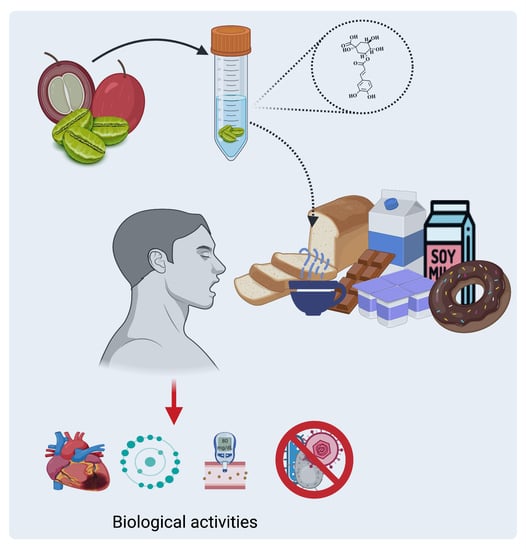
Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review
Abstract
:1. Introduction
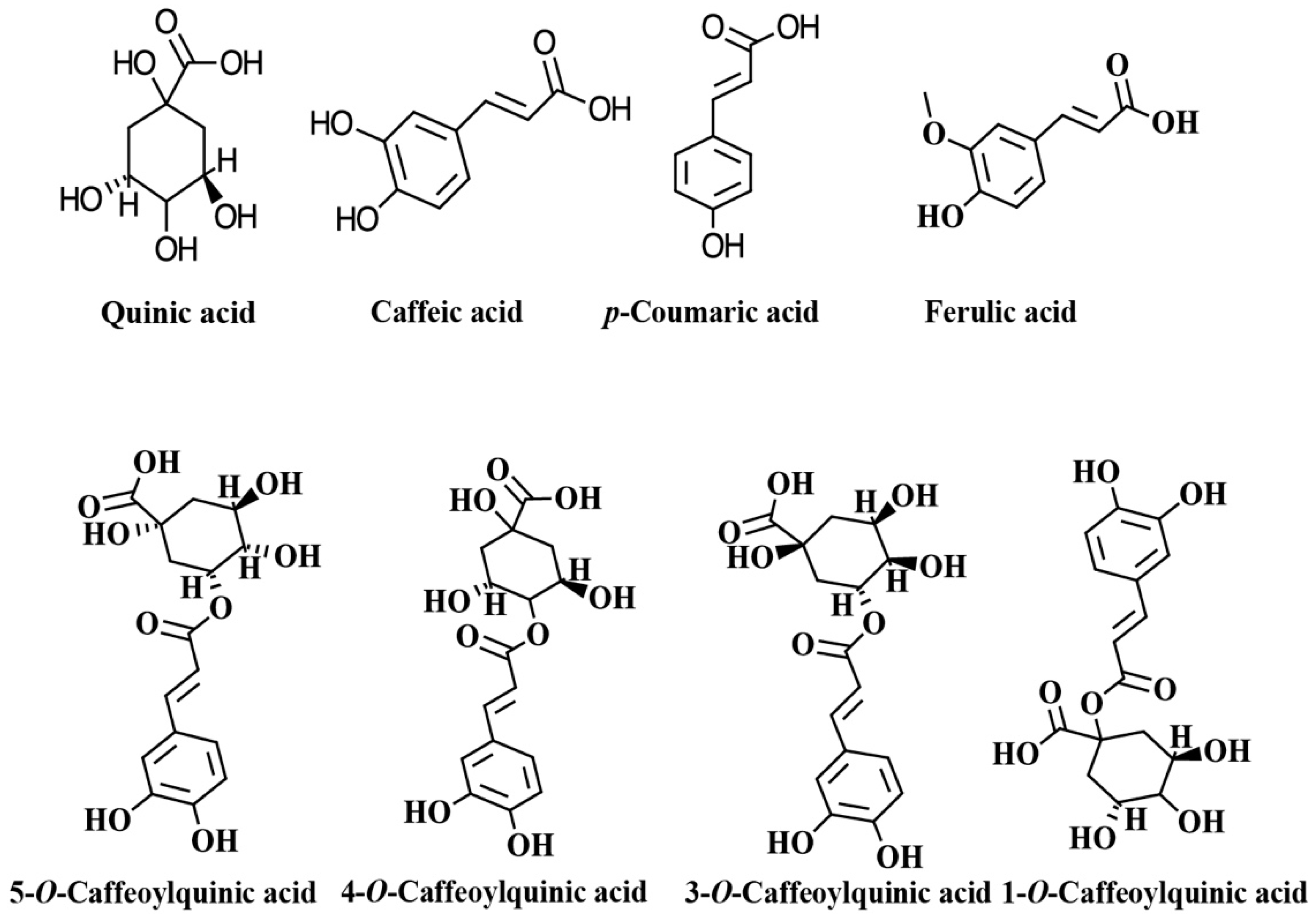
2. Dietary Sources of Chlorogenic Acids (CGAs)
Coffee as a Source of CGAs
3. Extraction of Chlorogenic Acids (CGAs) from Coffee
3.1. Organic Solvent Extraction
3.2. Pulsed Electric Field Extraction
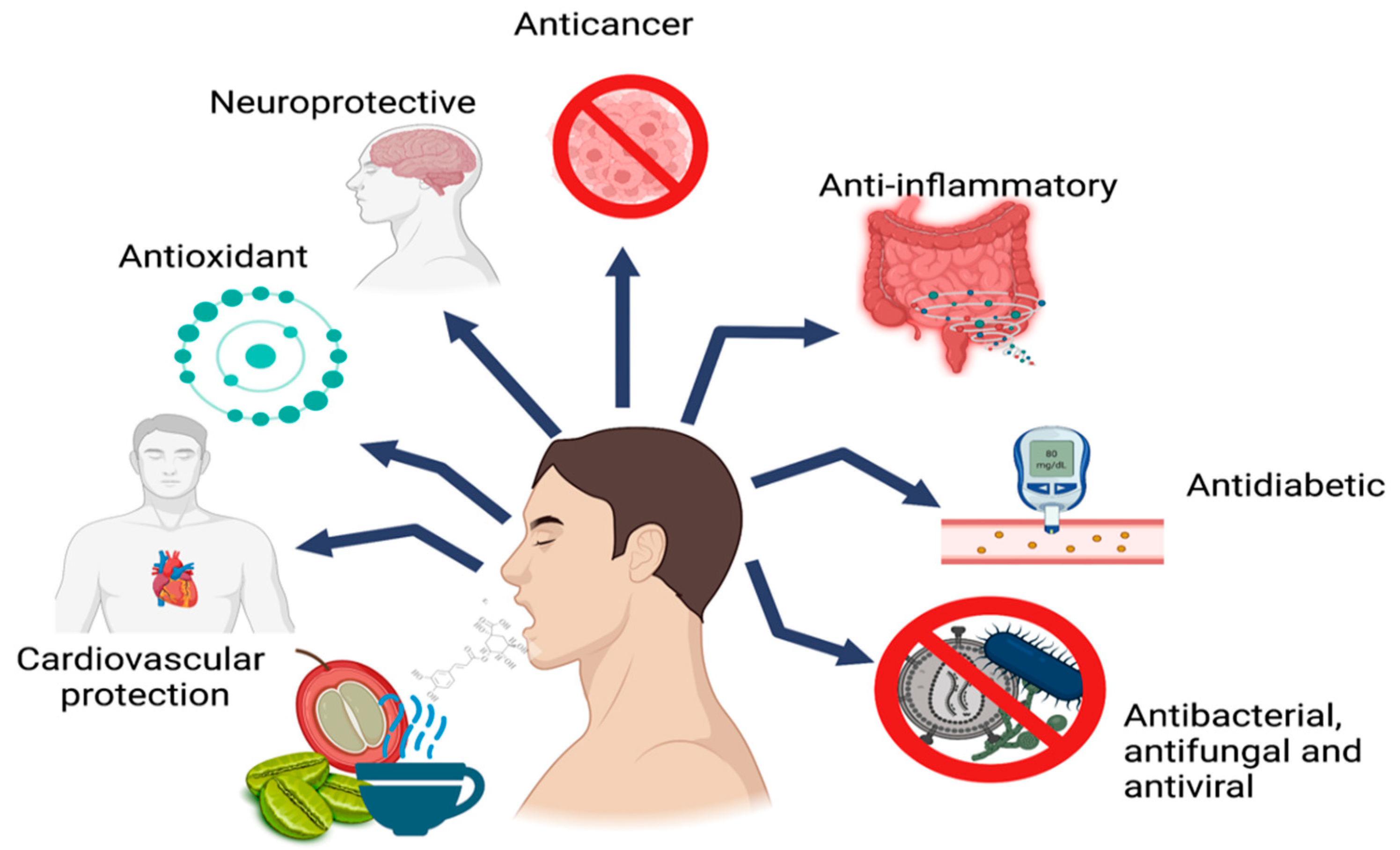
4. Biological Activities of CGAs
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Activity
4.3. Neuroprotective Activity
4.4. Anticancer Activity
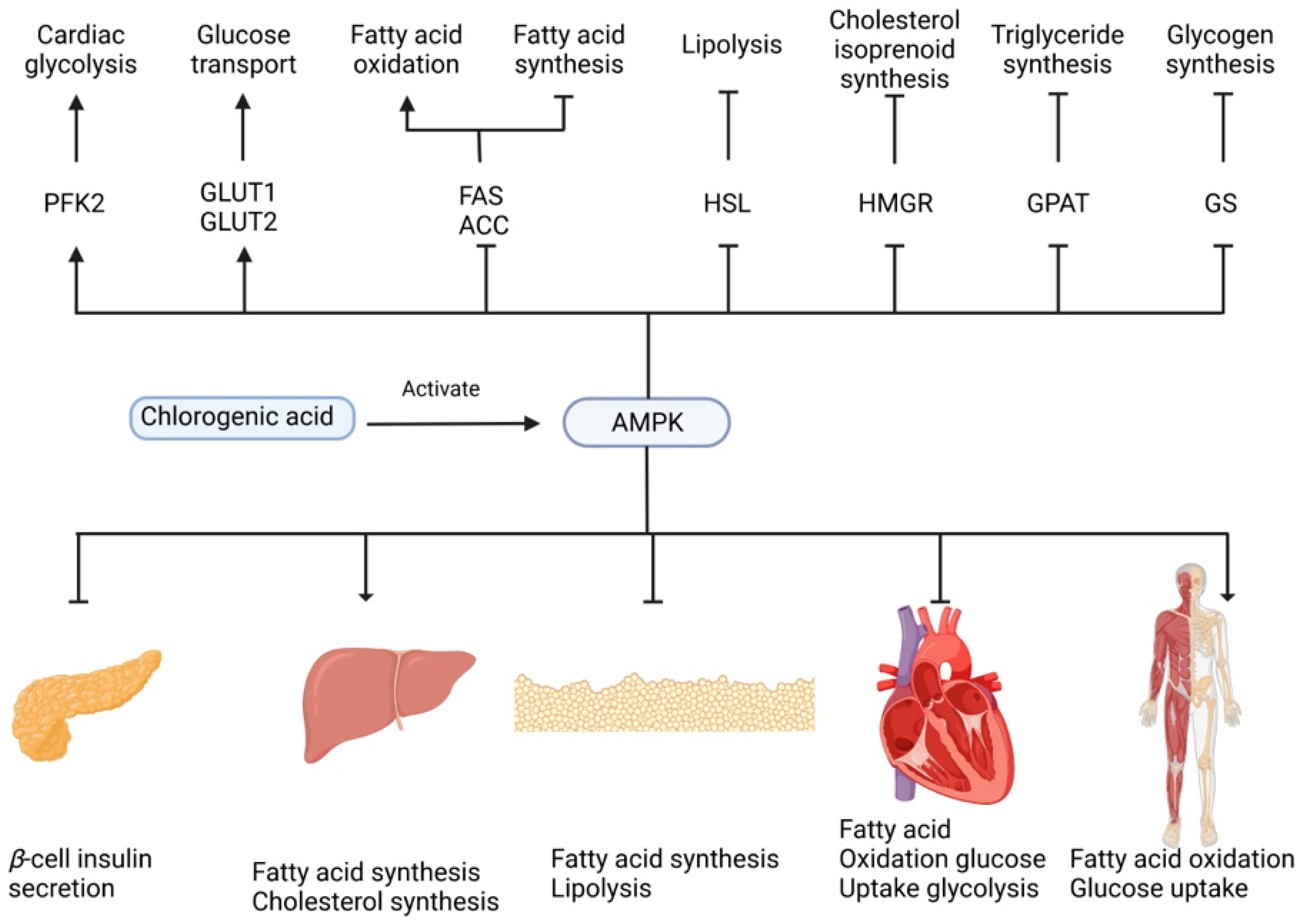
4.5. Antidiabetic Activity
4.6. Cardiovascular Protection Activity
4.7. Antibacterial, Antifungal, and Antiviral Activity
4.8. Other Bioactivities
4.8.1. Hepatoprotective Activity
4.8.2. Potential Prebiotic Activity
5. Bioavailability of CGAs
5.1. Absorption of CGAs
5.2. Metabolization of CGAs
6. Incorporation of CGAs into Food Matrices
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The impact of coffee and its selected bioactive compounds on the development and progression of colorectal cancer in vivo and in vitro. Molecules 2018, 23, 3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 Diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Wang, L.N.; Wang, W.; Hattori, M.; Daneshtalab, M.; Ma, C.M. Synthesis, Anti-HCV, antioxidant and reduction of intracellular reactive oxygen species generation of a chlorogenic acid analogue with an amide bond replacing the ester bond. Molecules 2016, 21, 737. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Li, J.; Gao, Z.; Wang, D.; Wang, H.; Wu, J. Chlorogenic acid inhibits lipid deposition by regulating the enterohepatic FXR-FGF15 pathway. BioMed Res. Int. 2022, 2022, 4919153. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Smith, P.; Kiong Ho, C.; Takagi, Y.; Djaballah, H.; Shuman, S. Nanomolar inhibitors of Trypanosoma brucei RNA triphosphatase. MBio 2016, 7, e00058-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hee, S.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-α-induced secretion of Interleukin-8 from Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; An, L.; Gao, L.Y.; Bai, J.P.; Wang, J.; Meng, W.H.; Ren, T.S.; Zhao, Q.C. Compound MQA, a caffeoylquinic acid derivative, protects against NMDA-induced neurotoxicity and potential mechanisms in vitro. CNS Neurosci. Ther. 2015, 21, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2006, 55, 185–190. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Bhattacharya, P.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Chatterjee, T.K. Hypoglycemic and antihyperglycemic activity of leaf extract of Pluchea indica Less. Orient. Pharm. Exp. Med. 2006, 6, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Moore, P.S.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.J.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antivir. Chem. Chemother. 2016, 4, 235–240. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of Caffeoyl quinic acid derivatives from the roots of Dipsacus asper wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Teixeira Filho, J.; Wagner, R.; Teixeira Godoy, H. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019, 82, 103244. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of chlorogenic acids from agri-food wastes: Updates on green extraction techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Cicco, N.; Linsalata, V. Antioxidant activities of artichoke phenolics. Acta Hortic. 2005, 681, 421–428. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in Coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Wald, B.; Wray, V.; Galensa, R.; Herrmann, K. Malonated flavonol glycosides and 3,5-dicaffeoylquinic acid from pears. Phytochemistry 1989, 28, 663–664. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P.E. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 6, 5772–5778. [Google Scholar] [CrossRef]
