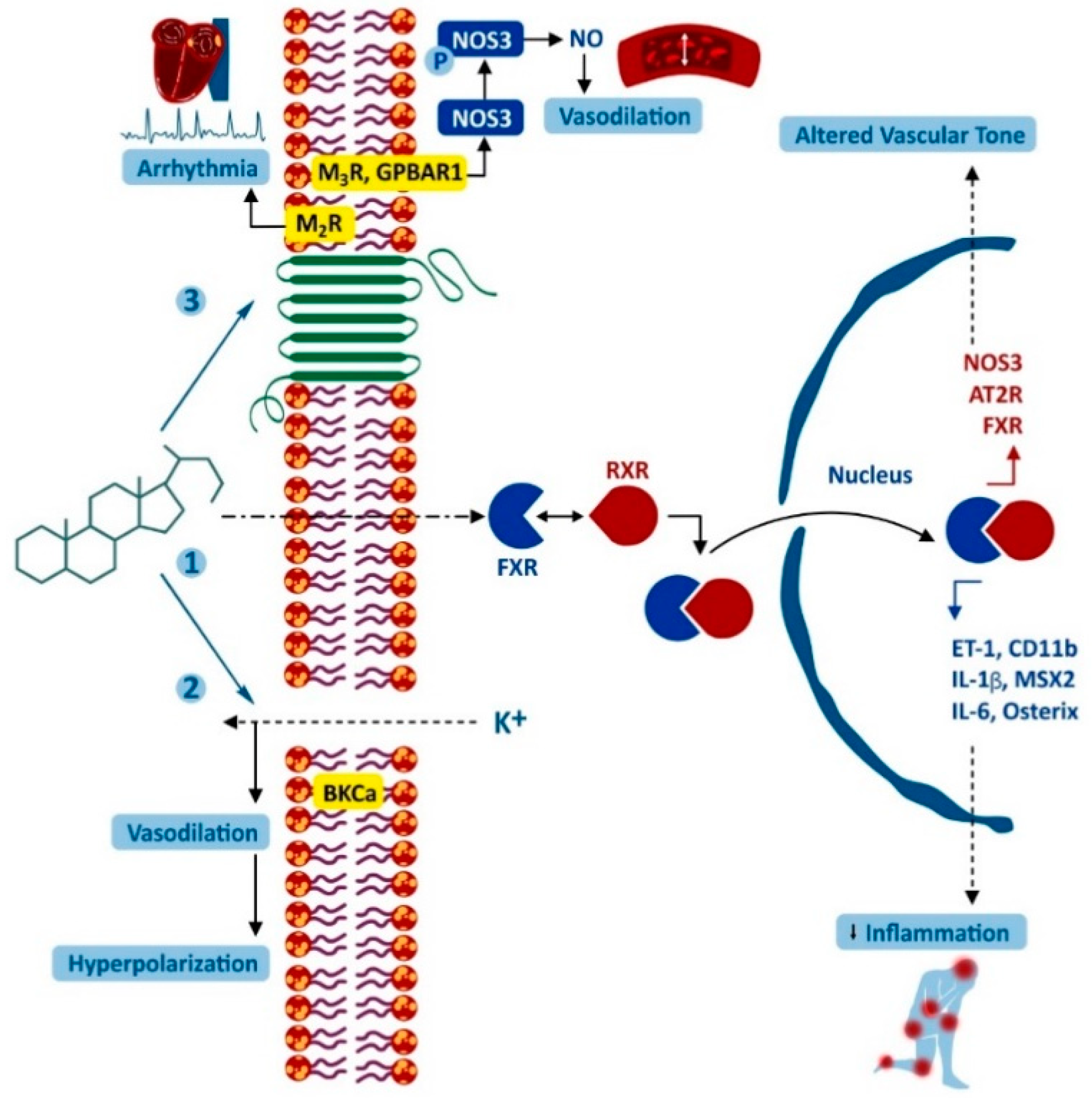
3. The Effect of Bile Acids on the Cardiovascular System
4. The Influence of Bile Acids on the Hypothalamic–Pituitary–Adrenal Axis
5. The Role of Bile Acids in the Nervous System
6. Aspects of the Involvement of Bile Acids in the Pathogenesis of Intestinal Disease
6.1. Bile Acids and Intestinal Microbiota
6.2. Bile Acids and Colon Tumors
6.3. The Gastrointestinal Tract after Cholecystectomy
7. Possible Effects of Bile Acids on the Growth of Tumor Cells
8. The Role of Bile Acids in the Pathogenesis of Liver Diseases
9. Potential Role of Bile Acids in the Development of Lung Diseases
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russell, D.W. The Enzymes, Regulation, and Genetics of Bile Acid Synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef] [Green Version]
- Evangelakos, I.; Heeren, J.; Verkade, E.; Kuipers, F. Role of Bile Acids in Inflammatory Liver Diseases. Semin. Immunopathol. 2021, 43, 577–590. [Google Scholar] [CrossRef]
- Khurana, S.; Raufman, J.P.; Pallone, T.L. Bile Acids Regulate Cardiovascular Function. Clin. Transl. Sci. 2011, 4, 210–218. [Google Scholar] [CrossRef]
- Lieu, T.; Jayaweera, G.; Bunnett, N.W. GPBA: A GPCR for Bile Acids and an Emerging Therapeutic Target for Disorders of Digestion and Sensation: GPBA (TGR5) Bile Acid Receptor. Br. J. Pharmacol. 2014, 171, 1156–1166. [Google Scholar] [CrossRef]
- Ackerman, H.D.; Gerhard, G.S. Bile Acids in Neurodegenerative Disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Aldhahrani, A.; Verdon, B.; Ward, C.; Pearson, J. Effects of Bile Acids on Human Airway Epithelial Cells: Implications for Aerodigestive Diseases. ERJ Open Res. 2017, 3, 00107–02016. [Google Scholar] [CrossRef] [Green Version]
- Nasr, A.O.; Robb, W.; Walsh, T.N. Review article; duodeno-gastro-esophageal reflux combined and isolated. Am. Med. J. 2013, 4, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Brodlie, M.; Aseeri, A.; Lordan, J.L.; Robertson, A.G.N.; McKean, M.C.; Corris, P.A.; Griffin, S.M.; Manning, N.J.; Pearson, J.P.; Ward, C. Bile Acid Aspiration in People with Cystic Fibrosis before and after Lung Transplantation. Eur. Respir. J. 2015, 46, 1820–1823. [Google Scholar] [CrossRef] [Green Version]
- Biagioli, M.; Fiorucci, S. Bile Acid Activated Receptors: Integrating Immune and Metabolic Regulation in Non-Alcoholic Fatty Liver Disease. Liver Res. 2021, 5, 119–141. [Google Scholar] [CrossRef]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and Function of the Bile Acid Receptor TGR5 in Kupffer Cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile Acids Activated Receptors Regulate Innate Immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef] [Green Version]
- Lou, G.; Ma, X.; Fu, X.; Meng, Z.; Zhang, W.; Wang, Y.D.; Van Ness, C.; Yu, D.; Xu, R.; Huang, W. GPBAR1/TGR5 Mediates Bile Acid-Induced Cytokine Expression in Murine Kupffer Cells. PLoS ONE 2014, 9, e93567. [Google Scholar] [CrossRef] [Green Version]
- Biagioli, M.; Carino, A.; Cipriani, S.; Francisci, D.; Marchianò, S.; Scarpelli, P.; Sorcini, D.; Zampella, A.; Fiorucci, S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J. Immunol. 2017, 199, 2718–2733. [Google Scholar] [CrossRef] [Green Version]
- Keitel, V.; Reinehr, R.; Gatsios, P.; Rupprecht, C.; Görg, B.; Selbach, O.; Häussinger, D.; Kubitz, R. The G-Protein Coupled Bile Salt Receptor TGR5 Is Expressed in Liver Sinusoidal Endothelial Cells. Hepatology 2007, 45, 3695–3704. [Google Scholar] [CrossRef]
- Spatz, M.; Ciocan, D.; Merlen, G.; Rainteau, D.; Humbert, L.; Gomes-Rochette, N.; Hugot, C.; Trainel, N.; Mercier-Nomé, F.; Domenichini, S.; et al. Bile Acid-Receptor TGR5 Deficiency Worsens Liver Injury in Alcohol-Fed Mice by Inducing Intestinal Microbiota Dysbiosis. JHEP Rep. 2021, 3, 2100230. [Google Scholar] [CrossRef]
- Jourdainne, V.; Péan, N.; Doignon, I.; Humbert, L.; Rainteau, D.; Tordjmann, T. The Bile Acid Receptor TGR5 and Liver Regeneration. Dig. Dis. 2015, 33, 3319–3326. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Jha, P.; Müller, M.; Agostinelli, L.; Saccomanno, S.; Auer, N.; Deutschmann, A.; Zöhrer, C.; Auwerx, J.; Marzioni, M.; et al. P389 protective role of membrane bile acid receptor tgr5 (gpbar1) in ddc-induced sclerosing cholangitis in mice. J. Hepatol. 2014, 60, S197–S198. [Google Scholar] [CrossRef]
- Yang, Z.H.; Liu, F.; Zhu, X.R.; Suo, F.Y.; Jia, Z.J.; Yao, S.K. Altered Profiles of Fecal Bile Acids Correlate with Gut Microbiota and Inflammatory Responses in Patients with Ulcerative Colitis. World J. Gastroenterol. 2021, 27, 243609–2433629. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell. Metab. 2016, 24, 141–191. [Google Scholar] [CrossRef] [Green Version]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile Acid Metabolites Control TH17 and Treg Cell Differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial Metabolism of Bile Acids Promotes Generation of Peripheral Regulatory T Cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 2115–2126. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. The Road Ahead. Cell 2001, 104, 4503–4516. [Google Scholar] [CrossRef] [Green Version]
- González, N.A.; Castrillo, A. Liver X Receptors as Regulators of Macrophage Inflammatory and Metabolic Pathways. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 8982–8994. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Said, O.; Ehrlich, Y.; Meddings, J.B.; Shaffer, E.A.; Bomzon, A. On the in Vitro Vasoactivity of Bile Acids: The in Vitro Vasoactivity of Bile Acids. Br. J. Pharmacol. 2000, 131, 3387–3398. [Google Scholar] [CrossRef] [Green Version]
- Pols, T.W.H.; Nomura, M.; Harach, T.; Lo Sasso, G.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R.; et al. TGR5 Activation Inhibits Atherosclerosis by Reducing Macrophage Inflammation and Lipid Loading. Cell Metab. 2011, 14, 6747–6757. [Google Scholar] [CrossRef] [Green Version]
- Barchetta, I. Expression of TGR5 in Adipose Tissue in Relation to Metabolic Impairment and Adipose Tissue Dysfunction in Human Obesity. Metab. Target Organ Damage 2021, 4, 517. [Google Scholar] [CrossRef]
- Hageman, J.; Herrema, H.; Groen, A.K.; Kuipers, F. A Role of the Bile Salt Receptor FXR in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 81519–81528. [Google Scholar] [CrossRef]
- Vasavan, T.; Ferraro, E.; Ibrahim, E.; Dixon, P.; Gorelik, J.; Williamson, C. Heart and Bile Acids–Clinical Consequences of Altered Bile Acid Metabolism. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 41345–41355. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Z.; Han, K.; Zhao, Y.; Li, Y.; Li, D.; Wang, X.; Li, X.; Sun, S.; Lin, F.; et al. Correlation between Mitochondrial Dysfunction, Cardiovascular Diseases, and Traditional Chinese Medicine. Evid. Based Complement. Alternat. Med. 2020, 2020, 2902136. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.P.; Peng, S.Y. Relation between Bile Acids and Myocardial Damage in Obstructive Jaundice. World J. Gastroenterol. 1997, 3, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P. Cholic acid and the heart: In vitro studies of the effect on heart rate and myocardial contractility in the rat. Clin. Exp. Pharmacol. Physiol. 1978, 5, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yuan, A.; Shan, P.; Gao, E.; Wang, X.; Wang, Y.; Lau, W.B.; Koch, W.; Ma, X.L.; He, B. Cardiomyocyte-Expressed Farnesoid-X-Receptor Is a Novel Apoptosis Mediator and Contributes to Myocardial Ischaemia/Reperfusion Injury. Eur. Heart J. 2013, 34, 1834–1845. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P.; Pasdois, P. The Role of the Mitochondrial Permeability Transition Pore in Heart Disease. Biochim. Biophys. Acta 2009, 1787, 111402–111415. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.S.; Mathur, B.; Eblimit, Z.; Vasquez, H.; Taegtmeyer, H.; Karpen, S.J.; Penny, D.J.; Moore, D.D.; Anakk, S. Bile Acid Excess Induces Cardiomyopathy and Metabolic Dysfunctions in the Heart. Hepatology 2017, 65, 1189–1201. [Google Scholar] [CrossRef]
