The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts
Abstract
1. Introduction
2. Results
2.1. Total Flavonoids and Polysaccharides
2.2. HPLC Analysis
2.3. HPLC-MS Analysis
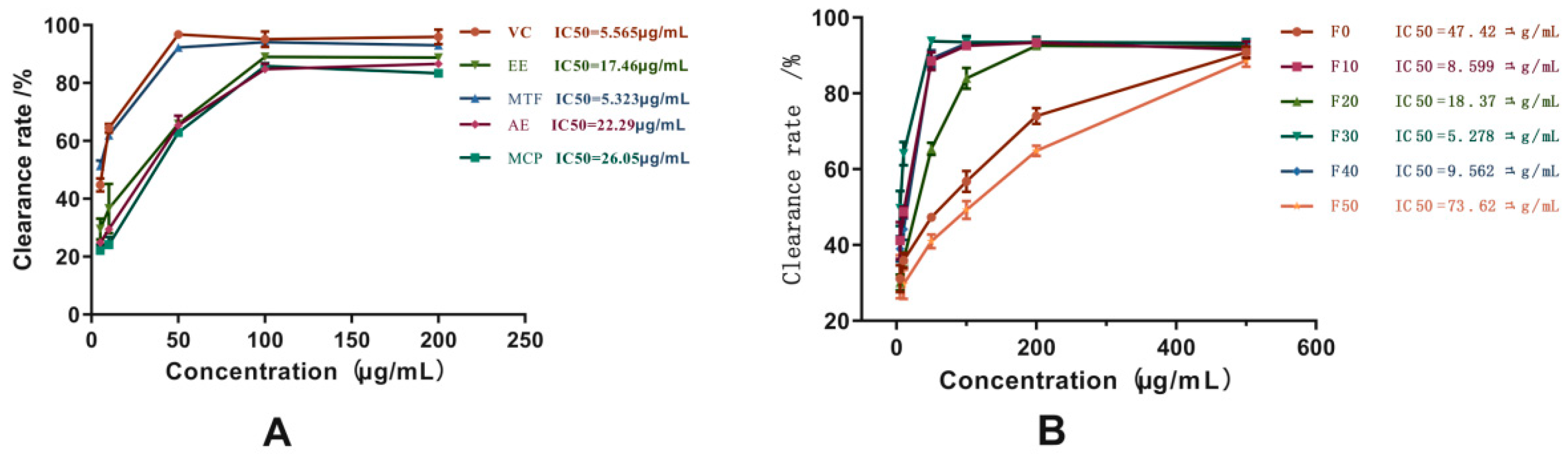
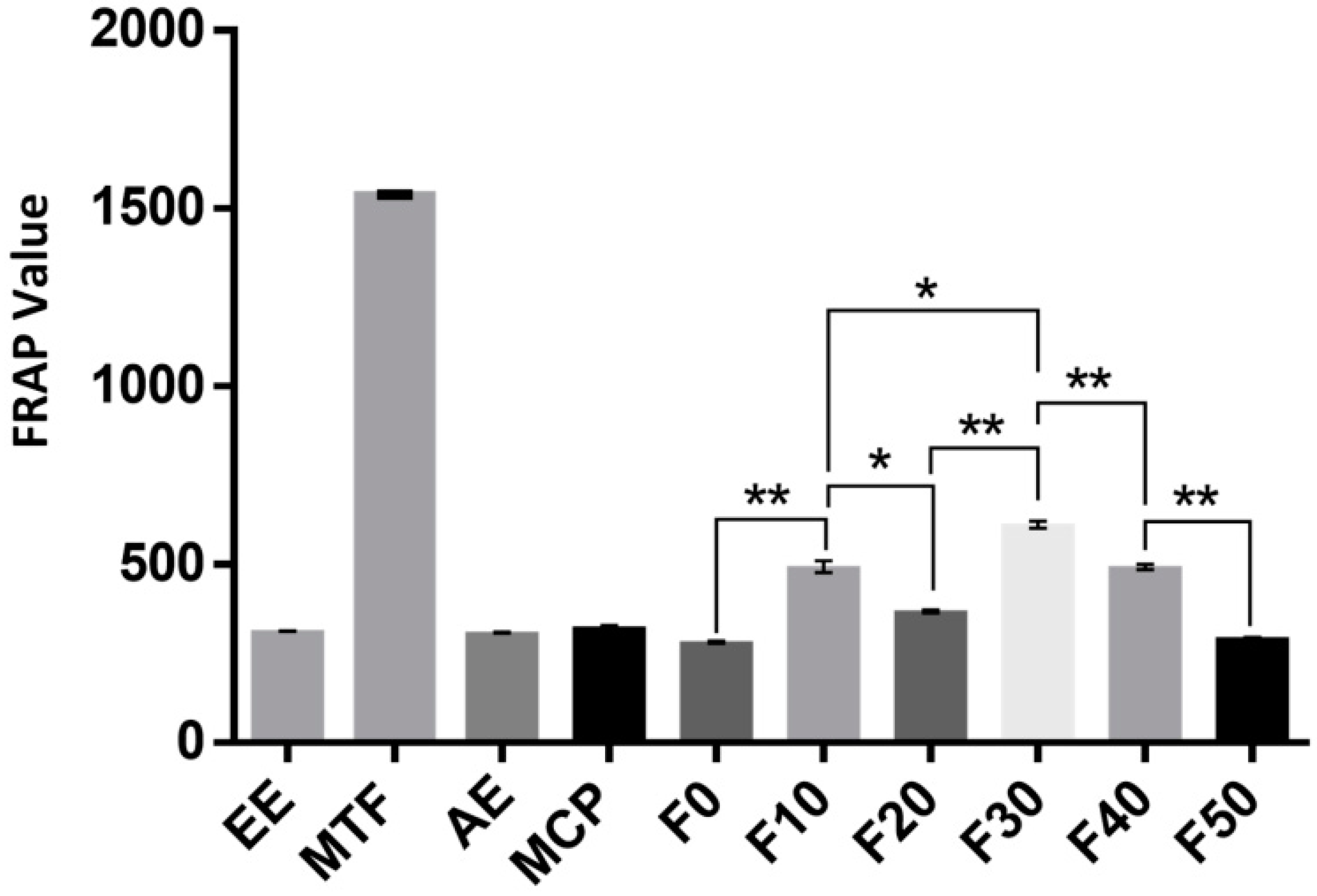
2.4. Antioxidant Activities
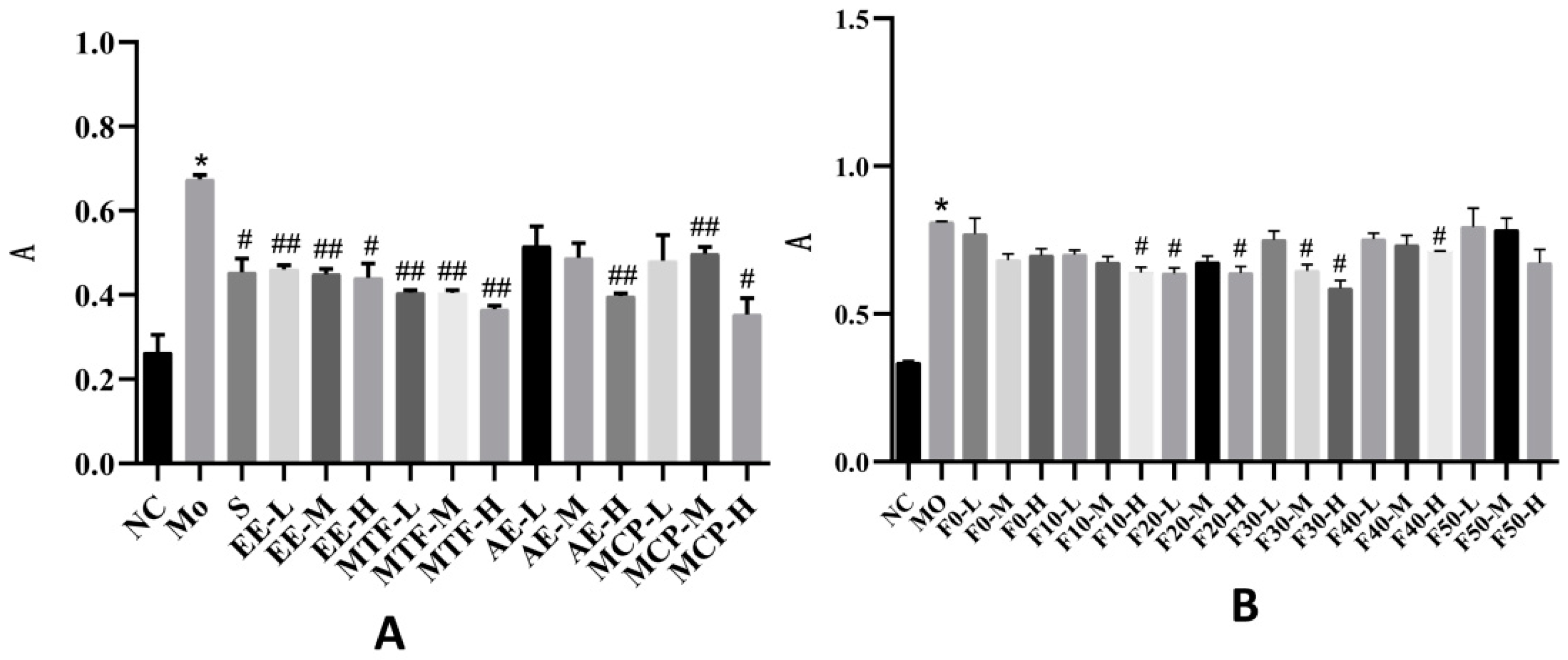
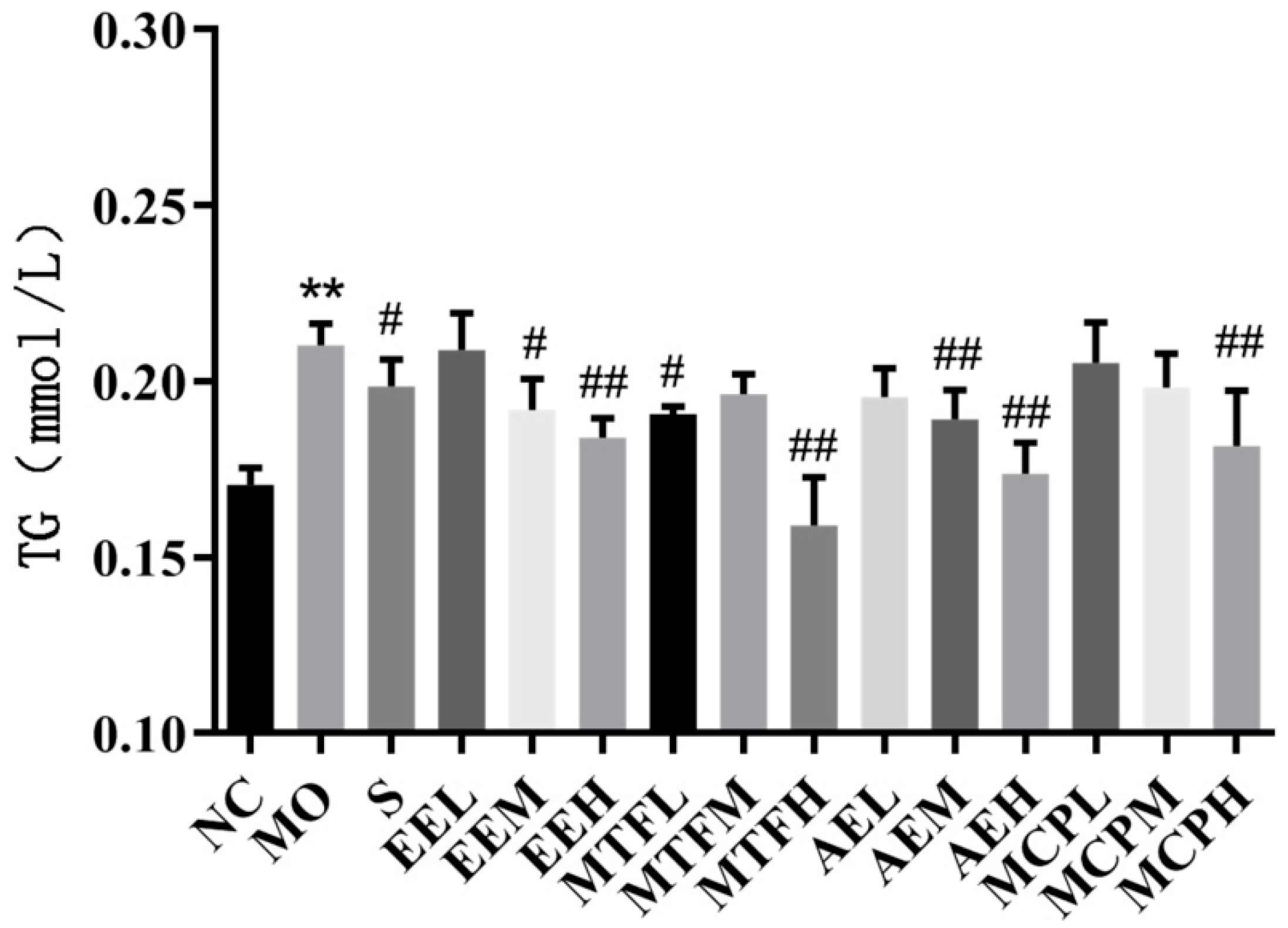
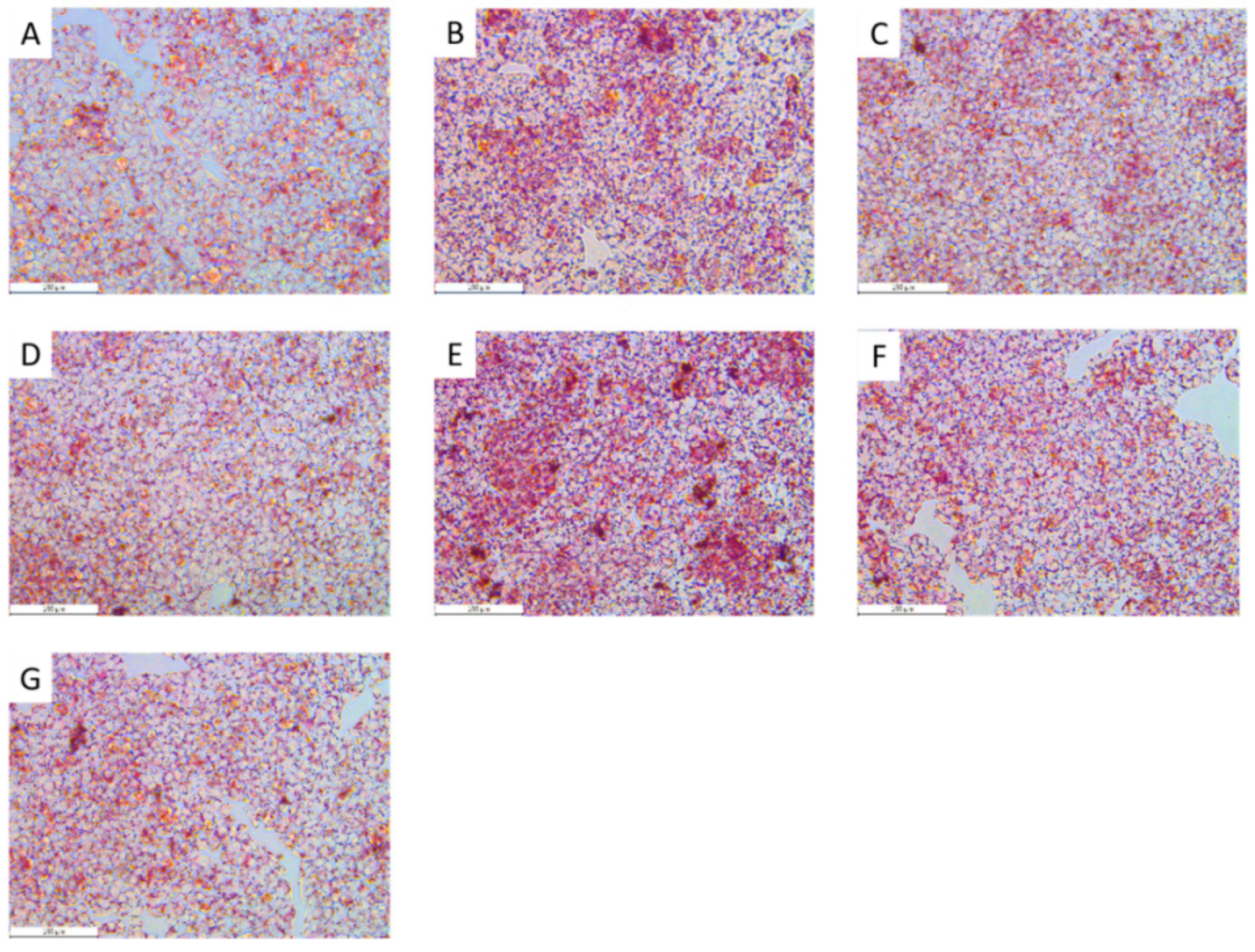
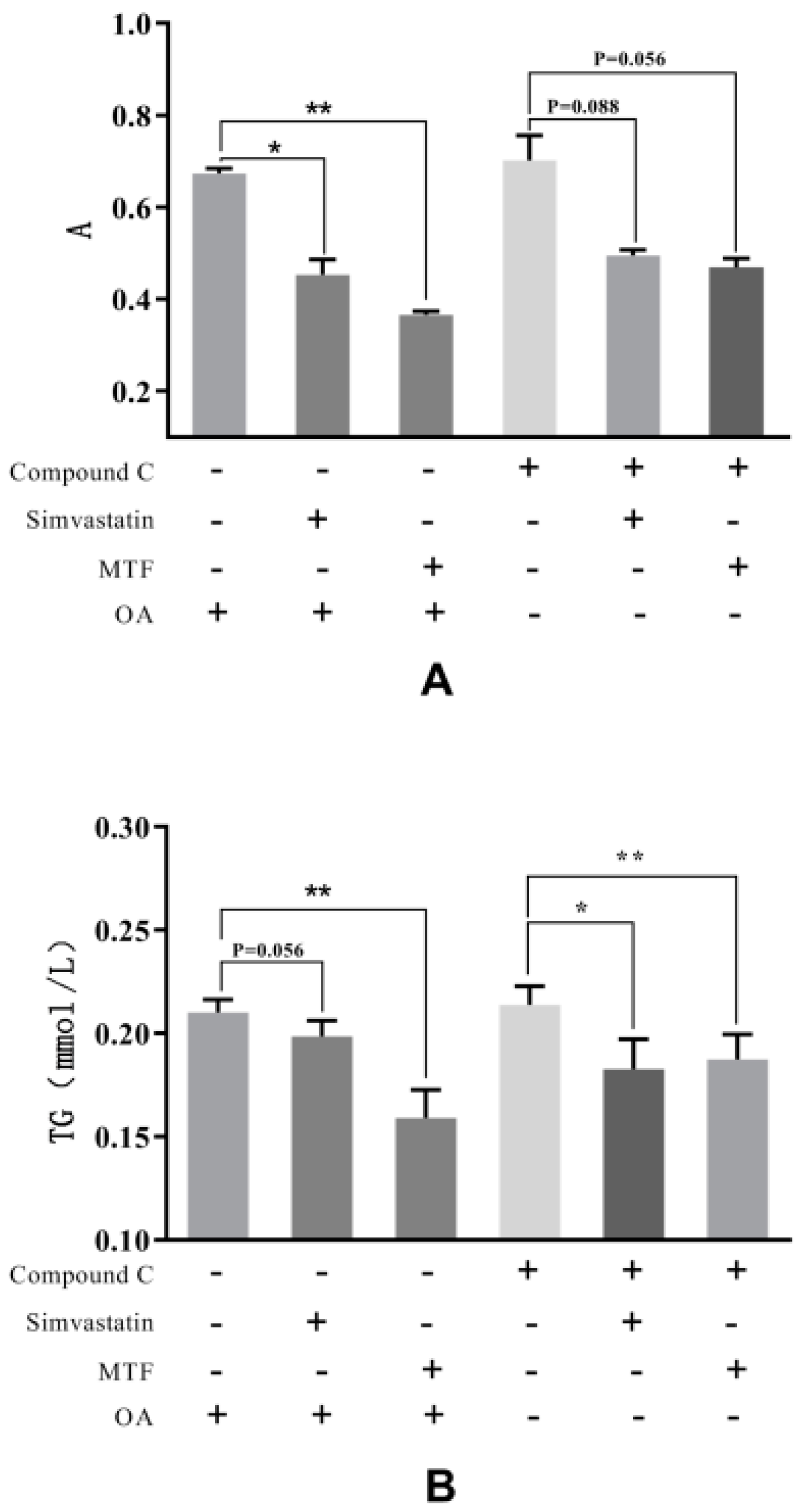
2.5. The Oleic Acid (OA)-Induced HepG2 Model
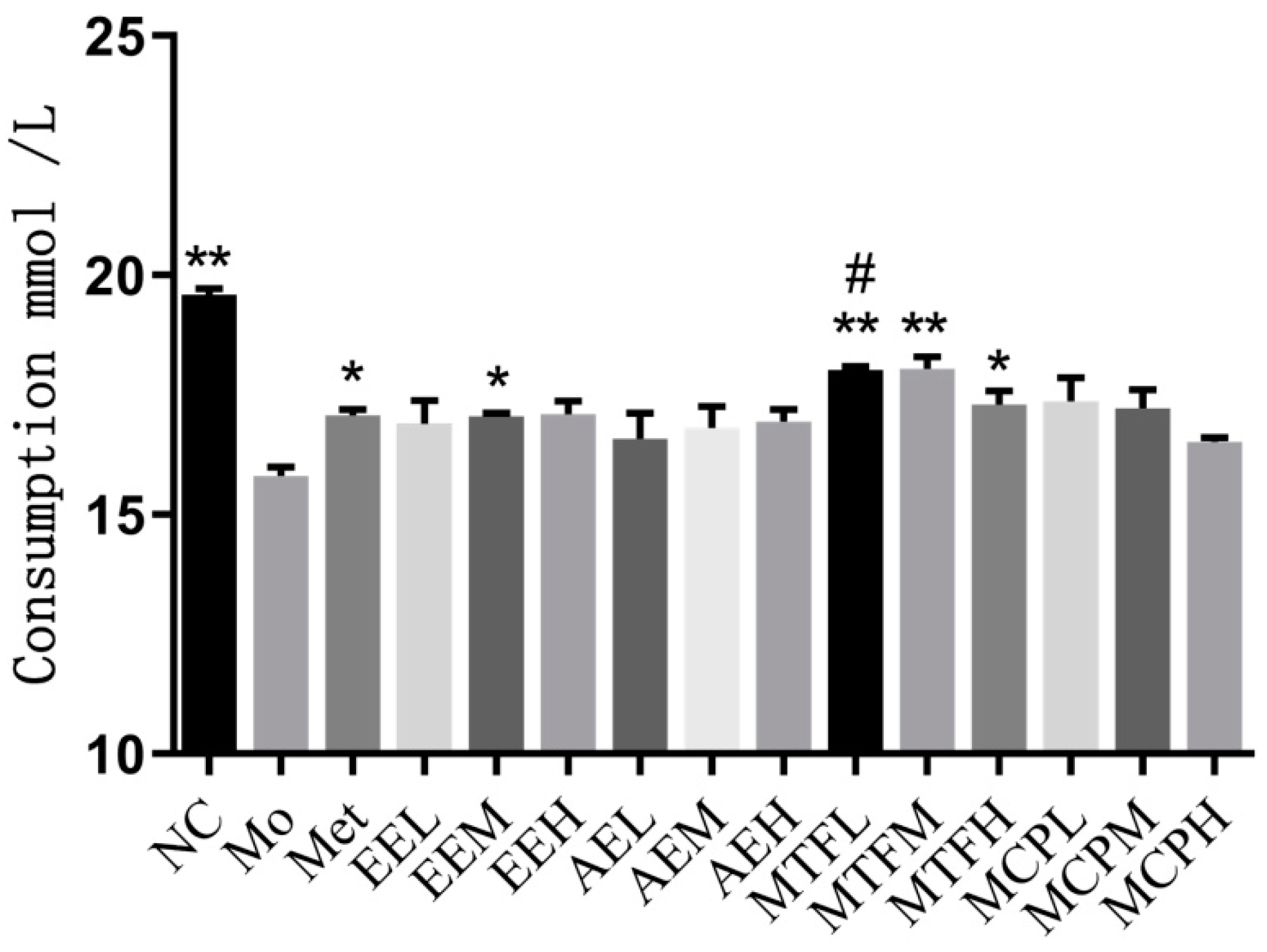
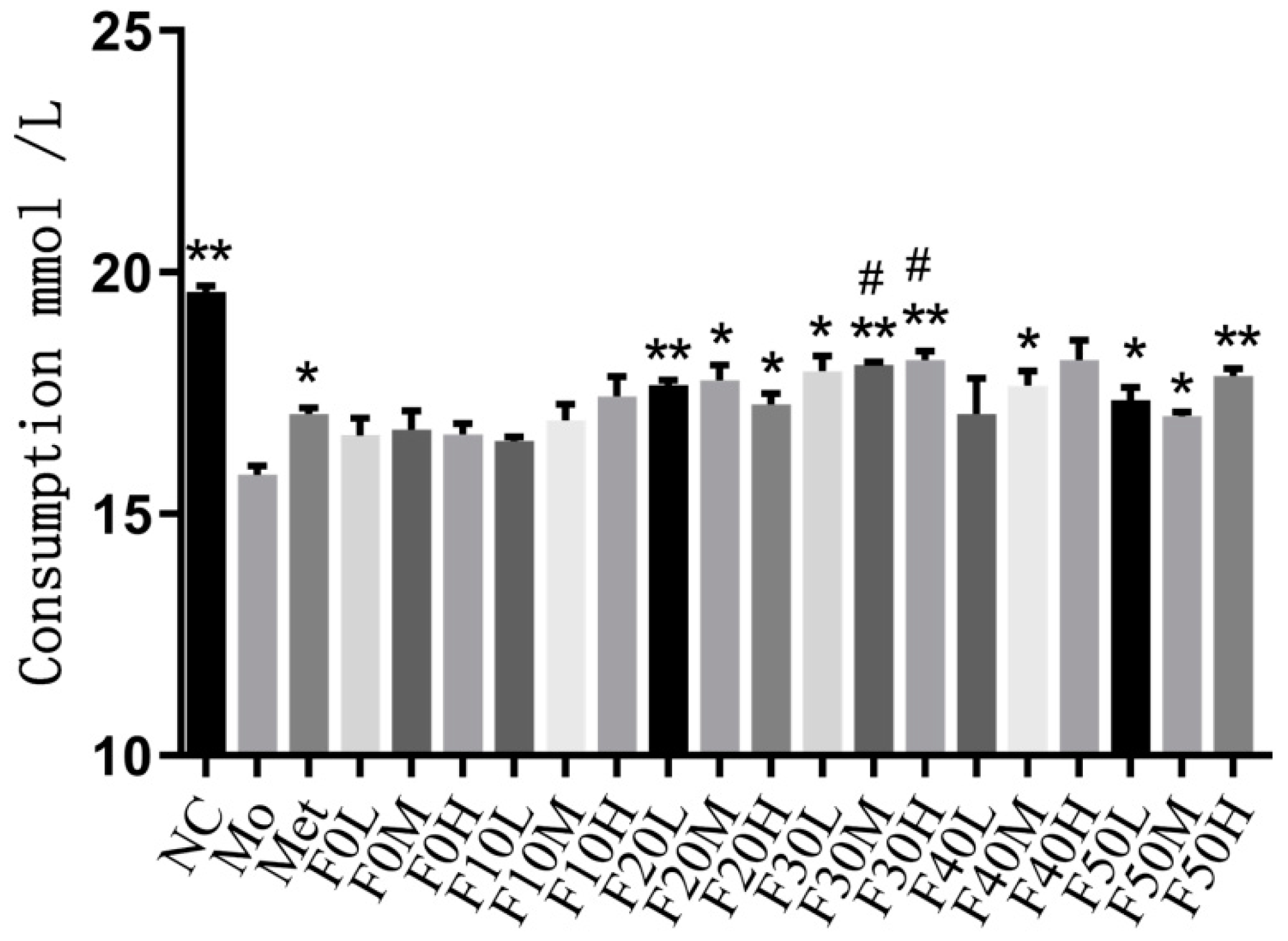
2.6. Glucose Intake in IR-HepG2 Cells
2.7. Statistical Correlations
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction Procedure
4.3. Chemical Composition Analysis
4.3.1. Total Flavonoids and Polysaccharides
4.3.2. HPLC Analysis
4.3.3. HPLC-MS Analysis
4.4. Antioxidant Activity
4.4.1. Antiradical Activity
4.4.2. FRAP Assay
4.5. HepG2 Cell Lipid Accumulation
4.5.1. Cell Culture
4.5.2. MTT Cytotoxicity Assay
4.5.3. Lipid Accumulation and TG Content
4.5.4. The AMPK Inhibitor (Compound C)
4.6. Insulin Resistance of HepG2 Cell
4.7. Spectral Correlations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yen, G.-C.; Duh, P.-D.; Hung, Y.-L. Contributions of Major Components to the Antimutagenic Effect of Hsian-tsao (Mesona procumbens Hemsl.). J. Agric. Food Chem. 2001, 49, 5000–5004. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.-J.; Ong, J.-W.; Lu, C.-C.; Hsu, C.-L.; Lin, J.-H.; Liao, J.-W.; Yen, G.-C. Hypouricemic effects of Mesona procumbens Hemsl. through modulating xanthine oxidase activity in vitro and in vivo. Food Funct. 2016, 7, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Chusak, C.; Thilavech, T.; Adisakwattana, S. Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am. J. Chin. Med. 2014, 42, 315–336. [Google Scholar] [CrossRef]
- Lai, L.-S.; Chou, S.-T.; Chao, W.-W. Studies on the Antioxidative Activities of Hsian-tsao (Mesona procumbens Hemsl) Leaf Gum. J. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Yen, G.-C. Antioxidant Activity of Phenolic Compounds Isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Abhari, F.M. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 11, 117–124. [Google Scholar] [CrossRef]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D.; Wang, Y.; Dong, M. Salidroside suppresses nonsmall cell lung cancer cells proliferation and migration via microRNA-103-3p/Mzb1. Anti-Cancer Drugs 2020, 31, 663–671. [Google Scholar] [CrossRef]
- Yin, P.; Wei, Y.; Wang, X.; Zhu, M.; Feng, J. Roles of Specialized Pro-Resolving Lipid Mediators in Cerebral Ischemia Reperfusion Injury. Front. Neurol. 2018, 9, 617. [Google Scholar] [CrossRef]