- Somers, T.C.; Vérette, E.; Pocock, K.F. Hydroxycinnamate esters of Vitis vinifera: Changes during white vinification, and effects of exogenous enzymic hydrolysis. J. Sci. Food Agric. 1987, 40, 67–78. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Strack, D.; Hartfeld, F.; Austenfeld, F.A.; Grotjahn, L.; Wray, V. Coumaroyl-, caffeoyl- and feruloyltartronates and their accumulation in Mung bean. Phytochemistry 1985, 24, 147–150. [Google Scholar] [CrossRef]
- Donovan, J.L.; Meyer, A.S.; Waterhouse, A.L. Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica). J. Agric. Food Chem. 1998, 46, 1247–1252. [Google Scholar] [CrossRef]
- Malmberg, A.G.; Theander, O. Determination of chlorogenic acid in potato tubers. J. Agric. Food Chem. 1985, 33, 549–551. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Coffee and its by-products as sources of bioactive compounds. In Coffee: Production, Consumption and Health Benefits, 1st ed.; Massey, J.L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–28. [Google Scholar]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; de Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Silva, C.T.; de Souza, M.C.; Machado, A.P.D.F.; do Nascimento, R.D.P.; da Cunha, D.T.; Bezerra, R.M.N.; Rostagno, M.A. Thermal stability and sensory evaluation of a bioactive extract from roasted coffee (Coffea arabica) beans added at increasing concentrations to conventional bread. J. Food Process. Preserv. 2021, 45, e15955. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.; Alonso Campos, A.; Valerio Alfaro, G.; González-Ríos, O.; Villeneuve, P.; Figueroa-Espinoza, M. Isolation of green coffee chlorogenic acids using activated carbon. J. Food Compos. Anal. 2014, 33, 55–58. [Google Scholar] [CrossRef]
- Upadhyay, R.; Ramalakshmi, K.; Jagan Mohan Rao, L. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, Z.; Li, F.; Li, X. An effective method for the extraction and purification of chlorogenic acid from ramie (Boehmeria nivea L.) leaves using acidic ionic liquids. Ind. Crops Prod. 2016, 89, 78–86. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Podsȩdek, A.; Zyzelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009, 228, 913–922. [Google Scholar] [CrossRef]
- Phongsupa, J.; Yawootti, A.; Wattanutchariya, W. Chlorogenic acid extraction of local coffee beans by pulsed electric field. AIP Conf. Proc. 2021, 2397, 020004. [Google Scholar] [CrossRef]
- Bilge, G.; Yurdakul, M.; Buzrul, S.; Bulut, O. Evaluation of the effect of pulsed electric field on coffee arabica beans. Food Bioproc. Technol. 2022, 15, 1073–1081. [Google Scholar] [CrossRef]
- Madhava Naidu, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar] [CrossRef]
- Rakotomalala, J.J. Diversité Biochimique des Caféiers: Analyse des Acides Hydroxycinnamiques, Bases Puriques et Diterpènes Glycosidiques. Particularités des Caféiers Sauvages de la Région Malgache (Mascarocoffea chev.), 1993. Ph.D. Thesis, Universite Montpellier II, Montpellier, France, 1992. [Google Scholar]
