Application of Dual-Enhanced Surface-Enhanced Raman Scattering Probe Technology in the Diagnosis of Tumor Cells in Vitro
Abstract
:1. Introduction
2. Material and Methods
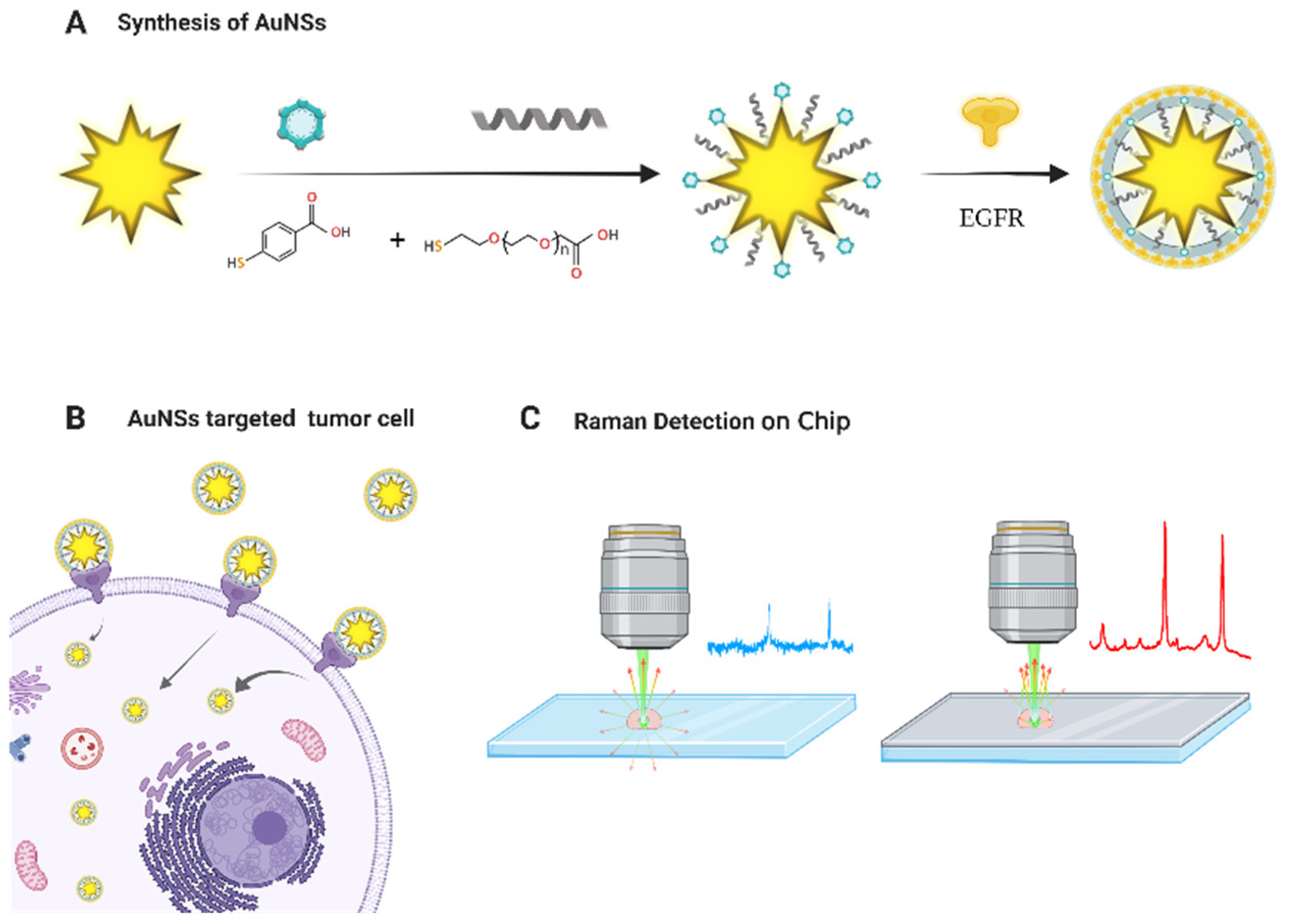
2.1. Synthesis of AuNSs
2.2. Surface Functional Modification and Optimization of AuNSs
2.3. Cytotoxicity Test
2.4. Raman Experiment
2.4.1. Surface-Functionalized AuNSs for Raman Detection of Cells in Vitro
2.4.2. Raman Chip
3. Results and Discussion
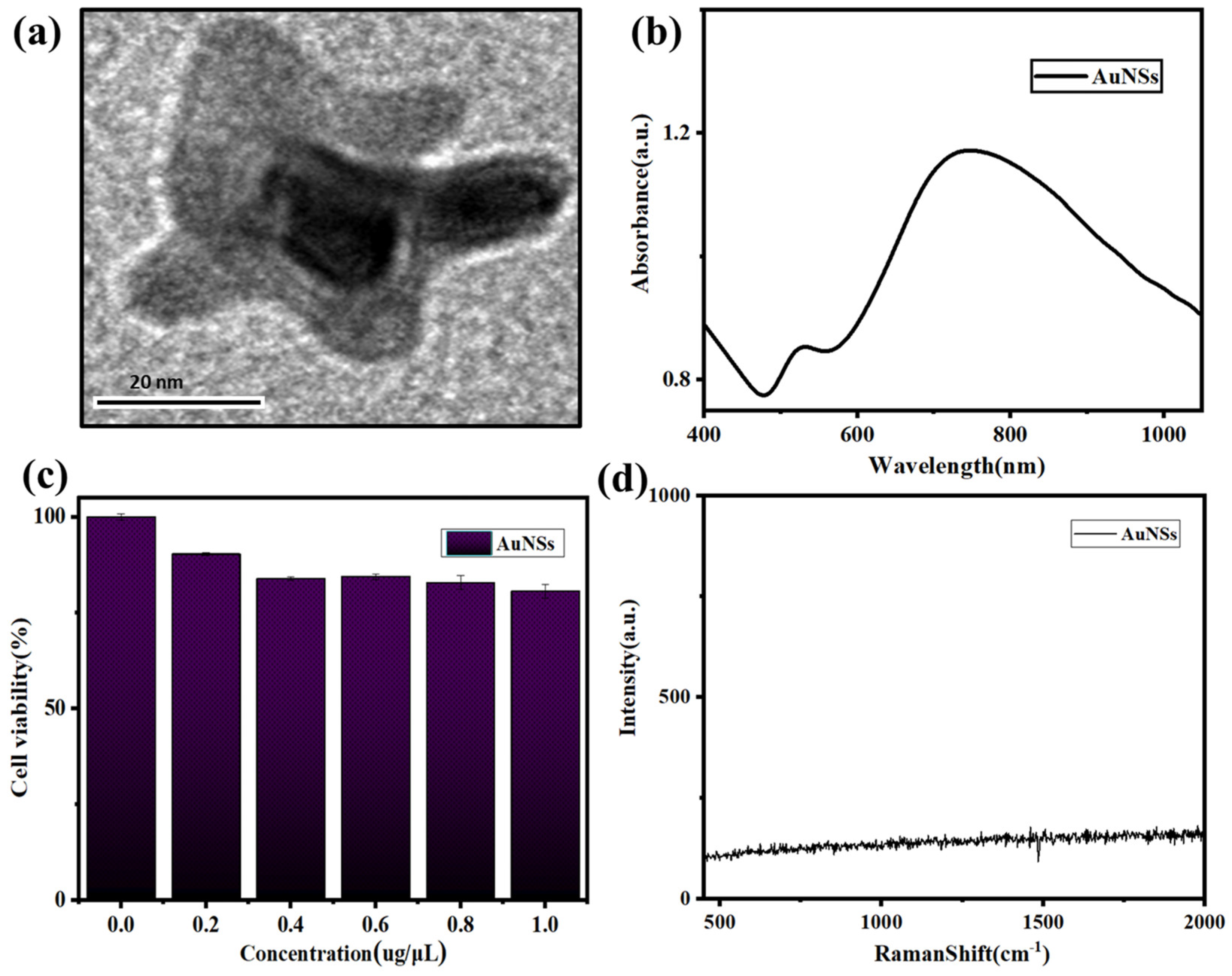
3.1. Characterization of AuNSs
3.2. Synthesis and Characterization of Functional Nanomaterials
3.3. Enhancement of the Physical Signals of Functional Nanomaterials
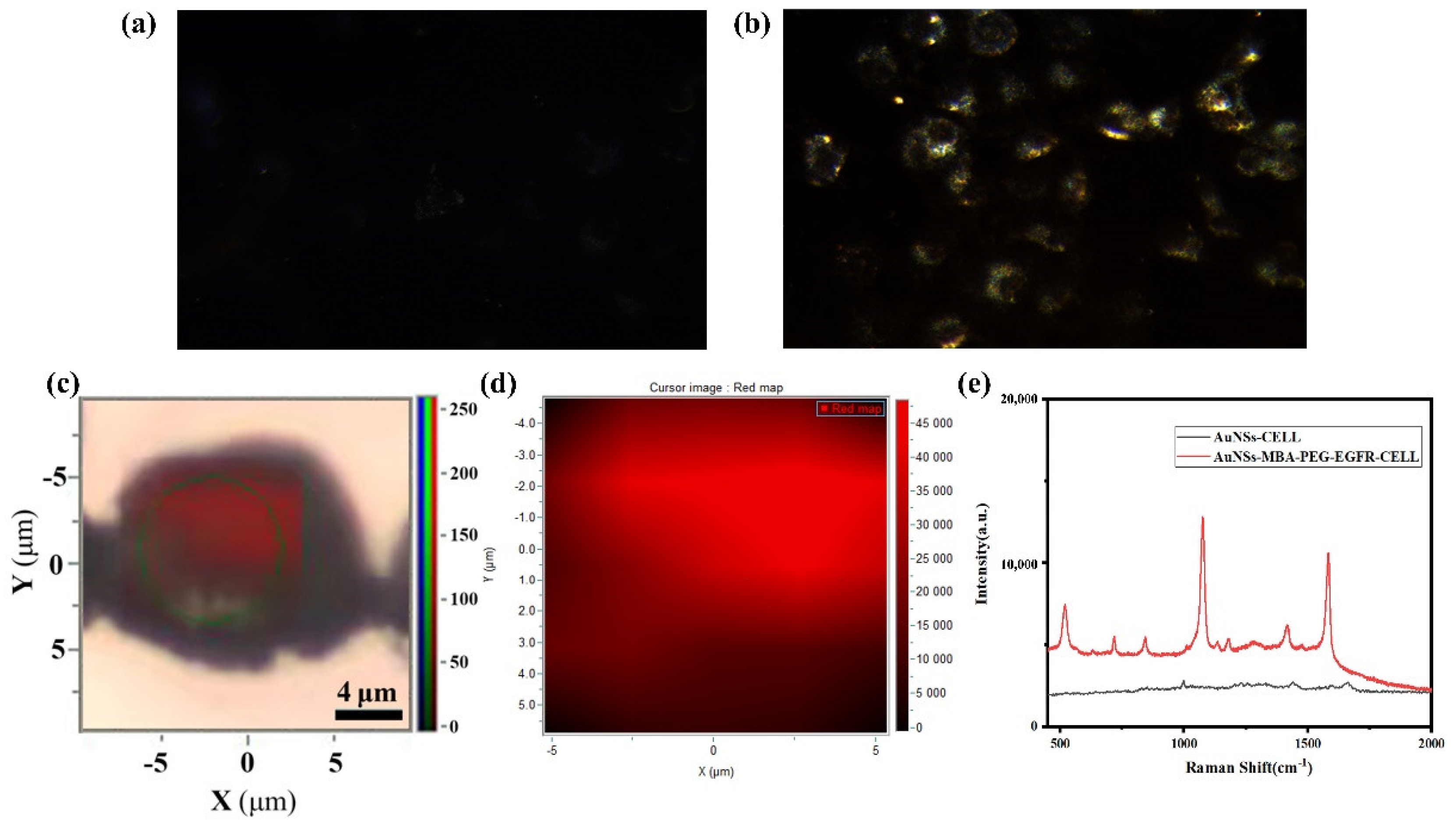
3.4. Functional Nanomaterials for In Vitro Cell Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Zheng, J.; Wu, A. An efficient strategy for circulating tumor cell detection: Surface-enhanced Raman spectroscopy. J. Mater. Chem. B 2020, 8, 3316–3326. [Google Scholar] [CrossRef]
- Blatt, J.; McLean, T.W.; Castellino, S.M.; Burkhart, C.N. A review of contemporary options for medical management of hemangiomas, other vascular tumors, and vascular malformations. Pharmacol. Ther. 2013, 139, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M. Targeted therapy in cancer. Cancer Chemother. Pharmacol. 2015, 76, 1113–1132. [Google Scholar] [CrossRef] [Green Version]
- Juratli, T.A.; Schackert, G.; Krex, D. Current status of local therapy in malignant gliomas—A clinical review of three selected approaches. Pharmacol. Ther. 2013, 139, 341–358. [Google Scholar] [CrossRef]
- Pretto, F.; Neri, D. Pharmacotherapy of metastatic melanoma: Emerging trends and opportunities for a cure. Pharmacol. Ther. 2013, 139, 405–411. [Google Scholar] [CrossRef]
