68Ga-Labeled [Leu13ψThz14]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake
Abstract
1. Introduction
2. Results
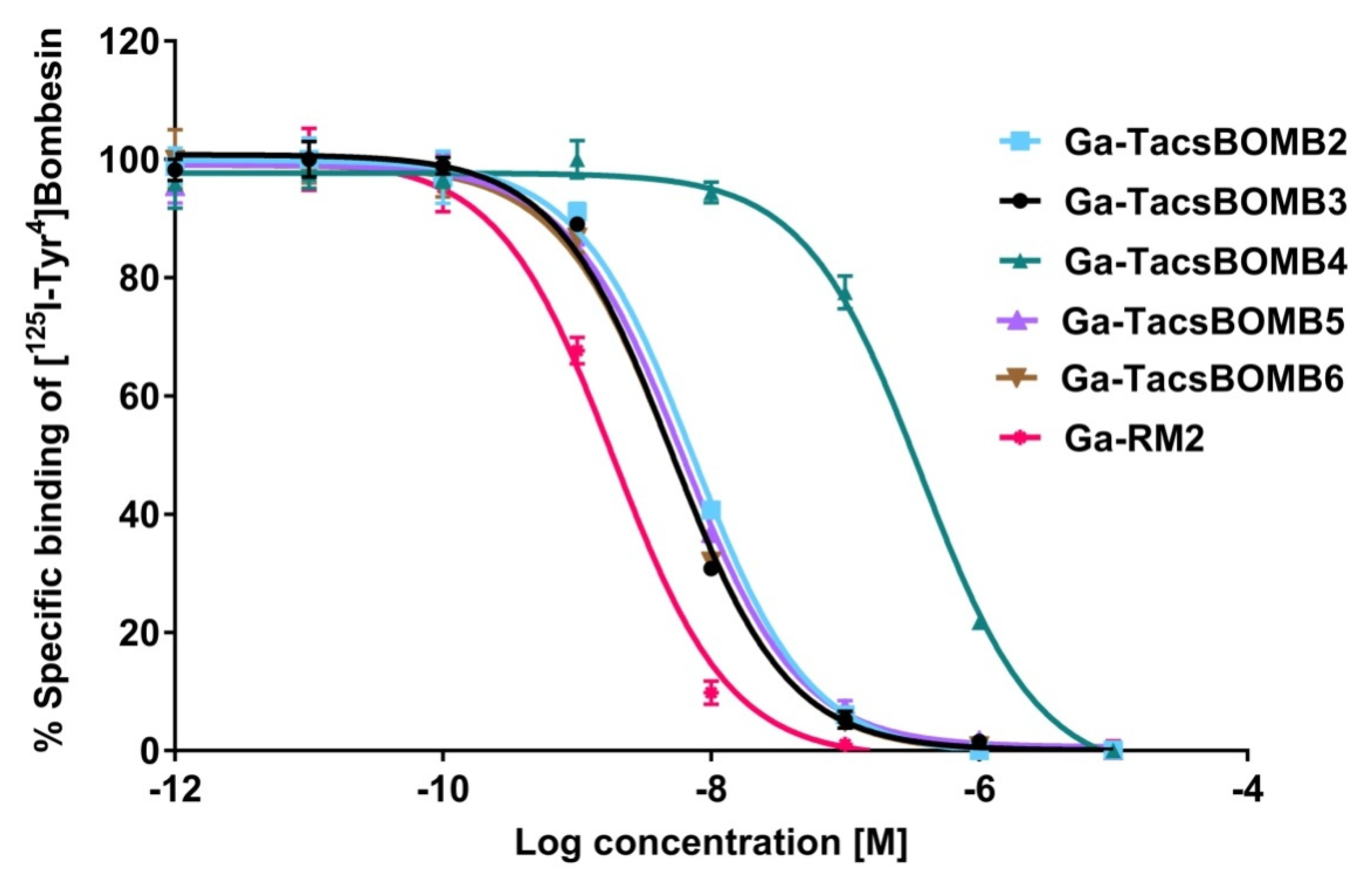
2.1. Binding Affinity, Antagonist Characterization and Hydrophilicity
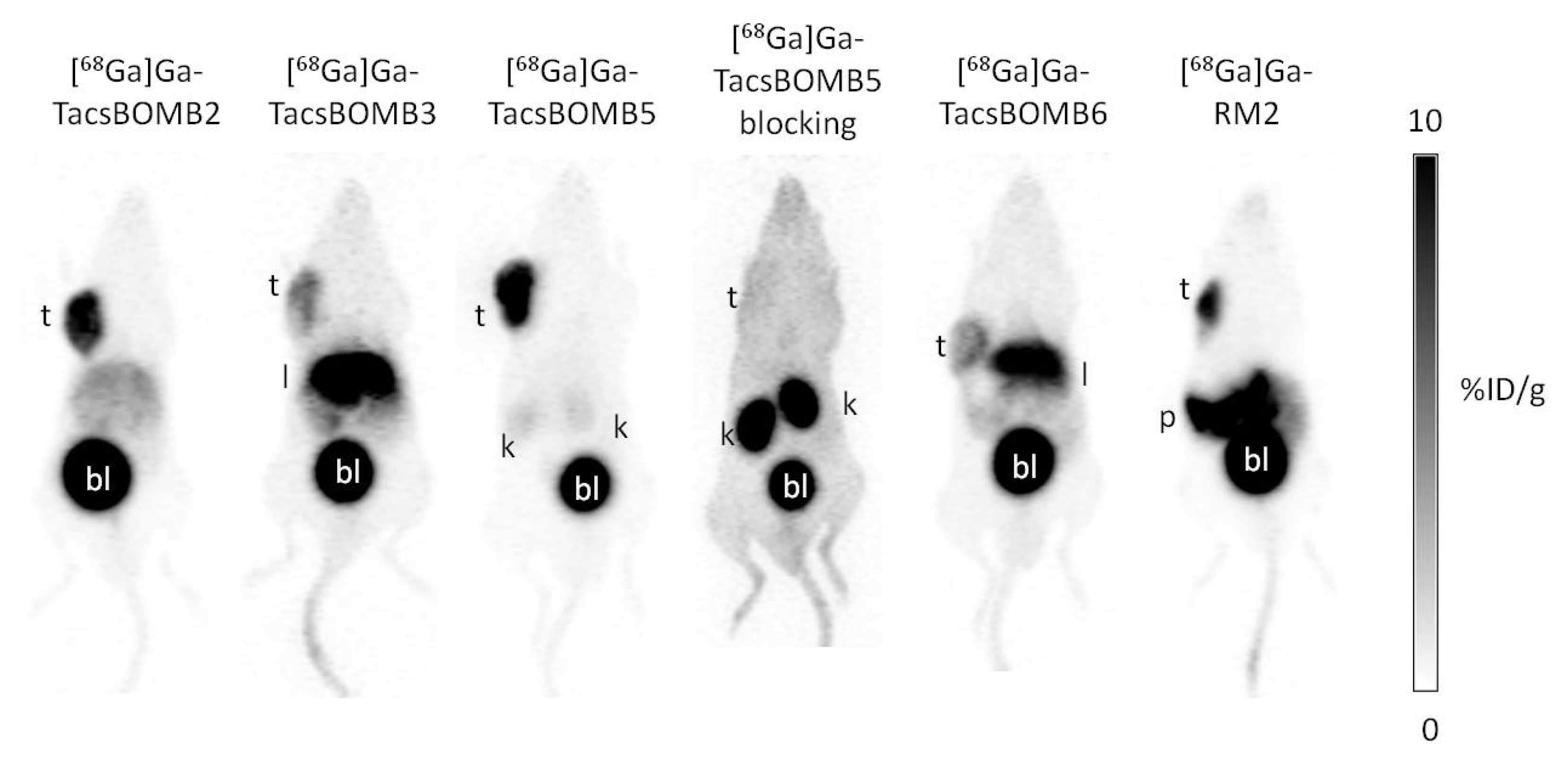
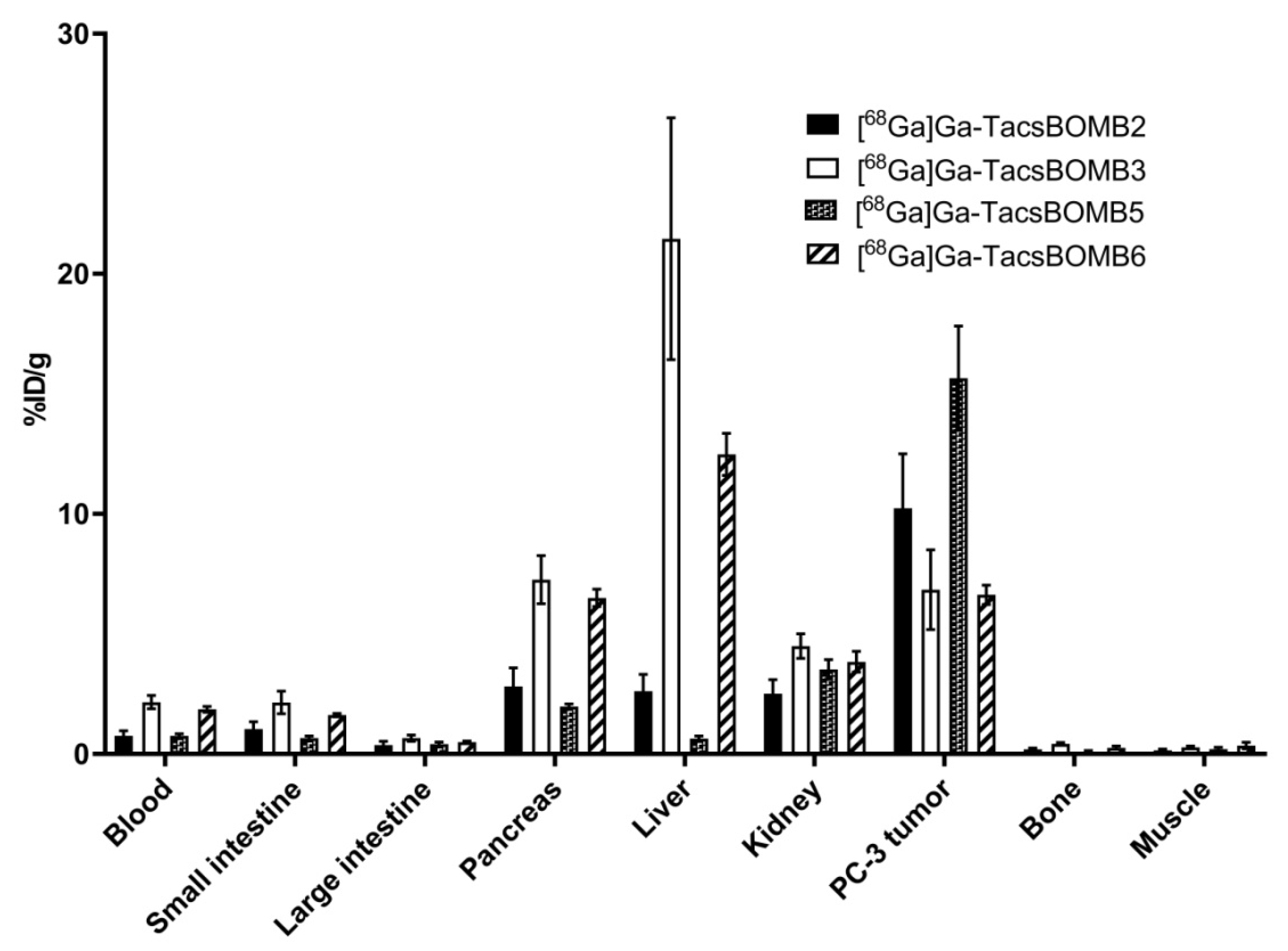
2.2. PET Imaging and Ex Vivo Biodistribution
2.3. In Vivo Stability
3. Discussion
4. Materials and Methods
4.1. Synthesis of GRPR-Targeting Ligands
4.2. LogD7.4 Measurement
4.3. Cell Culture
4.4. Fluorometric Calcium Release Assay
4.5. In Vitro Competition Binding Assay
4.6. Ex Vivo Biodistribution, PET/CT Imaging and In Vivo Stability Studies
4.7. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, R.; Battey, J.; Spindel, E.; Benya, R. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Hajri, A.; Koenig, M.; Balboni, G.; Damgé, C. Expression and characterization of gastrin-releasing peptide receptor in normal and cancerous pancreas. Pancreas 1996, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.; Zia, F.; Venugopal, R.; Fagarasan, M.; Oie, H.; Hu, V. GRP receptors are present in non small cell lung cancer cells. J. Cell Biochem. 1996, 63, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.; Woodhouse, L.; Jones-Blackett, S.; Miller, G.; Primrose, J. High-affinity binding sites for gastrin-releasing peptide on human colorectal cancer tissue but not uninvolved mucosa. Br. J. Cancer 1995, 71, 1087–1089. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gugger, M.; Reubi, J.C. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am. J. Pathol. 1999, 155, 2067–2076. [Google Scholar] [CrossRef]