- Ferreira, M.; Coxito, P.M.; Sardão, V.A.; Palmeira, C.M.; Oliveira, P.J. Bile Acids Are Toxic for Isolated Cardiac Mitochondria: A Possible Cause for Hepatic-Derived Cardiomyopathies? Cardiovasc. Toxicol. 2005, 5, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Rainer, P.P.; Primessnig, U.; Harenkamp, S.; Doleschal, B.; Wallner, M.; Fauler, G.; Stojakovic, T.; Wachter, R.; Yates, A.; Groschner, K.; et al. Bile Acids Induce Arrhythmias in Human Atrial Myocardium—Implications for Altered Serum Bile Acid Composition in Patients with Atrial Fibrillation. Heart 2013, 99, 221685–221692. [Google Scholar] [CrossRef]
- Liu, L.; Panzitt, K.; Racedo, S.; Wagner, M.; Platzer, W.; Zaufel, A.; Theiler-Schwetz, V.; Obermayer-Pietsch, B.; Müller, H.; Höfler, G.; et al. Bile Acids Increase Steroidogenesis in Cholemic Mice and Induce Cortisol Secretion in Adrenocortical H295R Cells via S1 PR 2, ERK and SF–1. Liver Int. 2019, 39, 112112–112123. [Google Scholar] [CrossRef] [Green Version]
- Frey, F.J. Impaired 11β-Hydroxysteroid Dehydrogenase Contributes to Renal Sodium Avidity in Cirrhosis: Hypothesis or Fact? Hepatology 2006, 44, 4795–4801. [Google Scholar] [CrossRef]
- McNeilly, A.D.; Macfarlane, D.P.; O’Flaherty, E.; Livingstone, D.E.; Mitić, T.; McConnell, K.M.; McKenzie, S.M.; Davies, E.; Reynolds, R.M.; Thiesson, H.C.; et al. Bile Acids Modulate Glucocorticoid Metabolism and the Hypothalamic–Pituitary–Adrenal Axis in Obstructive Jaundice. J. Hepatol. 2010, 52, 5705–5711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, A.J.; Díaz, M.B.; Reimann, A.; Klement, J.; Walcher, T.; Krones-Herzig, A.; Strobel, O.; Werner, J.; Peters, A.; Kleyman, A.; et al. Molecular Control of Systemic Bile Acid Homeostasis by the Liver Glucocorticoid Receptor. Cell Metab. 2011, 14, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, D.R.; Schmidt, S.; Holmstrom, S.R.; Makishima, M.; Yu, R.T.; Cummins, C.L.; Mangelsdorf, D.J.; Kliewer, S.A. AKR1B7 Is Induced by the Farnesoid X Receptor and Metabolizes Bile Acids. J. Biol. Chem. 2011, 286, 42425–42432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Wang, T.; Lan, Y.; Yang, L.; Pan, W.; Zhu, Y.; Lv, B.; Wei, Y.; Shi, H.; Wu, H.; et al. Deletion of Mouse FXR Gene Disturbs Multiple Neurotransmitter Systems and Alters Neurobehavior. Front. Behav. Neurosci. 2015, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and Neurological Diseases: An Endocrine View. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef] [Green Version]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D Receptor and 1α-Hydroxylase in Human Brain. J. Chem. Neuroanat. 2005, 29, 121–130. [Google Scholar] [CrossRef]
- Buell, J.S.; Dawson-Hughes, B. Vitamin D and Neurocognitive Dysfunction: Preventing “D”Ecline? Mol. Aspects Med. 2008, 29, 6415–6422. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef]
- Ikura, T.; Ito, N. Crystal Structure of the Vitamin D Receptor Ligand-Binding Domain with Lithocholic Acids. In Vitamins and Hormones; Elsevier: Berlin, Germany, 2016; Volume 100, pp. 117–136. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, J.M. Transcriptional Regulation of Hepatic Autophagy by Nuclear Receptors. Cells 2022, 11, 620. [Google Scholar] [CrossRef]
- Li, R.; Guo, E.; Yang, J.; Li, A.; Yang, Y.; Liu, S.; Liu, A.; Jiang, X. 1,25(OH)2D3 Attenuates Hepatic Steatosis by Inducing Autophagy in Mice: 1,25(OH)2D3 Attenuates Hepatic Steatosis. Obesity 2017, 25, 3561–3571. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Xu, Y.; You, K.; Zhang, J.; Yang, F.; Li, Y. Calcitriol Alleviates Ethanol-Induced Hepatotoxicity via AMPK/MTOR-Mediated Autophagy. Arch. Biochem. Biophys. 2021, 697, 108694. [Google Scholar] [CrossRef] [PubMed]
- D’Aldebert, E.; Biyeyeme Bi Mve, M.; Mergey, M.; Wendum, D.; Firrincieli, D.; Coilly, A.; Fouassier, L.; Corpechot, C.; Poupon, R.; Housset, C.; et al. Bile Salts Control the Antimicrobial Peptide Cathelicidin Through Nuclear Receptors in the Human Biliary Epithelium. Gastroenterology 2009, 136, 41435–41443. [Google Scholar] [CrossRef]
- Han, S.; Chiang, J.Y.L. Mechanism of Vitamin D Receptor Inhibition of Cholesterol 7α-Hydroxylase Gene Transcription in Human Hepatocytes. Drug Metab. Dispos. 2009, 37, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, D.S. Neurosteroids: Endogenous Role in the Human Brain and Therapeutic Potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Keitel, V.; Görg, B.; Bidmon, H.J.; Zemtsova, I.; Spomer, L.; Zilles, K.; Häussinger, D. The Bile Acid Receptor TGR5 (GPBAR-1) Acts as a Neurosteroid Receptor in Brain. Glia 2010, 58, 151794–151805. [Google Scholar] [CrossRef]
- Schubring, S.R.; Fleischer, W.; Lin, J.S.; Haas, H.L.; Sergeeva, O.A. The Bile Steroid Chenodeoxycholate Is a Potent Antagonist at NMDA and GABAA Receptors. Neurosci. Lett. 2012, 506, 2322–2326. [Google Scholar] [CrossRef]
- Yanovsky, Y.; Schubring, S.R.; Yao, Q.; Zhao, Y.; Li, S.; May, A.; Haas, H.L.; Lin, J.-S.; Sergeeva, O.A. Waking Action of Ursodeoxycholic Acid (UDCA) Involves Histamine and GABAA Receptor Block. PLoS ONE 2012, 7, e42512. [Google Scholar] [CrossRef]
- Silva, S.L.; Vaz, A.R.; Diógenes, M.J.; van Rooijen, N.; Sebastião, A.M.; Fernandes, A.; Silva, R.F.M.; Brites, D. Neuritic Growth Impairment and Cell Death by Unconjugated Bilirubin Is Mediated by NO and Glutamate, Modulated by Microglia, and Prevented by Glycoursodeoxycholic Acid and Interleukin-10. Neuropharmacology 2012, 62, 72398–72408. [Google Scholar] [CrossRef]
- Palmela, I.; Correia, L.; Silva, R.F.M.; Sasaki, H.; Kim, K.S.; Brites, D.; Brito, M.A. Hydrophilic Bile Acids Protect Human Blood-Brain Barrier Endothelial Cells from Disruption by Unconjugated Bilirubin: An in Vitro Study. Front. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile Acids Permeabilize the Blood Brain Barrier after Bile Duct Ligation in Rats via Rac1-Dependent Mechanisms. Dig. Liver Dis. 2014, 46, 6527–6534. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Gu, G.; Wang, J.; Chai, Y.; Fan, Y.; Yang, M.; Xu, X.; Gao, W.; Li, F.; Yin, D.; et al. Administration of Tauroursodeoxycholic Acid Attenuates Early Brain Injury via Akt Pathway Activation. Front. Cell. Neurosci. 2017, 11, 193. [Google Scholar] [CrossRef]
- Romero-Ramírez, L.; Nieto-Sampedro, M.; Yanguas-Casás, N. Tauroursodeoxycholic Acid: More than Just a Neuroprotective Bile Conjugate. Neural Regen. Res. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.; Sassani, M.; Buckley, E.; Moll, S.; Anton, A.; Appleby, M.; Maru, S.; Taylor, R.; McNeill, A.; Hoggard, N.; et al. Ursodeoxycholic Acid as a Novel Disease-Modifying Treatment for Parkinson’s Disease: Protocol for a Two-Centre, Randomised, Double-Blind, Placebo-Controlled Trial, The “UP” Study. BMJ Open 2020, 10, e038911. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated Bile Acids in Rat Brain: Analytical Method Based on LC/ESI-MS/MS with Chemical Derivatization and Estimation of Their Origin by Comparison to Serum Levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Tobin, R.; Dusio, G.; Smith, J.; Shin, H.; Newell-Rogers, K.; Grant, S.; DeMorrow, S. TGR5 Signaling Reduces Neuroinflammation during Hepatic Encephalopathy. J. Neurochem. 2015, 135, 3565–3576. [Google Scholar] [CrossRef]