- Dibert, K.; Cros, E.; Andrieu, J. Solvent extraction of oil and chlorogenic acid from green coffee. Part II: Kinetic data. J. Food Eng. 1989, 10, 199–214. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef] [Green Version]
- Amato, A.; Terzo, S.; Mulè, F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 2019, 12, 608. [Google Scholar] [CrossRef] [Green Version]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of chlorogenic acid against Diabetes Mellitus and its complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zheng, S.; Sheng, Y.; Miao, T.; Xu, J.; Xu, W.; Huang, K.; Zhao, C. Chlorogenic acid ameliorates obesity by preventing energy balance shift in high-fat diet induced obese mice. J. Sci. Food Agric. 2021, 101, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ghadieh, H.E.; Smiley, Z.N.; Kopfman, M.W.; Najjar, M.G.; Hake, M.J.; Najjar, S.M. Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice. Nutr. Metab. 2015, 12, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 1717. [Google Scholar] [CrossRef]
- Zheng, G.; Qiu, Y.; Zhang, Q.F.; Li, D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br. J. Nutr. 2014, 112, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Ding, Y.; Li, L.; Ge, M.; Ban, G.; Yang, H.; Dai, J.; Zhang, L. Effects and mechanism of chlorogenic acid on weight loss. Curr. Pharm. Biotechnol. 2020, 21, 1099–1106. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Sales, A.L.; Depaula, J.; Mellinger Silva, C.; Cruz, A.; Lemos Miguel, M.A.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, H.; Burger, H.J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age- related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, N.; Kayano, S.I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Laranjinha, J.A.N.; Almeida, L.M.; Madeira, V.M.C. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494. [Google Scholar] [CrossRef]
- Gordon, M.H.; Wishart, K. Effects of chlorogenic acid and bovine serum albumin on the oxidative stability of low density lipoproteins in vitro. J. Agric. Food Chem. 2010, 58, 5828–5833. [Google Scholar] [CrossRef]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on x-ray induced dna damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Du, W.Y.; Xiao, Y.; Yao, J.J.; Hao, Z.; Zhao, Y.B. Involvement of NADPH oxidase in high-dose phenolic acid-induced pro-oxidant activity on rat mesenteric venules. Exp. Ther. Med. 2017, 13, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-O-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [Green Version]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) complex of plant phenolic chlorogenic acid: Antioxidant, antimicrobial and structural studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubĩa, P.; Lahera, V.; Lũo, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 Cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 Mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced Cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-ΚB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.; Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxidative Med. Cell. Longev. 2016, 2016, 1923754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardener, S.L.; Rainey-Smith, S.R.; Villemagne, V.L.; Fripp, J.; Doré, V.; Bourgeat, P.; Taddei, K.; Fowler, C.; Masters, C.L.; Maruff, P.; et al. Higher coffee consumption is associated with slower cognitive decline and less cerebral Aβ-Amyloid accumulation over 126 months: Data from the Australian imaging, biomarkers, and lifestyle study. Front. Aging Neurosci. 