- Chiechio, S.; Zammataro, M.; Barresi, M.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P. A Standardized Extract Prepared from Red Orange and Lemon Wastes Blocks High-Fat Diet-Induced Hyperglycemia and Hyperlipidemia in Mice. Molecules 2021, 26, 4291. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Baldini, F.; Salis, A.; Damonte, G.; Daher, A.; Voci, A.; Vergani, L. Antisteatotic and antioxidant activities of Thymbra spicata L. extracts in hepatic and endothelial cells as in vitro models of non-alcoholic fatty liver disease. J. Ethnopharmacol. 2019, 239, 111919. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, X.; Liu, J.; Fan, X.; Jin, Y.; Gu, J.; Liang, J.; Liang, X.; Wang, C. Isoquercetin improves inflammatory response in rats following ischemic stroke. Front. Neurosci. 2021, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Adisakwattana, S.; Henry, C.J. Effects of grass jelly on glycemic control: Hydrocolloids may inhibit gut carbohydrase. Asia Pac. J. Clin. Nutr. 2018, 27, 336–340. [Google Scholar]
- Huang, H.-C.; Chuang, S.-H.; Wu, Y.-C.; Chao, P.-M. Hypolipidaemic function of Hsian-tsao tea (Mesona procumbens Hemsl.): Working mechanisms and active components. J. Funct. Foods 2016, 26, 217–227. [Google Scholar] [CrossRef]