- Nizamutdinov, D.; DeMorrow, S.; McMillin, M.; Kain, J.; Mukherjee, S.; Zeitouni, S.; Frampton, G.; Bricker, P.C.S.; Hurst, J.; Shapiro, L.A. Hepatic Alterations Are Accompanied by Changes to Bile Acid Transporter-Expressing Neurons in the Hypothalamus after Traumatic Brain Injury. Sci. Rep. 2017, 7, 140112. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, Bile Acid, and Cholesterol Transporters: Function and Regulation. Pharmacol. Rev. 2010, 62, 11–96. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, V.; Contin, M.; Fernández, M.A.; Lemberg, A. Bile Acids Content in Brain of Common Duct Ligated Rats. Annu. Hepatol. 2012, 11, 6930–6934. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile Acid Signaling Pathways from the Enterohepatic Circulation to the Central Nervous System. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef] [Green Version]
- Kremer, A.E.; Namer, B.; Bolier, R.; Fischer, M.J.; Oude Elferink, R.P.; Beuers, U. Pathogenesis and Management of Pruritus in PBC and PSC. Dig. Dis. 2015, 33 (Suppl. 2), 164–175. [Google Scholar] [CrossRef] [Green Version]
- Krähenbühl, S.; Talos, C.; Fischer, S.; Reichen, J. Toxicity of Bile Acids on the Electron Transport Chain of Isolated Rat Liver Mitochondria. Hepatology 1994, 19, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jin, C.; Li, X.; Wang, F.; McKeehan, W.L.; Luo, Y. Differential Specificity of Endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in Complex with KLB. PLoS ONE 2012, 7, e33870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile Acids Are Important Direct and Indirect Regulators of the Secretion of Appetite- and Metabolism-Regulating Hormones from the Gut and Pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Meaney, S.; Heverin, M.; Panzenboeck, U.; Ekström, L.; Axelsson, M.; Andersson, U.; Diczfalusy, U.; Pikuleva, I.; Wahren, J.; Sattler, W.; et al. Novel Route for Elimination of Brain Oxysterols across the Blood-Brain Barrier: Conversion into 7α-Hydroxy-3-Oxo-4-Cholestenoic Acid. J. Lipid Res. 2007, 48, 4944–4951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 3332–3338. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Duan, Z. Bile Acids and the Potential Role in Primary Biliary Cirrhosis. Digestion 2016, 94, 3145–3153. [Google Scholar] [CrossRef]
- Garmendia, J.; Frankel, G.; Crepin, V.F. Enteropathogenic and Enterohemorrhagic Escherichia Coli Infections: Translocation, Translocation, Translocation. Infect. Immun. 2005, 73, 52573–52585. [Google Scholar] [CrossRef] [Green Version]
- Begley, M.; Gahan, C.G.M.; Hill, C. Bile Stress Response in Listeria Monocytogenes LO28: Adaptation, Cross-Protection, and Identification of Genetic Loci Involved in Bile Resistance. Appl. Environ. Microbiol. 2002, 68, 126005–126012. [Google Scholar] [CrossRef] [Green Version]
- Prouty, A.M.; Gunn, J.S. Salmonella Enterica Serovar Typhimurium Invasion Is Repressed in the Presence of Bile. Infect. Immun. 2000, 68, 126763–126769. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, K.P.; Chanin, R.B.; Sistrunk, J.R.; Rasko, D.A.; Fink, P.J.; Barry, E.M.; Nataro, J.P.; Faherty, C.S. Analysis of Shigella Flexneri Resistance, Biofilm Formation, and Transcriptional Profile in Response to Bile Salts. Infect. Immun. 2017, 85, 1067–1082. [Google Scholar] [CrossRef] [Green Version]
- Faherty, C.S.; Redman, J.C.; Rasko, D.A.; Barry, E.M.; Nataro, J.P. Shigella Flexneri Effectors OspE1 and OspE2 Mediate Induced Adherence to the Colonic Epithelium Following Bile Salts Exposure: OspE1 and OspE2 Enhance Adherence to Epithelial Cells. Mol. Microbiol. 2012, 85, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Malik-Kale, P.; Parker, C.T.; Konkel, M.E. Culture of Campylobacter Jejuni with Sodium Deoxycholate Induces Virulence Gene Expression. J. Bacteriol. 2008, 190, 72286–72297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solheim, M.; Aakra, Å.; Vebø, H.; Snipen, L.; Nes, I.F. Transcriptional Responses of Enterococcus Faecalis V583 to Bovine Bile and Sodium Dodecyl Sulfate. Appl. Environ. Microbiol. 2007, 73, 185767–518774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Francis, M.B.; Allen, C.A.; Shrestha, R.; Sorg, J.A. Bile Acid Recognition by the Clostridium Difficile Germinant Receptor, CspC, Is Important for Establishing Infection. PLoS Pathog. 2013, 9, e1003356. [Google Scholar] [CrossRef]
- Sorg, J.A.; Sonenshein, A.L. Inhibiting the Initiation of Clostridium Difficile Spore Germination Using Analogs of Chenodeoxycholic Acid, a Bile Acid. J. Bacteriol. 2010, 192, 194983–194990. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Fukiya, S.; Yokota, A. Comprehensive Evaluation of the Bactericidal Activities of Free Bile Acids in the Large Intestine of Humans and Rodents. J. Lipid Res. 2017, 58, 61143–61152. [Google Scholar] [CrossRef] [Green Version]
- Sannasiddappa, T.H.; Lund, P.A.; Clarke, S.R. In Vitro Antibacterial Activity of Unconjugated and Conjugated Bile Salts on Staphylococcus Aureus. Front. Microbiol. 2017, 8, 1581. [Google Scholar] [CrossRef] [Green Version]
- Prete, R.; Long, S.L.; Gallardo, A.L.; Gahan, C.G.; Corsetti, A.; Joyce, S.A. Beneficial Bile Acid Metabolism from Lactobacillus Plantarum of Food Origin. Sci. Rep. 2020, 10, 11165. [Google Scholar] [CrossRef] [Green Version]
- Faillie, J.L.; Yu, O.H.; Yin, H.; Hillaire-Buys, D.; Barkun, A.; Azoulay, L. Association of Bile Duct and Gallbladder Diseases with the Use of Incretin-Based Drugs in Patients with Type 2 Diabetes Mellitus. JAMA Intern. Med. 2016, 176, 101474. [Google Scholar] [CrossRef]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorakova, K.; Garewal, H. Bile Acids as Carcinogens in Human Gastrointestinal Cancers. Mutat. Res. Mutat. Res. 2005, 589, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The Bile Acid Receptor FXR Is a Modulator of Intestinal Innate Immunity. J. Immunol. 2009, 183, 106251–106261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M. Advances in Understanding of Bile Acid Diarrhea. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Nadeau, A.; Tremaine, W.J.; Lamsam, J.; Burton, D.; Odunsi, S.; Sweetser, S.; Singh, R. Measurement of Serum 7α-Hydroxy-4-Cholesten-3-One (or 7αC4), a Surrogate Test for Bile Acid Malabsorption in Health, Ileal Disease and Irritable Bowel Syndrome Using Liquid Chromatography-Tandem Mass Spectrometry. Neurogastroenterol. Motil. 2009, 21, 7734–7743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, A.; Arasaradnam, R. Bile Acid Diarrhoea: Pathophysiology, Diagnosis and Management. Frontline Gastroenterol. 2021, 12, 6500–6507. [Google Scholar] [CrossRef]
- Li, W.T.; Luo, Q.Q.; Wang, B.; Chen, X.; Yan, X.J.; Qiu, H.Y.; Chen, S.L. Bile Acids Induce Visceral Hypersensitivity via Mucosal Mast Cell–to–Nociceptor Signaling That Involves the Farnesoid X Receptor/Nerve Growth Factor/Transient Receptor Potential Vanilloid 1 Axis. FASEB J. 2019, 33, 22435–22450. [Google Scholar] [CrossRef] [Green Version]
- Centuori, S.M.; Martinez, J.D. Differential Regulation of EGFR–MAPK Signaling by Deoxycholic Acid (DCA) and Ursodeoxycholic Acid (UDCA) in Colon Cancer. Dig. Dis. Sci. 2014, 59, 102367–210380. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.W.; Kennaway, E.L.; Kennaway, N.M. Production of Tumours in Mice by Deoxycholic Acid. Nature 1940, 145, 3677627. [Google Scholar] [CrossRef]