2021, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s Disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Azheimer Association. 2022 Alzheimer’s Disease Facts and Figures; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Basurto-Islas, G.; Blanchard, J.; Tung, Y.C.; Fernandez, J.R.; Voronkov, M.; Stock, M.; Zhang, S.; Stock, J.B.; Iqbal, K. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2701–2712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-Amyloid production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; He, Y. Coffee intake and the incident risk of cognitive disorders: A dose–response meta-analysis of nine prospective cohort studies. Clin. Nutr. 2017, 36, 730–736. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and Adenosine A2a receptor antagonists prevent β-Amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef]
- Rosso, A.; Mossey, J.; Lippa, C.F. Caffeine: Neuroprotective functions in cognition and Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2008, 23, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Song, X.; Li, F.; Tan, X.; Sun-Waterhouse, D.; Li, D. Caffeic acid phenethyl ester reversed cadmium-induced cell death in hippocampus and cortex and subsequent cognitive disorders in mice: Involvements of AMPK/SIRT1 pathway and amyloid-tau-neuroinflammation axis. Food Chem. Toxicol. 2020, 144, 111636. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Jang, Y.J.; Hwang, M.K.; Kang, N.J.; Lee, K.W.; Lee, H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2009, 661, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Sarroca, S.; Gatius, A.; Rodríguez-Farré, E.; Vilchez, D.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2014, 51, 466–479. [Google Scholar] [CrossRef]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Panta, R.; Miyares, M. Influence of coffee and its components on breast cancer: A review. Asian Pac. J. Trop. Dis. 2016, 6, 827–831. [Google Scholar] [CrossRef]
- Li, Y.M.; Peng, J.; Li, L.Z. Coffee consumption associated with reduced risk of oral cancer: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 381–389.e1. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A. Coffee consumption and the risk of cancer: An overview. Cancer Lett. 2009, 277, 121–125. [Google Scholar] [CrossRef]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator Protein-1, NF-ΚB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef] [Green Version]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef]
- Boettler, U.; Volz, N.; Pahlke, G.; Teller, N.; Kotyczka, C.; Somoza, V.; Stiebitz, H.; Bytof, G.; Lantz, I.; Lang, R.; et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol. Nutr. Food Res. 2011, 55, 798–802. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas 2021- 10th Edition|IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Salazar-Martinez, E.; Willett, W.C.; Ascherio, A.; Manson, J.A.E.; Leitzmann, M.F.; Stampfer, M.J.; Hu, F.B. Coffee consumption and risk for type 2 Diabetes Mellitus. Ann. Intern. Med. 2004, 140, 1–8. [Google Scholar] [CrossRef]
- Agardh, E.E.; Carlsson, S.; Ahlbom, A.; Efendic, S.; Grill, V.; Hammar, N.; Hilding, A.; Östenson, C.G. Coffee consumption, type 2 Diabetes and impaired glucose tolerance in Swedish men and women. J. Intern. Med. 2004, 255, 645–652. [Google Scholar] [CrossRef]