- Huang, H.-T.; Liaw, C.-C.; Chiou, C.-T.; Kuo, Y.-H.; Lee, K.-T. Triterpene Acids from Mesona procumbens Exert Anti-inflammatory Effects on LPS-Stimulated Murine Macrophages by Regulating the MAPK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6271–6280. [Google Scholar] [CrossRef]
- Widyaningsih, T.D. Cytotoxic effect of water, ethanol and ethyl acetate extract of black cincau (Mesona Palustris BL) against hela cell culture. APCBEE Procedia 2012, 2, 110–114. [Google Scholar] [CrossRef][Green Version]
- Handayani, D.; Widyaningsih, T.D.; Wijayanti, N.; Etika, M. Black Grass Jelly (Mesona Palustris Bl) Effervescent powder has anti-dyslipidemia in high cholesterol diet-fed rats and antioxidant activity. Res. J. Life Sci. 2017, 4, 159–167. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Thilavech, T.; Chusak, C. Mesona Chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement. Altern. Med. 2014, 14, 130. [Google Scholar] [CrossRef]
- Guo, C.; Shangguan, Y.; Zhang, M.; Ruan, Y.; Xue, G.; Ma, J.; Yang, J.; Qiu, L. Rosmarinic acid alleviates ethanol-induced lipid accumulation by repressing fatty acid biosynthesis. Food Funct. 2020, 11, 2094–2106. [Google Scholar] [CrossRef]
- Pattananandecha, T.; Apichai, S.; Julsrigival, J.; Ungsurungsie, M.; Samuhasaneetoo, S.; Chulasiri, P.; Kwankhao, P.; Pitiporn, S.; Ogata, F.; Kawasaki, N.; et al. Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yoshioka, Y.; Yuan, S.; Horiuchi, Y.; Yamashita, Y.; Croft, K.D.; Ashida, H. Enzymatically modified isoquercitrin promotes energy metabolism through activating AMPKα in male C57BL/6 mice. Food Funct. 2019, 10, 5188–5202. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shao, S.; Xiao, F.; Zhang, H.; Zhang, R.; Wang, M.; Li, G.; Yan, M. Platycodon grandiflorum extract: Chemical composition and whitening, antioxidant, and anti-inflammatory effects. RSC Adv. 2021, 11, 10814–10826. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Wang, Y.-L.; Wang, M.-L.; Wang, H.-Y. Screening and identification of the metabolites in rat urine and feces after oral administration of Lycopus lucidus Turcz extract by UHPLC−Q-TOF-MS mass spectrometry. J. Chromatogr. B 2016, 1027, 64–73. [Google Scholar] [CrossRef]
- Karar, M.G.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) Leaves, Fruits and their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 2572-0406. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Shi, S.; Yu, Q.; Liu, M.; Zhang, Z. Identification of cerebrospinal fluid metabolites as biomarkers for neurobrucellosis by liquid chromatography-mass spectrometry approach. Bioengineered 2022, 13, 6996–7010. [Google Scholar] [CrossRef]