- Terenghi, M.; Elviri, L.; Careri, M.; Mangia, A.; Lobinski, R. Multiplexed determination of protein biomarkers using metal−tagged antibodies and size exclusion chromatography−−Inductively coupled plasma mass spectrometry. Anal. Chem. 2009, 81, 9440–9448. [Google Scholar] [CrossRef]
- Xu, M.J.; Johnson, D.E.; Grandis, J.R. EGFR−targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017, 36, 463–473. [Google Scholar] [CrossRef]
- Ilkhani, H.; Sarparast, M.; Noori, A.; Zahra Bathaie, S.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Gao, M.; Zhang, X. High-sensitive bioorthogonal SERS tag for live cancer cell imaging by self-assembling core-satellites structure gold-silver nanocomposite. Talanta 2017, 172, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Chon, J.W.; Clayton, A.H. Inhibiting EGFR clustering and cell proliferation with gold nanoparticles. Small 2015, 11, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Nowsheen, S.; Aziz, K.; Georgakilas, A.G. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol. Ther. 2013, 139, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, H.−Y.; Wang, M.; Wang, Y.; Tao, L.; Qian, W. Au nanoparticle decorated resin microspheres: Synthesis and application in electrochemical cytosensors for sensitive and selective detection of lung cancer A549 cells. RSC Adv. 2015, 5, 24615–24624. [Google Scholar] [CrossRef]
- Song, L.; Falzone, N.; Vallis, K.A. EGF-coated gold nanoparticles provide an efficient nano-scale delivery system for the molecular radiotherapy of EGFR−positive cancer. Int. J. Radiat. Biol. 2016, 92, 716–723. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liu, T.Y.; Chen, P.J.; Chang, P.H.; Chen, S.Y. A High-Sensitivity and Low-Power Theranostic Nanosystem for Cell SERS Imaging and Selectively Photothermal Therapy Using Anti-EGFR-Conjugated Reduced Graphene Oxide/Mesoporous Silica/AuNPs Nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef]