- Preston, S.R.; Woodhouse, L.F.; Gokhale, J.; Miller, G.V.; Primrose, J.N. Characterization of a bombesin/gastrin-releasing peptide receptor on a human gastric-cancer cell line. Int. J. Cancer 1994, 57, 734–741. [Google Scholar] [CrossRef]
- Markwalder, R.; Reubi, J.C. Gastrin-releasing peptide receptors in the human prostate: Relation to neoplastic transformation. Cancer Res. 1999, 59, 1152–1159. [Google Scholar]
- Shimoda, J. Effects of bombesin and its antibody on growth of human prostatic carcinoma cell lines. Jpn. J. Urol. 1992, 83, 1459–1468. [Google Scholar] [CrossRef][Green Version]
- Qin, Y.; Ertl, T.; Cai, R.-Z.; Halmos, G.; Schally, A.V. Inhibitory effect of bombesin receptor antagonist RC-3095 on the growth of human pancreatic cancer cells in vivo and in vitro. Cancer Res. 1994, 54, 1035–1041. [Google Scholar]
- Baum, R.; Prasad, V.; Mutloka, N.; Frischknecht, M.; Maecke, H.; Reubi, J. Molecular imaging of bombesin receptors in various tumors by Ga-68 AMBA PET/CT: First results. J. Nucl. Med. 2007, 48 (Suppl. 2), 79. [Google Scholar]
- Kähkönen, E.; Jambor, I.; Kemppainen, J.; Lehtiö, K.; Grönroos, T.J.; Kuisma, A.; Luoto, P.; Sipilä, H.J.; Tolvanen, T.; Alanen, K. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin. Cancer Res. 2013, 19, 5434–5443. [Google Scholar] [CrossRef] [PubMed]
- Stoykow, C.; Erbes, T.; Maecke, H.R.; Bulla, S.; Bartholomä, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G. Gastrin-releasing peptide receptor imaging in breast cancer using the receptor antagonist 68Ga-RM2 and PET. Theranostics 2016, 6, 1641. [Google Scholar] [CrossRef] [PubMed]