- Fu, T.; Coulter, S.; Yoshihara, E.; Oh, T.G.; Fang, S.; Cayabyab, F.; Zhu, Q.; Zhang, T.; Leblanc, M.; Liu, S.; et al. FXR Regulates Intestinal Cancer Stem Cell Proliferation. Cell 2019, 176, 51098. [Google Scholar] [CrossRef] [Green Version]
- Akare, S.; Martinez, J.D. Bile Acid Induces Hydrophobicity-Dependent Membrane Alterations. Biochim. Biophys. Acta 2005, 1735, 159–167. [Google Scholar] [CrossRef]
- Bernstein, C.; Holubec, H.; Bhattacharyya, A.K.; Nguyen, H.; Payne, C.M.; Zaitlin, B.; Bernstein, H. Carcinogenicity of Deoxycholate, a Secondary Bile Acid. Arch. Toxicol. 2011, 85, 8863–8871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.K.; Cho, J.H.; Kim, E.; Kim, Y.J. Ursodeoxycholic Acid Inhibits the Proliferation of Colon Cancer Cells by Regulating Oxidative Stress and Cancer Stem-like Cell Growth. PLoS ONE 2017, 12, e0181183. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H.; Kim, B.G.; Lee, K.L.; Kim, J.W.; Koh, S.J. Tauroursodeoxycholic Acid Attenuates Colitis-Associated Colon Cancer by Inhibiting Nuclear Factor KappaB Signaling: Effects of TUDCA in Colitis. J. Gastroenterol. Hepatol. 2019, 34, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Nie, Y.; Yu, J.; Wong, C.C. Microbial Metabolites in Colorectal Cancer: Basic and Clinical Implications. Metabolites 2021, 11, 159. [Google Scholar] [CrossRef]
- Powell, A.A.; LaRUE, J.M.; Batta, A.K.; Martinez, J.D. Bile Acid Hydrophobicity Is Correlated with Induction of Apoptosis and/or Growth Arrest in HCT116 Cells. Biochem. J. 2001, 356, 481–486. [Google Scholar] [CrossRef]
- Sips, F.L.P.; Eggink, H.M.; Hilbers, P.A.J.; Soeters, M.R.; Groen, A.K.; van Riel, N.A.W. In Silico Analysis Identifies Intestinal Transit as a Key Determinant of Systemic Bile Acid Metabolism. Front. Physiol. 2018, 9, 631. [Google Scholar] [CrossRef]
- Berr, F.; Stellaard, F.; Pratschke, E.; Paumgartner, G. Effects of Cholecystectomy on the Kinetics of Primary and Secondary Bile Acids. J. Clin. Investig. 1989, 83, 1541–1550. [Google Scholar] [CrossRef] [Green Version]
- Barrera, F.; Azócar, L.; Molina, H.; Schalper, K.A.; Ocares, M.; Liberona, J.; Villarroel, L.; Pimentel, F.; Pérez-Ayuso, R.M.; Nervi, F.; et al. Effect of Cholecystectomy on Bile Acid Synthesis and Circulating Levels of Fibroblast Growth Factor 19. Annu. Hepatol. 2015, 14, 710–721. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Li, L.; Ai, M.; Gong, Z.; He, Y.; Dong, Y.; Xu, S.; Wang, J.; Jin, B.; et al. Cholecystectomy Can Increase the Risk of Colorectal Cancer: A Meta-Analysis of 10 Cohort Studies. PLoS ONE 2017, 12, e0181852. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Xu, J.; Zhang, Y.; Chen, G.; Zhang, Y.; Huang, Q.; Liu, Y. Bacterial Alterations in Post-Cholecystectomy Patients Are Associated with Colorectal Cancer. Front. Oncol. 2020, 10, 1418. [Google Scholar] [CrossRef]
- Vinikoor, L.C.; Robertson, D.J.; Baron, J.A.; Silverman, W.B.; Sandler, R.S. Cholecystectomy and the Risk of Recurrent Colorectal Adenomas. Cancer Epidemiol. Biomark. Amp. Prev. 2007, 16, 1523–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer through Senescence Secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The Senescence-Associated Secretome as an Indicator of Age and Medical Risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10−/− Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Vacante, M.; Ciuni, R.; Basile, F.; Biondi, A. Gut Microbiota and Colorectal Cancer Development: A Closer Look to the Adenoma-Carcinoma Sequence. Biomedicines 2020, 8, 489. [Google Scholar] [CrossRef]
- Baiocchi, L.; Zhou, T.; Liangpunsakul, S.; Lenci, I.; Santopaolo, F.; Meng, F.; Kennedy, L.; Glaser, S.; Francis, H.; Alpini, G. Dual Role of Bile Acids on the Biliary Epithelium: Friend or Foe? Int. J. Mol. Sci. 2019, 20, 1869. [Google Scholar] [CrossRef] [Green Version]
- Roma, M.G.; Sanchez Pozzi, E.J. Oxidative Stress: A Radical Way to Stop Making Bile. Annu. Hepatol. 2008, 7, 16–33. [Google Scholar] [CrossRef]
- Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Regulation of CAR and PXR Expression in Health and Disease. Cells 2020, 9, 2395. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [Green Version]
- Steib, C.J.; Hartmann, A.C.; Hesler, C.V.; Benesic, A.; Hennenberg, M.; Bilzer, M.; Gerbes, A.L. Intraperitoneal LPS Amplifies Portal Hypertension in Rat Liver Fibrosis. Lab. Investig. 2010, 90, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Bräsen, J.H.; Mederacke, Y.; Schmitz, J.; Diahovets, K.; Khalifa, A.; Hartleben, B.; Person, F.; Wiech, T.; Steenbergen, E.; Großhennig, A.; et al. Cholemic Nephropathy Causes Acute idney Injury and Is Accompanied by Loss of Aquaporin 2 in Collecting Ducts. Hepatology 2019, 69, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acids: Regulation of Synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. In Advances in Pharmacology; Elsevier: Berlin, Germany, 2015; Volume 74, pp. 263–302. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, Y.; Bouscarel, B.; Ikegami, T.; Honda, A.; Doy, M.; Ceryak, S.; Fukushima, S.; Yoshida, S.; Shoda, J.; Tanaka, N. Selective Inhibition of CYP27A1 and of Chenodeoxycholic Acid Synthesis in Cholestatic Hamster Liver. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2002, 1588, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Quezada, M.; Levine, P.; Han, Y.; McDaniel, K.; Zhou, T.; Lin, E.; Glaser, S.; Meng, F.; Francis, H.; et al. Functional Role of Cellular Senescence in Biliary Injury. Am. J. Pathol. 2015, 185, 602–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lleo, A.; Leung, P.S.C.; Hirschfield, G.M.; Gershwin, E.M. The Pathogenesis of Primary Biliary Cholangitis: A Comprehensive Review. Semin. Liver Dis. 2020, 40, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Ninfole, E.; Benedetti, A.; Maroni, L.; Marzioni, M. Aging-Related Molecular Pathways in Chronic Cholestatic Conditions. Front. Med. 2020, 6, 332. [Google Scholar] [CrossRef] [Green Version]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte Pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Melero, S.; Spirlì, C.; Zsembery, Á.; Medina, J.F.; Joplin, R.E.; Duner, E.; Zuin, M.; Neuberger, J.M.; Prieto, J.; Strazzabosco, M. Defective Regulation of Cholangiocyte Cl−/HCO−3 and Na+/H+ Exchanger Activities in Primary Biliary Cirrhosis: Defective Regulation of Cholangiocyte Cl−/HCO−3 and Na+/H+ Exchanger Activities in Primary Biliary Cirrhosis. Hepatology 2002, 35, 1513–1521. [Google Scholar] [CrossRef]
- Salas, J.T.; Banales, J.M.; Sarvide, S.; Recalde, S.; Ferrer, A.; Uriarte, I.; Oude Elferink, R.P.J.; Prieto, J.; Medina, J.F. Ae2a,b-Deficient Mice Develop Antimitochondrial Antibodies and Other Features Resembling Primary Biliary Cirrhosis. Gastroenterology 2008, 134, 1482–1493. [Google Scholar] [CrossRef]
- Colombo, C.; Battezzati, P.; Strazzabosco, M.; Podda, M. Liver and Biliary Problems in Cystic Fibrosis. Semin. Liver Dis. 1998, 18, 227–235. [Google Scholar] [CrossRef]
- Jahan, A.; Chiang, J.Y.L. Cytokine Regulation of Human Sterol 12α-Hydroxylase (CYP8B1) Gene. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G685–G695. [Google Scholar] [CrossRef]
- Dueland, S.; Reichen, J.; Everson, G.T.; Davis, R.A. Regulation of Cholesterol and Bile Acid Homoeostasis in Bile-Obstructed Rats. Biochem. J. 1991, 280, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Song, K.H.; Ellis, E.; Strom, S.; Chiang, J.Y.L. Hepatocyte Growth Factor Signaling Pathway Inhibits Cholesterol 7α-Hydroxylase and Bile Acid Synthesis in Human Hepatocytes. Hepatology 2007, 46, 1993–2002. [Google Scholar] [CrossRef]