- Wang, L.; Guo, T.; Lu, Q.; Yan, X.; Zhong, D.; Zhang, Z.; Ni, Y.; Han, Y.; Cui, D.; Li, X.; et al. Sea-urchin-like Au nanocluster with surface-enhanced raman scattering in detecting epidermal growth factor receptor (EGFR) mutation status of malignant pleural effusion. ACS Appl. Mater. Interfaces 2015, 7, 359–369. [Google Scholar] [CrossRef]
- D’Hollander, A.; Mathieu, E.; Jans, H.; Vande Velde, G.; Stakenborg, T.; van Dorpe, P.; Himmelreich, U.; Lagae, L. Development of nanostars as a biocompatible tumor contrast agent: Toward in vivo SERS imaging. Int. J. Nanomed. 2016, 11, 3703–3714. [Google Scholar] [CrossRef] [Green Version]
- Heidari, Z.; Sariri, R.; Salouti, M. Gold nanorods-bombesin conjugate as a potential targeted imaging agent for detection of breast cancer. J. Photochem. Photobiol. B 2014, 130, 40–46. [Google Scholar] [CrossRef]
- El−Sayed, I.H.; Huang, X.; El−Sayed, M.A. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005, 5, 829–834. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Guo, X.; Gu, J.; Zhang, L.; Jin, H.; Huang, H.; Li, J.; Huang, C. p85alpha promotes nucleolin transcription and subsequently enhances EGFR mRNA stability and EGF-induced malignant cellular transformation. Oncotarget 2016, 7, 16636–16649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.C.; Asakawa, A.; Li, Y.X.; Liu, I.M.; Amitani, H.; Cheng, J.T.; Inui, A. Opioid mu-receptors as new target for insulin resistance. Pharmacol. Ther. 2013, 139, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Wang, C.-R.C.; Shieh, D.-B.; Wei, C.