- Baratto, L.; Song, H.; Duan, H.; Hatami, N.; Bagshaw, H.; Buyyounouski, M.; Hancock, S.; Shah, S.A.; Srinivas, S.; Swift, P.; et al. PSMA- and GRPR-targeted PET: Results from 50 patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2021, 62, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Kurth, J.; Krause, B.J.; Schwarzenböck, S.M.; Bergner, C.; Hakenberg, O.W.; Heuschkel, M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: A radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Kaloudi, A.; Lymperis, E.; Giarika, A.; Kulkarni, H.R.; Klette, I.; Singh, A.; Krenning, E.P.; de Jong, M.; Maina, T. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: Preclinical and first clinical results. J. Nucl. Med. 2017, 58, 75–80. [Google Scholar] [CrossRef]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Vasanawala, S.; Gambhir, S.S.; Iagaru, A. Pilot comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in patients with biochemically recurrent prostate cancer. J. Nucl. Med. 2016, 57, 557–562. [Google Scholar] [CrossRef]
- Gasser, G.; Tjioe, L.; Graham, B.; Belousoff, M.J.; Juran, S.; Walther, M.; Künstler, J.; Bergmann, R.; Stephan, H.; Spiccia, L. Synthesis, copper(II) complexation, 64Cu-labeling, and bioconjugation of a new bis(2-pyridylmethyl) derivative of 1,4,7-triazacyclononane. Bioconjugate Chem. 2008, 19, 719–730. [Google Scholar] [CrossRef]
- Juran, S.; Walther, M.; Stephan, H.; Bergmann, R.; Steinbach, J.; Kraus, W.; Emmerling, F.; Comba, P. Hexadentate bispidine derivatives as versatile bifunctional chelate agents for copper(II) radioisotopes. Bioconjugate Chem. 2009, 20, 347–359. [Google Scholar] [CrossRef]
- Reile, H.; Cai, R.; Armatis, P.; Schally, A. New antagonists of bombesin gastrin-releasing peptide with C-terminal Leuψ(CH2N)Tac-NH2. Int. J. Oncol. 1995, 7, 749–754. [Google Scholar]