- Lin, W.Y.; Xaiver Pi-Sunyer, F.; Chen, C.C.; Davidson, L.E.; Liu, C.S.; Li, T.C.; Wu, M.F.; Li, C.I.; Chen, W.; Lin, C.C. Coffee consumption is inversely associated with type 2 Diabetes in Chinese. Eur. J. Clin. Investig. 2011, 41, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee consumption and risk of type 2 Diabetes Mellitus: An 11-year prospective study of 28 812 postmenopausal women. Arch. Intern. Med. 2006, 166, 1311–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomilehto, J.; Hu, G.; Bidel, S.; Lindström, J.; Jousilahti, P. Coffee consumption and risk of type 2 Diabetes Mellitus among middle-aged Finnish men and women. J. Am. Med. Assoc. 2004, 291, 1213–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dam, R.M. Coffee consumption and risk of type 2 Diabetes, cardiovascular diseasses, and cancer. Appl. Physiol. Nutr. Metab. 2008, 33, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Feskens, E.J.M. Coffee consumption and risk of type 2 Diabetes Mellitus. Lancet 2002, 360, 1477–1478. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid.-Based Complement. Altern. 2013, 11, 801457. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Man Ying Lee, C.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 Diabetes Mellitus a systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Sellars, E.A.; Farah, A.; Graham, T.E.; Wasserman, D.H. Effects of chronic coffee consumption on glucose kinetics in the conscious rat. Can. J. Physiol. Pharmacol. 2007, 85, 823–830. [Google Scholar] [CrossRef]
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5’AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 1999, 48, 1667–1671. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabol. 2005, 1, 15–25. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 April 2022).
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the Metabolic Syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic acid protects against OxLDL-induced oxidative damage and mitochondrial dysfunction by modulating SIRT1 in endothelial cells. Mol. Nutr. Food Res. 2018, 62, 1700928. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Hida, M.; Matsumoto, T.; Ikeuchi-Takahashi, Y.; Onishi, H.; Kobayashi, T. Effect of short-term polyphenol treatment on endothelial dysfunction and thromboxane A2 levels in streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 2014, 37, 1056–1061. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Kanegae, M.P.P.; da Fonseca, L.M.; Brunetti, I.L.; de Oliveira Silva, S.; Ximenes, V.F. The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: Contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. Biochem. Pharmacol. 2007, 74, 457–464. [Google Scholar] [CrossRef]
- Antonio, A.G.; Moraes, R.S.; Perrone, D.; Maia, L.C.; Santos, K.R.N.; Iório, N.L.P.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem. 2010, 118, 782–788. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Naghetini, C.C.; Santos, V.R.; Antonio, A.G.; Farah, A.; Glória, M.B.A. Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutans. Food Res. Int. 2012, 49, 459–461. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.I.; King, P.J.; Robinson, W.E. Anti-Human immunodeficiency virus activity of 3,4,5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol. Nutr. Food Res. 2006, 50, 396–400. [Google Scholar] [CrossRef]