- Lanzini, A. Intestinal Absorption of the Bile Acid Analogue 75Se-Homocholic Acid-Taurine Is Increased in Primary Biliary Cirrhosis and Reverts to Normal during Ursodeoxycholic Acid Administration. Gut 2003, 52, 1371–1375. [Google Scholar] [CrossRef] [Green Version]
- Jansen, P.L.M. New Therapies Target the Toxic Consequences of Cholestatic Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 277–285. [Google Scholar] [CrossRef]
- Shah, R.A.; Kowdley, K.V. Current and Potential Treatments for Primary Biliary Cholangitis. Lancet Gastroenterol. Hepatol. 2020, 5, 306–315. [Google Scholar] [CrossRef]
- Gomez-Ospina, N.; Potter, C.J.; Xiao, R.; Manickam, K.; Kim, M.-S.; Kim, K.H.; Shneider, B.L.; Picarsic, J.L.; Jacobson, T.A.; Zhang, J.; et al. Mutations in the Nuclear Bile Acid Receptor FXR Cause Progressive Familial Intrahepatic Cholestasis. Nat. Commun. 2016, 7, 10713. [Google Scholar] [CrossRef]
- Arroyo, M.; Crawford, J.M. Hepatitic Inherited Metabolic Disorders. Semin. Diagn. Pathol. 2006, 23, 182–189. [Google Scholar] [CrossRef]
- Sangkhathat, S.; Laochareonsuk, W.; Maneechay, W.; Kayasut, K.; Chiengkriwate, P. Variants Associated with Infantile Cholestatic Syndromes Detected in Extrahepatic Biliary Atresia by Whole Exome Studies: A 20-Case Series from Thailand. J. Pediatr. Genet. 2018, 7, 67–73. [Google Scholar] [CrossRef]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 107327481772924. [Google Scholar] [CrossRef]
- Liu, R.; Li, X.; Qiang, X.; Luo, L.; Hylemon, P.B.; Jiang, Z.; Zhang, L.; Zhou, H. Taurocholate Induces Cyclooxygenase-2 Expression via the Sphingosine 1-Phosphate Receptor 2 in a Human Cholangiocarcinoma Cell Line. J. Biol. Chem. 2015, 290, 30988–31002. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Wang, H.; Shi, Y.; Dong, Y.; Zhang, Y.; Wang, J. Impact of Bile Acids on the Growth of Human Cholangiocarcinoma via FXR. J. Hematol. Oncol. J. Hematol. Oncol. 2011, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Huang, X.; Yi, T.; Yen, Y.; Moore, D.D.; Huang, W. Spontaneous Development of Liver Tumors in the Absence of the Bile Acid Receptor Farnesoid X Receptor. Cancer Res. 2007, 67, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L. Negative Feedback Regulation of Bile Acid Metabolism: Impact on Liver Metabolism and Diseases: Hepatology elsewhere. Hepatology 2015, 62, 1315–1317. [Google Scholar] [CrossRef] [Green Version]
- Cave, M.C.; Clair, H.B.; Hardesty, J.E.; Falkner, K.C.; Feng, W.; Clark, B.J.; Sidey, J.; Shi, H.; Aqel, B.A.; McClain, C.J.; et al. Nuclear Receptors and Nonalcoholic Fatty Liver Disease. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2016, 1859, 1083–1099. [Google Scholar] [CrossRef] [Green Version]
- Urso, A.; D’Ovidio, F.; Xu, D.; Emala, C.W.; Bunnett, N.W.; Perez-Zoghbi, J.F. Bile Acids Inhibit Cholinergic Constriction in Proximal and Peripheral Airways from Humans and Rodents. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L264–L275. [Google Scholar] [CrossRef]
- Urso, A.; Perez-Zoghbi, J.; Nandakumar, R.; Cremers, S.; Bunnett, N.; Emala, C.; D’Ovidio, F. Aspirated Bile Acids Affect Lung Immunity and Function. In Transplantation; European Respiratory Society: Lausanne, Switzerland, 2019; p. 3359. [Google Scholar] [CrossRef]
- Chen, B.; You, W.J.; Liu, X.Q.; Xue, S.; Qin, H.; Jiang, H.D. Chronic Microaspiration of Bile Acids Induces Lung Fibrosis through Multiple Mechanisms in Rats. Clin. Sci. 2017, 131, 951–963. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Frisch, K.; Sørensen, M.; Munk, O.L.; Hofmann, A.F.; Horsager, J.; Schacht, A.C.; Erickson, M.; Shapiro, D.; Keiding, S. Obeticholic Acid Improves Hepatic Bile Acid Excretion in Patients with Primary Biliary Cholangitis. J. Hepatol. 2021, 74, 58–65. [Google Scholar] [CrossRef]
- Hofmann, A.F. The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef]
- Khurana, S.; Raina, H.; Pappas, V.; Raufman, J.-P.; Pallone, T.L. Effects of Deoxycholylglycine, a Conjugated Secondary Bile Acid, on Myogenic Tone and Agonist-Induced Contraction in Rat Resistance Arteries. PLoS ONE 2012, 7, e32006. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Okuda, Y.; Chisaki, K.; Shin, W.-S.; Iwasawa, K.; Morita, T.; Matsumoto, A.; Suzuki, J.; Suzuki, S.; Yamada, N.; et al. Bile Acids Increase Intracellular Ca2+ Concentration and Nitric Oxide Production in Vascular Endothelial Cells: Bile Acids and Endothelial Cells. Br. J. Pharmacol. 2000, 130, 1457–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alon, U.; Berant, M.; Mordechovitz, D.; Hashmonai, M.; Better, O.S. Effect of Isolated Cholaemia on Systemic Haemodynamics and Kidney Function in Conscious Dogs. Clin. Sci. Lond. Engl. 1982, 63, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomzon, A.; Finberg, J.P.; Tovbin, D.; Naidu, S.G.; Better, O.S. Bile Salts, Hypotension and Obstructive Jaundice. Clin. Sci. Lond. Engl. 1984, 67, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Pak, J.M.; Lee, S.S. Vasoactive Effects of Bile Salts in Cirrhotic Rats: In Vivo and in Vitro Studies. Hepatololy 1993, 18, 1175–1181. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Mu, Y.; Kuruba, R.; Ma, Z.; Wilson, A.; Alber, S.; Jiang, Y.; Stevens, T.; Watkins, S.; et al. Downregulation of Endothelin-1 by Farnesoid X Receptor in Vascular Endothelial Cells. Circ. Res. 2006, 98, 192–199. [Google Scholar] [CrossRef]
- Li, J.; Wilson, A.; Kuruba, R.; Zhang, Q.; Gao, X.; He, F.; Zhang, L.M.; Pitt, B.R.; Xie, W.; Li, S. FXR-Mediated Regulation of ENOS Expression in Vascular Endothelial Cells. Cardiovasc. Res. 2008, 77, 169–177. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.; Kuruba, R.; Gao, X.; Wilson, A.; Li, J.; Billiar, T.R.; Pitt, B.R.; Xie, W.; Li, S. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc. Res. 2008, 77, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ma, W.Q.; Fu, M.J.; Li, J.; Hu, C.H.; Chen, Y.; Zhou, M.M.; Gao, Z.J.; He, Y.L. Overview of Bile Acid Signaling in the Cardiovascular System. World J. Clin. Cases 2021, 9, 308–320. [Google Scholar] [CrossRef]
- Pols, T.W.H. TGR5 in Inflammation and Cardiovascular Disease. Biochem. Soc. Trans. 2014, 42, 244–249. [Google Scholar] [CrossRef]
- Charach, G.; Rabinovich, A.; Argov, O.; Weintraub, M.; Rabinovich, P. The Role of Bile Acid Excretion in Atherosclerotic Coronary Artery Disease. Int. J. Vasc. Med. 2012, 2012, 949672. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shu, S.; Cheng, L.; Hao, X.; Wang, L.; Wu, Y.; Yuan, Z.; Zhou, J. Fasting Serum Total Bile Acid Level Is Associated with Coronary Artery Disease, Myocardial Infarction and Severity of Coronary Lesions. Atherosclerosis 2020, 292, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanniman, E.A.; Lambert, G.; McCarthy, T.C.; Sinal, C.J. Loss of Functional Farnesoid X Receptor Increases Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice. J. Lipid Res. 2005, 46, 2595–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Lee, S.S.; Meddings, J.B. Effects of Altered Cardiac Membrane Fluidity on β-Adrenergic Receptor Signalling in Rats with Cirrhotic Cardiomyopathy. J. Hepatol. 1997, 26, 904–912. [Google Scholar] [CrossRef]