- Cai, R.; Reile, H.; Armatis, P.; Schally, A.V. Potent bombesin antagonists with C-terminal Leu-ψ(CH2-N)-Tac-NH2 or its derivatives. Proc. Natl. Acad. Sci. USA 1994, 91, 12664–12668. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Pinski, J.; Galvan, G.; Halmos, G.B.; Szepeshazi, K.R.; Gai, R.; Groot, K.; Vadillo-Buenfil, M.; Schally, A.V. Inhibition of growth of androgen-independent DU-145 prostate cancer in vivo by luteinising hormone-releasing hormone antagonist Cetrorelix and bombesin antagonists RC-3940-II and RC-3950-II. Eur. J. Cancer 1997, 33, 1141–1148. [Google Scholar] [CrossRef]
- Koppán, M.; Halmos, G.; Arencibia, J.M.; Lamharzi, N.; Schally, A.V. Bombesin/gastrin-releasing peptide antagonists RC-3095 and RC-3940-II inhibit tumor growth and decrease the levels and mRNA expression of epidermal growth factor receptors in H-69 small cell lung carcinoma. Cancer 1998, 83, 1335–1343. [Google Scholar] [CrossRef]
- Shirahige, Y.; Cai, R.-Z.; Szepeshazi, K.; Halmos, G.; Pinski, J.; Groot, K.; Schally, A. Inhibitory effect of bombesin/gastrin-releasing peptide (GRP) antagonists RC-3950-II and RC-3095 on MCF-7 MIII human breast cancer xenografts in nude mice. Biomed. Pharmacother. 1994, 48, 465–472. [Google Scholar] [CrossRef]
- Lau, J.; Rousseau, E.; Zhang, Z.; Uribe, C.F.; Kuo, H.-T.; Zeisler, J.; Zhang, C.; Kwon, D.; Lin, K.-S.; Bénard, F. Positron emission tomography imaging of the gastrin-releasing peptide receptor with a novel bombesin analogue. ACS Omega 2019, 4, 1470–1478. [Google Scholar] [CrossRef]