-W.; Liao, C.-K.; Poe, C.; Jhan, S.; Ding, A.-A.; Wu, Y.-N. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Optics Express 2008, 16, 18605–18615. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, D.; Long, T.; Yuan, L.; Qiu, S.; Li, D.; Mu, C.; Ge, L. pH-Sensitive nanoparticles based on amphiphilic imidazole/cholesterol modified hydroxyethyl starch for tumor chemotherapy. Carbohydr. Polym. 2022, 277, 118827. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef] [Green Version]
- Reina-Campos, M.; Moscat, J.; Diaz-Meco, M. Metabolism shapes the tumor microenvironment. Curr. Opin. Cell Biol. 2017, 48, 47–53. [Google Scholar] [CrossRef]
- Litti, L.; Colusso, A.; Pinto, M.; Ruli, E.; Scarsi, A.; Ventura, L.; Toffoli, G.; Colombatti, M.; Fracasso, G.; Meneghetti, M. SERRS multiplexing with multivalent nanostructures for the identification and enumeration of epithelial and mesenchymal cells. Sci. Rep. 2020, 10, 15805. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, J.; Yang, J.; Wang, J.Y.; Shen, X.; Song, S.S.; Wang, H.; He, H.; Wang, X.; Fan, S.; et al. Use of epidermal growth factor receptor antibody−gold cluster conjugates with good renal excretion in targeted cancer radiation treatment. J. Mater. Chem. B 2015, 3, 4735–4741. [Google Scholar] [CrossRef]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef] [Green Version]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Li, L.; Liao, M.; Chen, Y.; Shan, B.; Li, M. Surface-enhanced Raman spectroscopy (SERS) nanoprobes for ratiometric detection of cancer cells. J. Mater. Chem. B 2019, 7, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G. Quo vadis surface-enhanced Raman scattering? Phys. Chem. Chem. Phys. 2009, 11, 7348–7349. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, F.; Guo, X.; Chen, W.; Dong, C.; Zhang, J.; Zhang, J.; Wang, L. Gold nanostars for cancer cell-targeted SERS-imaging and NIR light-triggered plasmonic photothermal therapy (PPTT) in the first and second biological windows. J. Mater. Chem. B 2019, 7, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, C.; Lu, W.; Zhao, H.; Wang, M.; Zhang, M.; Chen, X.; Dong, J.; Han, X.; Qian, W. Surface Enhanced Raman Scattering Probes Based on Antibody Conjugated Au Nanostars for Distinguishing Lung Cancer Cells from Normal Cells. J. Nanosci. Nanotechnol. 2016, 16, 12161–12171. [Google Scholar] [CrossRef]
- Xiao, L.; Tian, X.; Harihar, S.; Li, Q.; Li, L.; Welch, D.R.; Zhou, A. Gd2O3-doped silica @ Au nanoparticles for in vitro imaging cancer biomarkers using surface-enhanced Raman scattering. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 218–225. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Kong, Y.; Chen, L.; Sheng, H.; Fei, Y.; Mi, L.; Li, B.; Ma, J. Application of Dual-Enhanced Surface-Enhanced Raman Scattering Probe Technology in the Diagnosis of Tumor Cells in Vitro. Molecules 2022, 27, 3582. https://doi.org/10.3390/molecules27113582
Zhao Y, Kong Y, Chen L, Sheng H, Fei Y, Mi L, Li B, Ma J. Application of Dual-Enhanced Surface-Enhanced Raman Scattering Probe Technology in the Diagnosis of Tumor Cells in Vitro. Molecules. 2022; 27(11):3582. https://doi.org/10.3390/molecules27113582
Chicago/Turabian StyleZhao, Yinping, Yawei Kong, Liwen Chen, Han Sheng, Yiyan Fei, Lan Mi, Bei Li, and Jiong Ma. 2022. "Application of Dual-Enhanced Surface-Enhanced Raman Scattering Probe Technology in the Diagnosis of Tumor Cells in Vitro" Molecules 27, no. 11: 3582. https://doi.org/10.3390/molecules27113582