- Honjo, S.; Kono, S.; Coleman, M.P.; Shinchi, K.; Sakurai, Y.; Todoroki, I.; Umeda, T.; Wakabayashi, K.; Imanishi, K.; Nishikawa, H.; et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J. Clin. Epidemiol. 2001, 54, 823–829. [Google Scholar] [CrossRef]
- Wadhawan, M.; Anand, A.C. Coffee and liver disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef] [Green Version]
- La Vecchia, C. Coffee, liver enzymes, cirrhosis and liver cancer. J. Hepatol. 2005, 42, 444–446. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421.e1. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology 2007, 132, 1740–1745. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Machado, S.R.; Parise, E.R.; de Carvalho, L. Coffee has hepatoprotective benefits in Brazilian patients with chronic Hepatitis C even in lower daily consumption than in American and European populations. Braz. J. Infect. Dis. 2014, 18, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Oyarzábal, I.S.; Gill, C.; Rowland, I. An exploratory study into the putative prebiotic activity of fructans isolated from Agave angustifolia and the associated anticancer activity. Anaerobe 2013, 22, 38–44. [Google Scholar] [CrossRef]
- Saha, D.C.; Reimer, R.A. long-term intake of a high prebiotic fiber diet but not high protein reduces metabolic risk after a high fat challenge and uniquely alters gut microbiota and hepatic gene expression. Nutr. Res. 2014, 34, 789–796. [Google Scholar] [CrossRef]
- Kumar, V.P.; Prashanth, K.V.H.; Venkatesh, Y.P. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa). Carbohydr. Polym. 2015, 117, 115–122. [Google Scholar] [CrossRef]
- Kelly-Quagliana, K.A.; Nelson, P.D.; Buddington, R.K. Dietary oligofructose and inulin modulate immune functions in mice. Nutr. Res. 2003, 23, 257–267. [Google Scholar] [CrossRef]
- Munjal, U.; Glei, M.; Pool-Zobel, B.L.; Scharlau, D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br. J. Nutr. 2009, 102, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 Diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef] [PubMed]
- Cluny, N.L.; Eller, L.K.; Keenan, C.M.; Reimer, R.A.; Sharkey, K.A. Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity 2015, 23, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Hicks, P.M.D.; Heaney, R.P.; Abrams, S.A. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr. Res. 2003, 23, 901–909. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Savige, G.S.; Reid, C.M. Effect of dietary prebiotic supplementation on advanced glycation, insulin resistance and inflammatory biomarkers in adults with pre-Diabetes: A study protocol for a double-blind placebo-controlled randomised crossover clinical trial. BMC Endocr. Disord. 2014, 14, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rechner, A.R.; Spencer, J.P.E.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Chlorogenic acids: Daily consumption through coffee, metabolism and potential health effects. In Coffee: Consumption and Health Implications, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 364–415. [Google Scholar] [CrossRef]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef] [Green Version]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Duarte, G. Bioavailability and metabolism of chlorogenic acids from coffee. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: Oxford, UK, 2015; pp. 789–801. [Google Scholar]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Williamson, G.; Renouf, M.; Marmet, C.; Steiling, H.; Dionisi, F.; Barron, D.; Melcher, R.; Richling, E. Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol. Nutr. Food Res. 2012, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (Acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development. Ital. J. Food Sci. 2016, 28, 338–352. [Google Scholar]