- Lin, L.; Xie, J.; Liu, S.; Shen, M.; Tang, W.; Xie, M. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 665–673. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K.A.; Majka, M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef]
- Choi, E.-H.; Chun, Y.-S.; Kim, J.; Ku, S.-K.; Jeon, S.; Park, T.-S.; Shim, S.-M. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J. Funct. Foods 2020, 72, 104072. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Teng, H.; Song, H.; Wu, X.; Xu, M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK. J. Funct. Foods 2015, 19, 487–494. [Google Scholar] [CrossRef]
- Alemán, A.; Pérez-García, S.; Fernández de Palencia, P.; Montero, M.P.; Gómez-Guillén, M.D. Physicochemical, Antioxidant, and Anti-Inflammatory Properties of Rapeseed Lecithin Liposomes Loading a Chia (Salvia hispanica L.). Seed Extract. Antioxid. 2021, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Lee, J.-J.; Kim, I.-S.; Kim, Y.; Park, J.-S.; Myung, C.-S. 7-O-Methylaromadendrin Stimulates Glucose Uptake and Improves Insulin Resistance in Vitro. Biol. Pharm. Bull. 2010, 33, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chandra, P.; Srivastava, M.; Mishra, D.K.; Kumar, B. Simultaneous quantitative determination of multiple bioactive markers in Ocimum sanctumobtained from different locations and its marketed herbal formulations using UPLC-ESI-MS/MS combined with principal component analysis. Phytochem. Anal. 2015, 26, 383–394. [Google Scholar] [CrossRef] [PubMed]