- Joubert, P. An in vivo investigation of the negative chronotropic effect of cholic acid in the rat. Clin. Exp. Pharmacol. Physiol. 1978, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raufman, J.P.; Chen, Y.; Zimniak, P.; Cheng, K. Deoxycholic Acid Conjugates Are Muscarinic Cholinergic Receptor Antagonists. Pharmacology 2002, 65, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yuan, G.; Xu, Z.; Lan, L.; Xin, W. Chenodeoxycholic and Deoxycholic Acids Induced Positive Inotropic and Negative Chronotropic Effects on Rat Heart. Naunyn. Schmiedebergs Arch. Pharmacol. 2021, 394, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Schultz, F.; Hasan, A.; Alvarez-Laviada, A.; Miragoli, M.; Bhogal, N.; Wells, S.; Poulet, C.; Chambers, J.; Williamson, C.; Gorelik, J. The Protective Effect of Ursodeoxycholic Acid in an in Vitro Model of the Human Fetal Heart Occurs via Targeting Cardiac Fibroblasts. Prog. Biophys. Mol. Biol. 2016, 120, 149–163. [Google Scholar] [CrossRef] [Green Version]
- von Haehling, S.; Schefold, J.C.; Jankowska, E.A.; Springer, J.; Vazir, A.; Kalra, P.R.; Sandek, A.; Fauler, G.; Stojakovic, T.; Trauner, M.; et al. Ursodeoxycholic Acid in Patients with Chronic Heart Failure: A Double-Blind, Randomized, Placebo-Controlled, Crossover Trial. J. Am. Coll. Cardiol. 2012, 59, 585–592. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; Ueland, T.; Broch, K.; Vincent, R.P.; Cross, G.F.; Dahl, C.P.; Aukrust, P.; Gullestad, L.; Hov, J.R.; Trøseid, M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017, 23, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, J.; Shevchuk, A.; de Swiet, M.; Lab, M.; Korchev, Y.; Williamson, C. Comparison of the Arrhythmogenic Effects of Tauro- and Glycoconjugates of Cholic Acid in an in Vitro Study of Rat Cardiomyocytes. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 867–870. [Google Scholar] [CrossRef]
- Desai, M.S.; Shabier, Z.; Taylor, M.; Lam, F.; Thevananther, S.; Kosters, A.; Karpen, S.J. Hypertrophic Cardiomyopathy and Dysregulation of Cardiac Energetics in a Mouse Model of Biliary Fibrosis. Hepatology 2010, 51, 2097–2107. [Google Scholar] [CrossRef] [Green Version]
- Kitai, T.; Tang, W.H.W. Gut Microbiota in Cardiovascular Disease and Heart Failure. Clin. Sci. 2018, 132, 85–91. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of Antibacterial Defense in the Small Intestine by the Nuclear Bile Acid Receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, A.F.; Eckmann, L. How Bile Acids Confer Gut Mucosal Protection against Bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 4333–4334. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.B.M.S.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile Acid Is a Host Factor That Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Isolation and Characterization of a Bile Acid Inducible 7α-Dehydroxylating Operon in Clostridium Hylemonae TN271. Anaerobe 2010, 16, 137–146. [Google Scholar] [CrossRef]
- Browne, H.P.; Forster, S.C.; Anonye, B.O.; Kumar, N.; Neville, B.A.; Stares, M.D.; Goulding, D.; Lawley, T.D. Culturing of ‘Unculturable’ Human Microbiota Reveals Novel Taxa and Extensive Sporulation. Nature 2016, 533, 543–546. [Google Scholar] [CrossRef] [Green Version]
- Philipp, B. Bacterial Degradation of Bile Salts. Appl. Microbiol. Biotechnol. 2011, 89, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, M. Cathelicidins, Multifunctional Peptides of the Innate Immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G.M. Regulation of Host Weight Gain and Lipid Metabolism by Bacterial Bile Acid Modification in the Gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [Green Version]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The Bile Acid Membrane Receptor TGR5 as an Emerging Target in Metabolism and Inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porez, G.; Prawitt, J.; Gross, B.; Staels, B. Bile Acid Receptors as Targets for the Treatment of Dyslipidemia and Cardiovascular Disease. J. Lipid Res. 2012, 53, 1723–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka-Tojo, M.; Tojo, T.; Izumi, T. Beyond Cholesterol Lowering: Pleiotropic Effects of Bile Acid Binding Resins Against Cardiovascular Disease Risk Factors in Patients with Metabolic Syndrome. Curr. Vasc. Pharmacol. 2008, 6, 271–281. [Google Scholar] [CrossRef]
- Ali, A.H.; Carey, E.J.; Lindor, K.D. Recent Advances in the Development of Farnesoid X Receptor Agonists. Ann. Transl. Med. 2015, 3, 5. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile Acids Lower Triglyceride Levels via a Pathway Involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [Green Version]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Smith, Z.; Ryerson, D.; Kemper, J.K. Epigenomic Regulation of Bile Acid Metabolism: Emerging Role of Transcriptional Cofactors. Mol. Cell. Endocrinol. 2013, 368, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Suzuki, Y.; Naito, H.; Yetti, H.; Kitamori, K.; Hayashi, Y.; Kaneko, R.; Nomura, M.; Yamori, Y.; Zaitsu, K.; et al. A Possible Role of Chenodeoxycholic Acid and Glycine-Conjugated Bile Acids in Fibrotic Steatohepatitis in a Dietary Rat Model. Dig. Dis. Sci. 2014, 59, 1490–1501. [Google Scholar] [CrossRef]
- Quintero, P.; Pizarro, M.; Solís, N.; Arab, J.P.; Padilla, O.; Riquelme, A.; Arrese, M. Bile Acid Supplementation Improves Established Liver Steatosis in Obese Mice Independently of Glucagon-like Peptide-1 Secretion. J. Physiol. Biochem. 2014, 70, 667–674. [Google Scholar] [CrossRef]
- Woolbright, B.L. Novel Insight into Mechanisms of Cholestatic Liver Injury. World J. Gastroenterol. 2012, 18, 4985. [Google Scholar] [CrossRef] [PubMed]
- Kirbas, O.; Biberoglu, E.H.; Kirbas, A.; Daglar, K.; Kurmus, O.; Danisman, N.; Biberoglu, K. Evaluation of Ventricular Repolarization in Pregnant Women with Intrahepatic Cholestasis. Int. J. Cardiol. 2015, 189, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Paumgartner, G. Ursodeoxycholic Acid in Cholestatic Liver Disease: Mechanisms of Action and Therapeutic Use Revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Kordes, C. Mechanisms of Tauroursodeoxycholate-Mediated Hepatoprotection. Dig. Dis. 2017, 35, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.H.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Mason, A.; Luketic, V.; Lindor, K.; Gordon, S.C.; Mayo, M.; Kowdley, K.V.; Vincent, C.; Bodhenheimer, H.C.; Parés, A.; et al. Efficacy of Obeticholic Acid in Patients with Primary Biliary Cirrhosis and Inadequate Response to Ursodeoxycholic Acid. Gastroenterology 2015, 148, 751–761.e8. [Google Scholar] [CrossRef] [Green Version]
- Úbeda, M.; Lario, M.; Muñoz, L.; Borrero, M.-J.; Rodríguez-Serrano, M.; Sánchez-Díaz, A.-M.; Del Campo, R.; Lledó, L.; Pastor, Ó.; García-Bermejo, L.; et al. Obeticholic Acid Reduces Bacterial Translocation and Inhibits Intestinal Inflammation in Cirrhotic Rats. J. Hepatol. 2016, 64, 1049–1057. [Google Scholar] [CrossRef]
- Laleman, W.; Trebicka, J.; Verbeke, L. Evolving Insights in the Pathophysiology of Complications of Cirrhosis: The Farnesoid X Receptor (FXR) to the Rescue? Hepatology 2016, 64, 1792–1794. [Google Scholar] [CrossRef]
- Vignozzi, L.; Morelli, A.; Cellai, I.; Filippi, S.; Comeglio, P.; Sarchielli, E.; Maneschi, E.; Vannelli, G.B.; Adorini, L.; Maggi, M. Cardiopulmonary Protective Effects of the Selective FXR Agonist Obeticholic Acid in the Rat Model of Monocrotaline-Induced Pulmonary Hypertension. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt B, 277–292. [Google Scholar] [CrossRef]