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef]
- Bagchi, D.; Moriyama, H.; Swaroop, A. Green Coffee Bean Extract in Human Health, 1st ed.; CRC-Press: Boca Raton, CA, USA, 2016; pp. 1–254. [Google Scholar] [CrossRef]
- Budryn, G.; Zyzelewicz, D.; Nebesny, E.; Oracz, J.; Krysiak, W. Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res. Int. 2013, 50, 149–160. [Google Scholar] [CrossRef]
- Corso, M.P.; Vignoli, J.A.; Benassi, M.D.T. Development of an instant coffee enriched with chlorogenic acids. J. Food Sci. Technol. 2016, 53, 1380–1388. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef]
- Mukkundur Vasudevaiah, A.; Chaturvedi, A.; Kulathooran, R.; Dasappa, I. Effect of green coffee extract on rheological, physico-sensory and antioxidant properties of bread. J. Food Sci. Technol. 2017, 54, 1827–1836. [Google Scholar] [CrossRef]
- Rahpeyma, E.; Sekhavatizadeh, S.S. Effects of encapsulated green coffee extract and canola oil on liquid Kashk quality. Foods Raw Mater. 2020, 8, 40–51. [Google Scholar] [CrossRef]
- Zohreh, D. Properties of dark chocolate enriched with free and encapsulated chlorogenic acids extracted from green coffee. Braz. J. Food Technol. 2020, 23, e2019118. [Google Scholar] [CrossRef]
- Pimpley, V.A.; Maity, S.; Murthy, P.S. Green coffee polyphenols in formulations of functional yoghurt and their quality attributes. Int. J. Dairy Technol. 2022, 75, 159–170. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT—Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ. Sci. 2018, 30, 278–282. [Google Scholar] [CrossRef]
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Rózyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement—Preliminary study. LWT—Food Sci. Technol. 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Source | Concentration (g/100 g) 1 (dm) | CGA Composition | References |
|---|---|---|---|
| Artichoke | 1–8 | 5-CQA, 1,5-DCQA 3,4-DCQA and DCQA | [24] |
| Artichoke leaves | 0.92 | CA, 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Sweet potato leaves | - | 3-CQA, 3,4-DCQA, 3,5-DCQA, 4,5-DCQA and 3,4,5-TCQA | [15] |
| White tea (Camelia sinensis) leaves | 1.64 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Green tea (Camelia sinensis) leaves | 1.32 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Yerba mate (Ilex paraguariensis) leaves and thalli | 9.19 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Green coffee beans | 4.10–11.3 2 | CQA, FQA and DCQA | [25] |
| Apples | 0.38 0–0.2 g/L (juice) | 3-CQA, 5-CQA, 4,5-DCQA | [26] |
| Pears | 0.28 0–0.24 g/L (juice) | 3-CQA, 5-CQA, 3,6-DCQA | [27] |
| Blueberries | 2 | 5-CQA, 3-FQA | [28] |
| Grapes | 0.15 | 5-CQA, CoQA | [29] |
| Spinach | 0.2 | p-CoQA | [30] |
| Beans and peas | 0.12 | p-CoQA | [31] |
| Stone fruits | 0.01–0.6 | p-CoQA, 5-CQA, FQA, 4,5-DCQA, 3,4-TCQA | [32] |
| Potato tubers | 0.5–1.2 | CQA; DCQA | [33] |
| Food Product | Technological Improvement | Extract Conditions | CGAs Content in Green Coffee Extract | Major Findings | Sensory Evaluation | References |
|---|---|---|---|---|---|---|