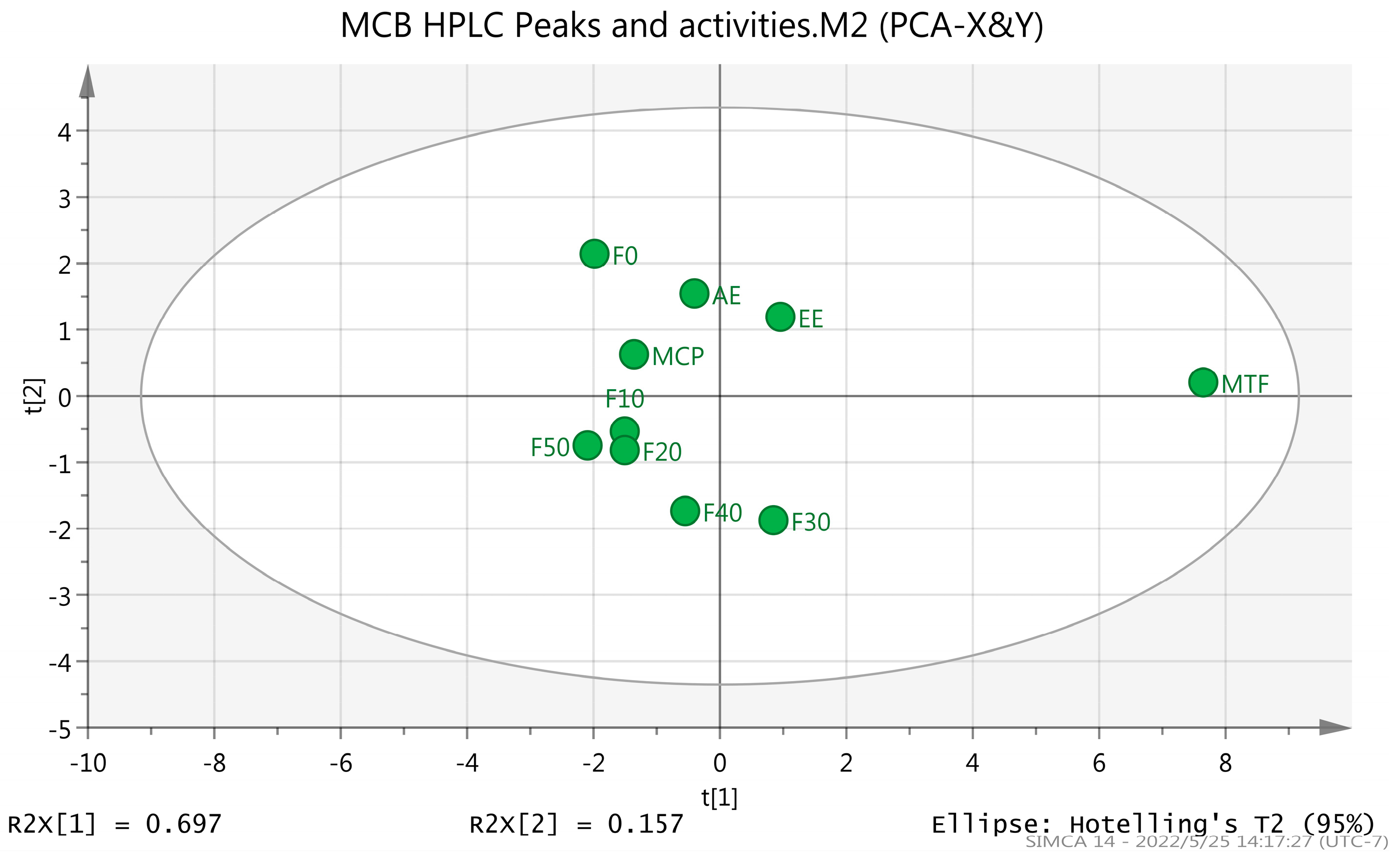
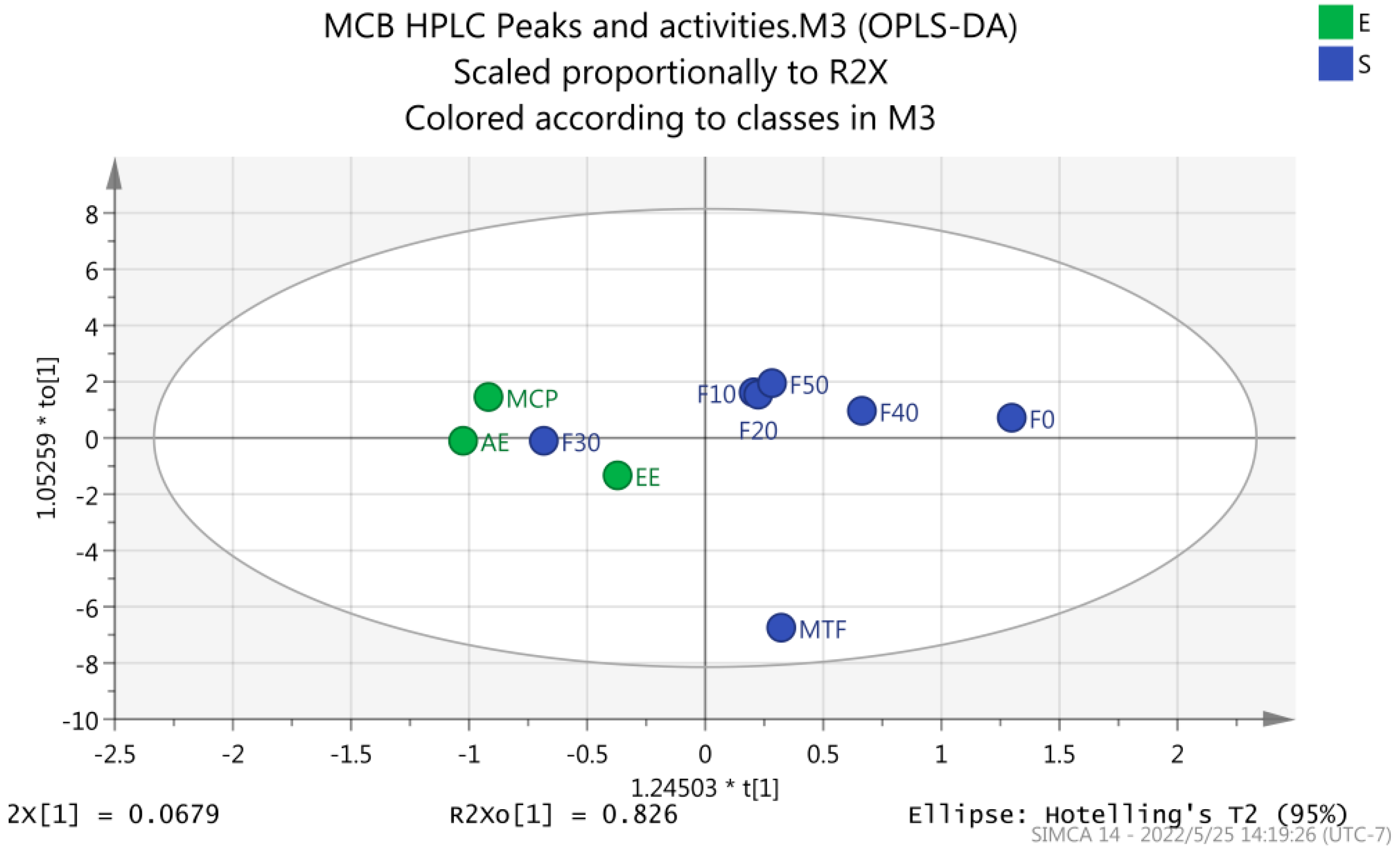
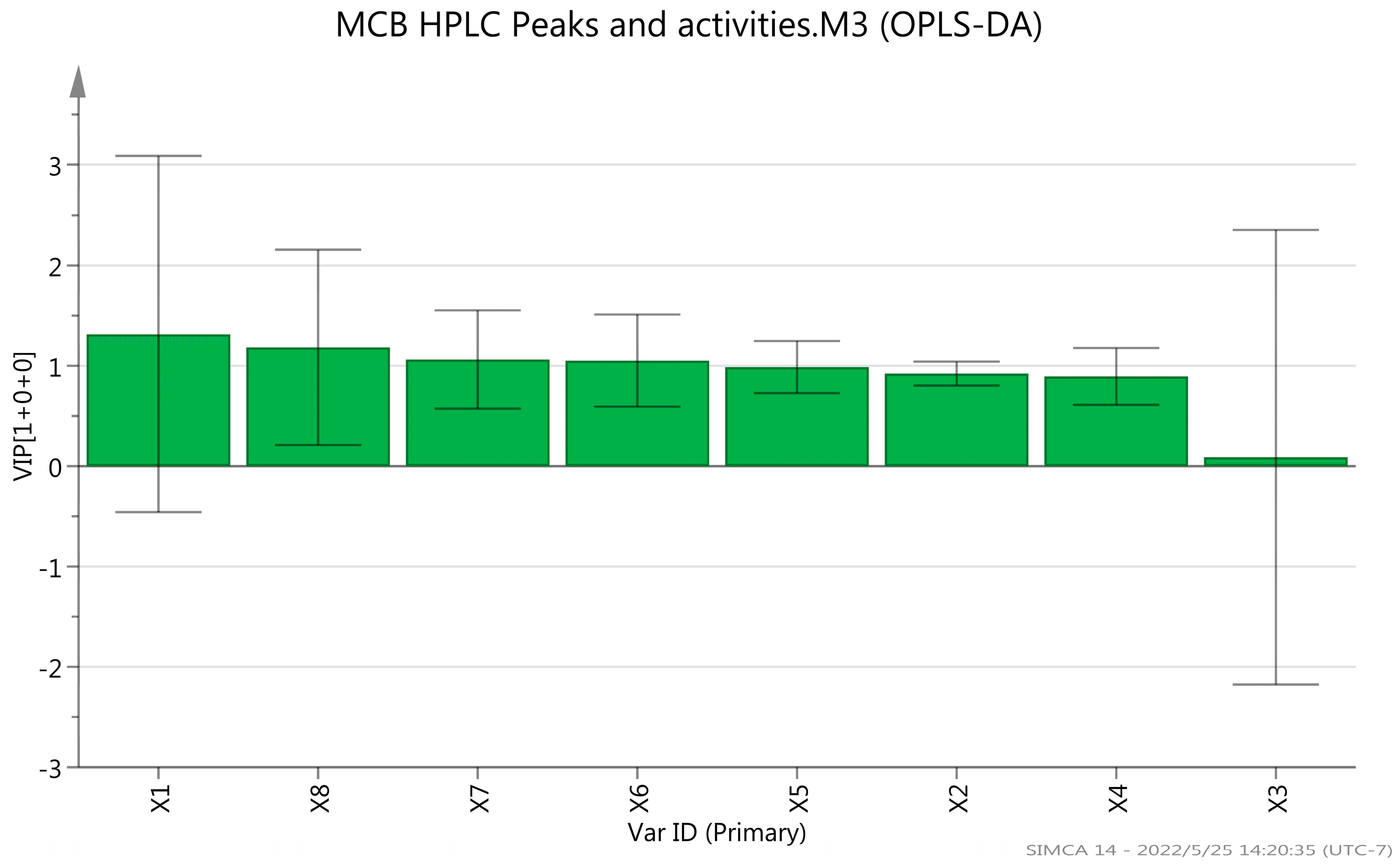
| Sample Name | Total Flavonoids (%) | Total Polysaccharides (%) |
|---|---|---|
| F0 | 5.74 | 35.27 |
| F10 | 31.73 | 12.14 |
| F20 | 30.73 | 6.69 |
| F30 | 65.27 | 9.32 |
| F40 | 29.53 | 9.79 |
| F50 | 16.95 | 4.99 |
| AE | 17.71 | 43.66 |
| MCP | 16.62 | 53.82 |
| EE | 24.30 | 28.59 |
| MTF | 78.52 | 18.83 |
| NO. | tR (Min) | Observed [M-H]-(m/z) | Formula | Fragment Ions | Compound | Ref. |
|---|---|---|---|---|---|---|
| Peak 1 | 14.647 | 178.62 | C9H8O4 | 178.62, 151.01, 134.70, 112.89 | Caffeic acid | Standard compound |
| Peak 2 | 20.04 | 615.20 | C28H24O16 | 615.20, 460.13, 391.10, 296.82, 182.90 | Quercetin 3-O-galactoside | Standard compound |
| Peak 3 | 21.86 | 301.06 | C15H10O7 | 301.06, 273.01, 150.97, 122.46 | Isoquercetin | Standard compound |
| Peak 4 | 27.56 | 447.10 | C21H20O11 | 447.10, 280.52, 242.83, 92.95 | Astragalin | Standard compound |
| Peak 5 | 30.553 | 358.56 | C18H16O8 | 358.56, 196.65, 162.79, 137.00 | Rosmarinic acid | Standard compound |
| Peak 6 | 26.27 | 592.86 | C27H30O15 | 592.86, 446.58, 326.59 | Aromadendrin-3-O-rutinoside | [24] |