- Bratanovic, I.J.; Zhang, C.; Zhang, Z.; Kuo, H.T.; Colpo, N.; Zeisler, J.; Merkens, H.; Uribe, C.; Lin, K.S.; Bénard, F. A Radiotracer for molecular imaging and therapy of gastrin-releasing peptide receptor–positive prostate cancer. J. Nucl. Med. 2022, 63, 424–430. [Google Scholar] [CrossRef]
- Höhne, A.; Mu, L.; Honer, M.; Schubiger, P.A.; Ametamey, S.M.; Graham, K.; Stellfeld, T.; Borkowski, S.; Berndorff, D.; Klar, U.; et al. Synthesis, 18F-Labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjugate Chem. 2008, 19, 1871–1879. [Google Scholar] [CrossRef]
- Richter, S.; Wuest, M.; Bergman, C.N.; Krieger, S.; Rogers, B.E.; Wuest, F. Metabolically stabilized 68Ga-NOTA-Bombesin for PET imaging of prostate cancer and influence of protease inhibitor phosphoramidon. Mol. Pharm. 2016, 13, 1347–1357. [Google Scholar] [CrossRef]
- Yraola, F.; Ventura, R.; Vendrell, M.; Colombo, A.; Fernàndez, J.C.; de la Figuera, N.; Fernández-Forner, D.; Royo, M.; Forns, P.; Albericio, F. A Re-evaluation of the Use of Rink, BAL, and PAL Resins and Linkers. QSAR Comb. Sci. 2004, 23, 145–152. [Google Scholar] [CrossRef]
- Lin, K.-S.; Pan, J.; Amouroux, G.; Turashvili, G.; Mesak, F.; Hundal-Jabal, N.; Pourghiasian, M.; Lau, J.; Jenni, S.; Aparicio, S. In vivo radioimaging of bradykinin receptor B1, a widely overexpressed molecule in human cancer. Cancer Res. 2015, 75, 387–393. [Google Scholar] [CrossRef]
- Kuo, H.-T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.-S.; Benard, F. Effects of linker modification on tumor-to-kidney contrast of 68Ga-labeled PSMA-targeted imaging probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.A.; Shams, S.; Khan, K.M. Thiazolidine esters: New potent urease inhibitors. J. Chem. Soc. Pak. 2014, 36, 858–864. [Google Scholar]