| Fried doughnuts | Dough stability | Heated at 110 °C for 15 min and Freeze-dried | 25.5 g/100 g | Dough stability was not affected during mixing and GCA showed high stability increasing antioxidant activity | No significant difference up to 1% of GCE addition (Score 5–4.9) | [175] |
| Instant coffee | Fortification | Heated at high pressure at 180 °C for percolation and extraction | 14.0 g/100 g | Enriched coffee with green coffee extract showed high antioxidant potential but decreased sensory score | No significant difference in C. arabica samples (Score 7.3–6.8) | [176] |
| Soymilk | Fortification | Heated aqueous extraction (1:10 w/v) at 100 °C for 1 h | N.A. | Phenolic compounds and antioxidant activity content increased significantly, and overall digestibility improved | No decrease in the acceptance level up to 0.25 mg/mL of CGA (Overall score 4.3–5.2) | [177] |
| Wheat bread | Dough stability and fortification | Heated aqueous extraction at 60, 70 and 80 °C for 1 h | 37.3 g/100 g | GCE addition increased CGAs and antioxidant activity in bread, baking quality was not affected. | Maximum level of GCE without adverse effect was 1.5% flour basis (Overall score 64–60) | [178] |
| Liquid Khask | Enrichment | Heated aqueous extraction (1:10 w/v) at 100 °C for 30 min and encapsulated with water and oil emulsion | 39.1 g/100 g | Encapsulated GCE protected color. pH remained unaffected and rheological properties were not affected and antioxidant activity highly increased | No significant difference up to 1% of encapsulated GCE addition(Score 4.7–4.9) | [179] |
| Dark chocolate | Enrichment | Heated aqueous extraction (1:5 w/v) at 80 °C for 30 and encapsulated | N.A. | Addition of CGAs (free and encapsulated) had no significant effect on dark chocolate color. However, the addition of free or encapsulated CGAs had a significant effect on chocolate flavor. This adverse effect of CGAs on chocolate flavor were lower in the case of encapsulated form addition | No significant difference in the bitterness up to 50.1 mg/5 kg of encapsulated CGAs (Score 1.5–2) | [180] |
| Yoghurt | Enrichment | Heated aqueous extraction (1:6 w/v) at 70 °C for 1 h. The extract was filtered and concentrated by evaporation (70 °C, 30 min) and spray drying | 46.5 g/100 g | Green coffee-enriched yoghurt have desirable pH (4.7), acidity, color, and minimum syneresis. The flavor, texture and other sensory attributes of yoghurt were improved. | Higher score in overall acceptance up to 2% w/v of GCE | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. https://doi.org/10.3390/molecules27113400
Rojas-González A, Figueroa-Hernández CY, González-Rios O, Suárez-Quiroz ML, González-Amaro RM, Hernández-Estrada ZJ, Rayas-Duarte P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules. 2022; 27(11):3400. https://doi.org/10.3390/molecules27113400
Chicago/Turabian StyleRojas-González, Alexis, Claudia Yuritzi Figueroa-Hernández, Oscar González-Rios, Mirna Leonor Suárez-Quiroz, Rosa María González-Amaro, Zorba Josué Hernández-Estrada, and Patricia Rayas-Duarte. 2022. "Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review" Molecules 27, no. 11: 3400. https://doi.org/10.3390/molecules27113400
APA StyleRojas-González, A., Figueroa-Hernández, C. Y., González-Rios, O., Suárez-Quiroz, M. L., González-Amaro, R. M., Hernández-Estrada, Z. J., & Rayas-Duarte, P. (2022). Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules, 27(11), 3400. https://doi.org/10.3390/molecules27113400