| Peak 7 | 32.057 | 520.87 | C24H26O13 | 520.87, 358.78, 196.81, 160.60,135.17 | Rosmarinic acid-3-O-glucoside | [25] |
| Peak 8 | 37.28 | 447.10 | C21H20O11 | 447.10, 264.59, 242.83, 150.90 | Kaempferol-7-O-glucoside | [26] |
| Sample | HepG2 Cell Viability IC50 (µg/mL) |
|---|---|
| EE | 731.1 |
| AE | 401.4 |
| MCP | - |
| MTF | - |
| F0 | - |
| F10 | 630.4 |
| F20 | 691.0 |
| F40 | 590.8 |
| F50 | 502.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Lu, X.; Yang, H.; Lin, C.; Li, L.; Ni, C.; Fang, Y.; Mo, S.; Zhan, R.; Yan, P. The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts. Molecules 2022, 27, 3423. https://doi.org/10.3390/molecules27113423
Xiao L, Lu X, Yang H, Lin C, Li L, Ni C, Fang Y, Mo S, Zhan R, Yan P. The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts. Molecules. 2022; 27(11):3423. https://doi.org/10.3390/molecules27113423
Chicago/Turabian StyleXiao, Luhua, Xiaoying Lu, Huilin Yang, Cuiqing Lin, Le Li, Chen Ni, Yuan Fang, Suifen Mo, Ruoting Zhan, and Ping Yan. 2022. "The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts" Molecules 27, no. 11: 3423. https://doi.org/10.3390/molecules27113423
APA StyleXiao, L., Lu, X., Yang, H., Lin, C., Li, L., Ni, C., Fang, Y., Mo, S., Zhan, R., & Yan, P. (2022). The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts. Molecules, 27(11), 3423. https://doi.org/10.3390/molecules27113423