- Amouroux, G.; Pan, J.; Jenni, S.; Zhang, C.; Zhang, Z.; Hundal-Jabal, N.; Colpo, N.; Liu, Z.; Bénard, F.; Lin, K.-S. Imaging human bradykinin B1 receptor with 68Ga-labeled [des-Arg10]kallidin derivatives: Effect of the linker on biodistribution and tumor uptake. Mol. Pharm. 2015, 12, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-S.; Amouroux, G.; Pan, J.; Zhang, Z.; Jenni, S.; Lau, J.; Hundal-Jabal, N.; Colpo, N.; Benard, F. Comparative studies of three gallium-68-labeled [des-Arg10]kallidin derivatives for imaging bradykinin B1 receptor expression with positron emission tomography. J. Nucl. Med. 2015, 56, 622–627. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, Z.; Merkens, H.; Zeisler, J.; Zhang, C.; Roxin, A.; Tan, R.; Bénard, F.; Lin, K.-S. 68Ga-Labeled [Leu13ψThz14]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake. Molecules 2022, 27, 3777. https://doi.org/10.3390/molecules27123777
Wang L, Zhang Z, Merkens H, Zeisler J, Zhang C, Roxin A, Tan R, Bénard F, Lin K-S. 68Ga-Labeled [Leu13ψThz14]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake. Molecules. 2022; 27(12):3777. https://doi.org/10.3390/molecules27123777
Chicago/Turabian StyleWang, Lei, Zhengxing Zhang, Helen Merkens, Jutta Zeisler, Chengcheng Zhang, Aron Roxin, Ruiyan Tan, François Bénard, and Kuo-Shyan Lin. 2022. "68Ga-Labeled [Leu13ψThz14]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake" Molecules 27, no. 12: 3777. https://doi.org/10.3390/molecules27123777
APA StyleWang, L., Zhang, Z., Merkens, H., Zeisler, J., Zhang, C., Roxin, A., Tan, R., Bénard, F., & Lin, K.-S. (2022). 68Ga-Labeled [Leu13ψThz14]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake. Molecules, 27(12), 3777. https://doi.org/10.3390/molecules27123777