- Voiosu, A.; Wiese, S.; Voiosu, T.; Bendtsen, F.; Møller, S. Bile Acids and Cardiovascular Function in Cirrhosis. Liver Int. 2017, 37, 1420–1430. [Google Scholar] [CrossRef] [Green Version]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Kong, B.; Zhu, Y.; Zhan, L.; Williams, J.A.; Tawfik, O.; Kassel, K.M.; Luyendyk, J.P.; Wang, L.; Guo, G.L. Small Heterodimer Partner Overexpression Partially Protects against Liver Tumor Development in Farnesoid X Receptor Knockout Mice. Toxicol. Appl. Pharmacol. 2013, 272, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Xie, G.; Jia, W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhana, L.; Nangia-Makker, P.; Arbit, E.; Shango, K.; Sarkar, S.; Mahmud, H.; Hadden, T.; Yu, Y.; Majumdar, A.P.N. Bile Acid: A Potential Inducer of Colon Cancer Stem Cells. Stem Cell Res. Ther. 2016, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Ridlon, J.M.; Wolf, P.G.; Gaskins, H.R. Taurocholic Acid Metabolism by Gut Microbes and Colon Cancer. Gut Microbes 2016, 7, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, N. Expression of COX2 and P53 in Rat Esophageal Cancer Induced by Reflux of Duodenal Contents. ISRN Gastroenterol. 2012, 2012, 914824. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, N. Effect of Pancreatic Juice and Bile Reflux to the Development of Esophageal Carcinogenesis in Rat Model. Clin. Oncol. 2018, 1, 1–6. [Google Scholar]
- Weingarden, A.R.; Dosa, P.I.; DeWinter, E.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Khoruts, A.; Sadowsky, M.J. Changes in Colonic Bile Acid Composition Following Fecal Microbiota Transplantation Are Sufficient to Control Clostridium Difficile Germination and Growth. PLoS ONE 2016, 11, e0147210. [Google Scholar] [CrossRef] [Green Version]
- Weingarden, A.R.; Chen, C.; Zhang, N.; Graiziger, C.T.; Dosa, P.I.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Sadowsky, M.J.; Khoruts, A. Ursodeoxycholic Acid Inhibits Clostridium Difficile Spore Germination and Vegetative Growth and Prevents the Recurrence of Ileal Pouchitis Associated with the Infection. J. Clin. Gastroenterol. 2016, 50, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Huo, X. Therapeutic and Chemopreventive Effects of Ursodeoxycholic Acid (UDCA): Potential Role in Patients with Barrett’s Esophagus. Gastro Open J. 2015, 1, 89–93. [Google Scholar] [CrossRef]
- Woods, D.F.; Flynn, S.; Caparrós-Martín, J.A.; Stick, S.M.; Reen, F.J.; O’Gara, F. Systems Biology and Bile Acid Signalling in Microbiome-Host Interactions in the Cystic Fibrosis Lung. Antibiotics 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; DeMorrow, S. Effects of Bile Acids on Neurological Function and Disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 3658–3668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of Gut Microbiome with Cognition in Hepatic Encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Attili, A.F.; Angelico, M.; Cantafora, A.; Alvaro, D.; Capocaccia, L. Bile Acid-Induced Liver Toxicity: Relation to the Hydrophobic-Hydrophilic Balance of Bile Acids. Med. Hypotheses 1986, 19, 57–69. [Google Scholar] [CrossRef]
- Zimber, A.; Zusman, I.; Bentor, R.; Pinus, H. Effects of Lithocholic Acid Exposure throughout Pregnancy on Late Prenatal and Early Postnatal Development in Rats. Teratology 1991, 43, 355–361. [Google Scholar] [CrossRef]
- Debruyne, P.R.; Bruyneel, E.A.; Li, X.; Zimber, A.; Gespach, C.; Mareel, M.M. The Role of Bile Acids in Carcinogenesis. Mutat. Res. 2001, 480, 359–369. [Google Scholar] [CrossRef]
- Costarelli, V.; Sanders, T.a.B. Plasma Deoxycholic Acid Concentration Is Elevated in Postmenopausal Women with Newly Diagnosed Breast Cancer. Eur. J. Clin. Nutr. 2002, 56, 925–927. [Google Scholar] [CrossRef] [Green Version]
- Zdebska, E.; Iolascon, A.; Spychalska, J.; Perrotta, S.; Lanzara, C.; Smolenska-Sym, G.; Koscielak, J. Abnormalities of erythrocyte glycoconjugates are identical in two families with congenital dyserythropoietic anemia type II with different chromosomal localizations of the disease gene. Haematologica 2007, 92, 427–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, M.J.; Marin, J.J.G.; Antelo, A.; Vazquez-Tato, J. Bile Acids: Chemistry, Physiology, and Pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef]

| BA Type | Effect | Probable Mechanism | Ref. |
|---|---|---|---|
| Cardiovascular effects | |||
| DCA, LCA in vitro | Mesenterial arterial dilatation | Endothelial S1PR2 stimulation, ↑ Ca2+ intracellular concentration and ↓ NO production | [153,154] |
| BA (not specified) or CDCA in serum | ↓ in mean arterial pressure and peripheral vascular resistance in cirrhosis. Could be involved in splanchnic hyperaemia and hyperdynamic syndrome | Endothelial FXR stimulation with ↑ eNOS and ↓ endothelin-1 and angiotensin-II receptor expression; ↓ vascular response to noradrenaline with DCA being the most potent inhibitor | [155,156,157,158,159,160,161] |
| Fasting BAs level in serum | Reversible association with atherosclerosis severity and the presence and severity of coronary artery disease, especially myocardial infarction | TGR5 stimulation with anti-inflammatory effect. Excess cholesterol excretion by secreting large amounts of BA into intestine. Activation of FXR (in animal models) | [162,163,164] |
| CDCA derivatives (oral administration) | Significantly ↓ aortic plaque formation and ↓ aortic expression of inflammatory factors (IL-6, IL-1, etc.) in apolipoprotein E-deficiency | Activation of FXR | [165] |
| Elevated serum BAs level in cirrhosis | Cirrhotic cardiomyopathy | Reduced fluidity of the myocardial membrane, resulting in adrenergic dysfunction and the inability to produce cAMP; ↓ myocardium contractility, apoptosis of cardiomyocytes, promoting myocardial ischemia/reperfusion injury, ↑ production of NO mediated by intracellular Ca2+ signaling | [161,166] |
| CA in cirrhosis/DCA and LCA in vitro and portal blood | Bradycardia | Altered cardiac membrane fluidity and decreased beta-adrenergic receptor signalling. DCA and LCA act as muscarinic antagonists | [166,167,168,169] |
| Non-UDCA/UDCA ratio in serum | Independent predictor of atrial fibrillation | ↑ portion of non-UDCA can change slow inward Na+ and Ca2+ currents and outward K+ currents, ↓ the duration of the action potential in cardiomyocytes predisposing to re-entry type arrythmia | [38,170,171] |
| Supraphysiological tauro-CA concentration in vitro and in intestine | A role in progressing of heart failure | Depolarization of the resting potential and inducing posterior depolarization of cells (reduced contractility and pacemaker activity). Decrease protein expression in heart tissue. | [172,173,174] |
| ↑ ratio of secondary BAs to primary BAs in intestine | -- | Indirect influence of the intestinal flora on the severity of HF hydrophobic BAs significantly alter mitochondrial bioenergetics | [37,172,175] |
| Intestinal microbiota modifications | |||
| Primary bile acids per os | Prevention of in overgrowth of aerobic and anaerobic bacteria in the ileum and cecum and of bacterial translocation | FXRα activation resulting in up-regulation of genes involved in mucosal defense in the ileum. Direct antimicrobial effects in high concentration of conjugated BAs | [176,177] |
| ↑ CA per os | ↑ in Firmicutes, especially groups capable of 7α-dehydroxylation, and ↓ of Bacteroidetes | Due to sustaining of 7α-dehydroxylating bacteria and antagonistic effect on other bacterial communities (↑ production of an antimicrobial compounds by these members, or use of BAs as an electron acceptor in metabolic pathways providing a net energy gain)/BA induce (bai) operon | [76,178,179] |
| Primary bile acids (tauro-CA) in intestine | Recovery of microbiota after dysbiosis induced by antibiotics or toxins | Provide homing signals to gut bacteria and promote germination of spores. This mechanism can be exploited by pathogens such as Clostridium difficile, germinating into a vegetative form | [120,180] |
| More hydrophobic bile acids (having two rather than three hydroxy groups) in intestine | Inhibition of bacterial overgrowth | Impair the membrane integrity. FXR activates genes involved in enteric protection (ANG1, iNOS). Induce ERK 1/2 pathway which activates the VDR and the synthesis of antimicrobial peptides cathelicidins | [85,181,182] |
| Metabolic effects | |||
| Dehydroxylated Bas in intestine | Significant reduction in host weight gain, plasma cholesterol, and liver triglycerides | Activation transcription of key genes involved in lipid metabolism (PPARγ, ANGPTL 4), cholesterol metabolism (ABCG 5/8), gastrointestinal homeostasis (REG 3γ), and circadian rhythm (DBP, PER1/2) in the liver or small intestine (probably through the FXRα activation) | [183] |
| BAs in intestine | ↑ energy expenditure in brown adipose tissue and muscle | TGR5 can stimulate glucagon-like protein 1, improving glucose tolerance and activating thyroid hormone | [184,185] |
| Improve glucose homeostasis and triglyceride control aspects of metabolic syndrome in animal models | Activation of FXRα | [186] | |
| Decreased concentration of BAs in intestine (in acid-binding resins application) | Stimulate the conversion of cholesterol to bile acids | Activation of FXRα | [187] |
| BAs or their synthetic derivatives per os | ↓ serum triglycerides and total cholesterol, inhibition of the atherosclerosis in a dose-dependent manner. | Activation of FXRα | [188] |
| CA and other FXRα agonists per os | ↑ serum HDL and phospholipids but decreased ApoA-1 (controversial results) | FXRα induction leading to ↓ SREBP1c (through SHP and LXRα/LXRβ) and triglyceride synthesis and VLDL level. FXRα induction leading to SR-B1 activation ↑ total and serum HDL cholesterol suggesting that reverse cholesterol transport is disrupted. Probable role of epigenetic mechanisms. | [189,190,191] |
| Role in fatty liver disease | |||
| Glycine-conjugated BAs | Positive correlation with macrovesicular steatosis score | Inhibition of CYP8B1 and stimulation of CYP7B1 expression in NASH livers (suggests a shift to alternative pathway of BAs synthesis) | [192] |
| Oral CA and UDCA | Improvement in hepatic steatosis | Under the stidy | [193] |
| Role in cholestatic liver disease | |||
| Accumulation of hydrophobic bile acids DCA and CDCA in the liver | Cholestatic liver injury | Membrane desorganisation stimulates production of reactive oxygen species and activation of NF-κB | [194] |
| Total BAs in fetal serum in intrahepatic cholestasis of pregnancy | Association with ventricular arrhythmia in pregnant women | Abnormal ventricular repolarization | [195] |
| Oral UDCA/tauro-UDCA | Protection of cholangiocytes against cytotoxicity of hydrophobic bile acids, stimulation of hepatobiliary secretion, and protection of hepatocytes against BAs- induced apoptosis | Modulation of the composition of mixed phospholipid-rich micelles, possibly, decrease in the concentration of hydrophobic bile acids in the cholangiocytes. Stimulation of Ca(2+)- and protein kinase C-alpha-dependent mechanisms and/or activation of p38 (MAPK) and extracellular signal-regulated kinases (ERK) resulting in insertion of transporter molecules (BSEP, MRP2) into the canalicular membrane and NTCP into the basolateral membrane. Inhibition of mitochondrial membrane permeability transition, and possibly, stimulation of a survival pathway. Counteraction with the action of toxic BAs reduces endoplasmic reticulum stress. TUDC initiates differentiation of multipotent mesenchymal stem cells. α5β1 integrins probably serve as sensors for TUDC with the downstream activation of focal adhesion kinase, c-SRC, the epidermal growth factor receptor and activation of the mitogen-activated protein kinases, ERKs and p38. | [196,197] |
| Obeticholic acid (a selective potent FXR agonist, structural CDCA analog) | Anticholestatic and antifibrotic properties in primary biliary cholangitis not responding to first-line treatment; ↓ portal pressure without a ↓ in mean arterial pressure. Protective cardiopulmonary effect in both cholestatic cirrhotic rat models. Ileal barrier function improvement, reduced bacterial translocation. | FXR activation with decreased BAs synthesis. Increased intrahepatic eNOS activity. Interaction with Kupffer cells and expression of IL-1 and TNFα with concomitant repression of CYP7A1 in hepatocytes. | [198,199,200,201,202,203] |
| Role in carcinogenesis | |||
| Accumulation DCA and CDCA in the liver | Hepatocellular carcinoma development | Stimulates production of reactive oxygen species and activation of RAS and NF-κB, proinflammatory or tumorogenic factors in the liver with subsequent downregulating of FXR and SHP—an important tumor suppressor. | [204,205,206] |
| ↑ levels of DCA, LCA in stool | Pro-carcinogenic potential in the colon | Generation of cancer stem cells probably through Wnt/β-catenin signaling | [207] |
| Tauro-CA in the colon | Pro-carcinogenic potential in the colon | Genotoxic effects are under investigation. Metabolism of taurine conjugated BAs by gut microbes generates a genotoxic hydrogen sulfide | [208] |
| BAs in duodenal refluctate | Esophageal dysplasia, squamous cell carcinoma and adenocarcinoma | Expression of COX2 and p53 in esophageal proliferating cells | [209,210] |
| Bowel diseases | |||
| Altered colonic BA (shift to CA and tauro-CA) in colon after antibiotic treatment | Association with several disease states, including recurrent C. difficile infection (with cases of CDI pouchitis) | A permissive environment in which the bacterium may thrive stimulate germination of C. difficile spores. CDCA, LCA, and UDCA inhibit germination of spores | [211,212] |
| Esophageal diseases | |||
| Oral UDCA | May protect against DNA damage induced by hydrophobic bile acids such as DCA in the metaplastic mucosa of patients with Barrett’s esophagus | UDCA counters the DNA damaging effects of DCA | [213] |
| Lung disease | |||
| Repeated microaspiration of CDCA, DCA, and LCA | Fibrotic changes in alveolar wall | Stimulation of fibrogenic mediator expression and activating TGF-β1/SMAD3 signaling and FXR | [150] |
| BAs in the lung tissue in cystic fibrosis | Association with inflammation and restructuring of the lung microbiota with a dominance of Proteobacteria | Tissue damage, bactericidal effect. | [214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulpekova, Y.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Synitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; Lapina, N.; et al. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules 2022, 27, 3401. https://doi.org/10.3390/molecules27113401
Shulpekova Y, Zharkova M, Tkachenko P, Tikhonov I, Stepanov A, Synitsyna A, Izotov A, Butkova T, Shulpekova N, Lapina N, et al. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules. 2022; 27(11):3401. https://doi.org/10.3390/molecules27113401
Chicago/Turabian StyleShulpekova, Yulia, Maria Zharkova, Pyotr Tkachenko, Igor Tikhonov, Alexander Stepanov, Alexandra Synitsyna, Alexander Izotov, Tatyana Butkova, Nadezhda Shulpekova, Natalia Lapina, and et al. 2022. "The Role of Bile Acids in the Human Body and in the Development of Diseases" Molecules 27, no. 11: 3401. https://doi.org/10.3390/molecules27113401
APA StyleShulpekova, Y., Zharkova, M., Tkachenko, P., Tikhonov, I., Stepanov, A., Synitsyna, A., Izotov, A., Butkova, T., Shulpekova, N., Lapina, N., Nechaev, V., Kardasheva, S., Okhlobystin, A., & Ivashkin, V. (2022). The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules, 27(11), 3401. https://doi.org/10.3390/molecules27113401






