Eco-Friendly Synthesis of PEtOz-PA: A Promising Polymer for the Formulation of Curcumin-Loaded Micelles
Abstract
:1. Introduction
2. Results
3. Results and Discussion
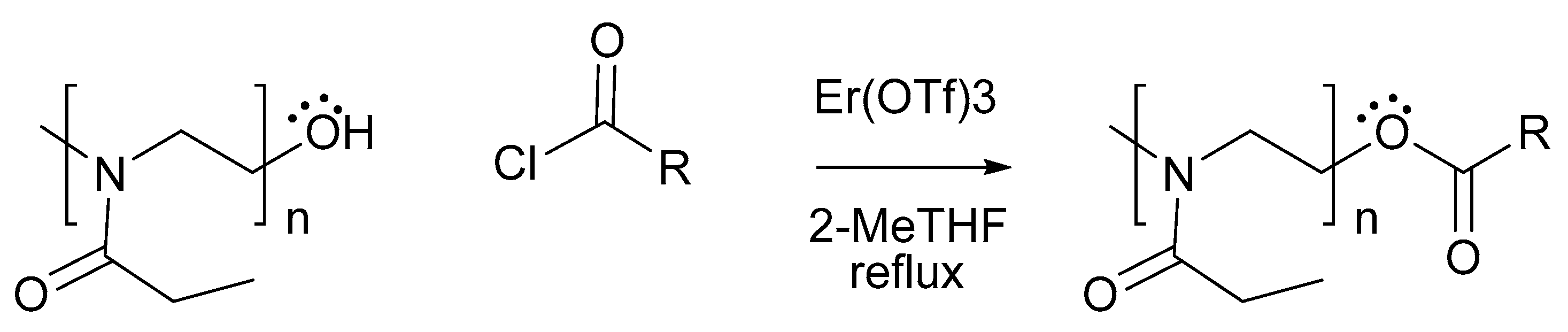
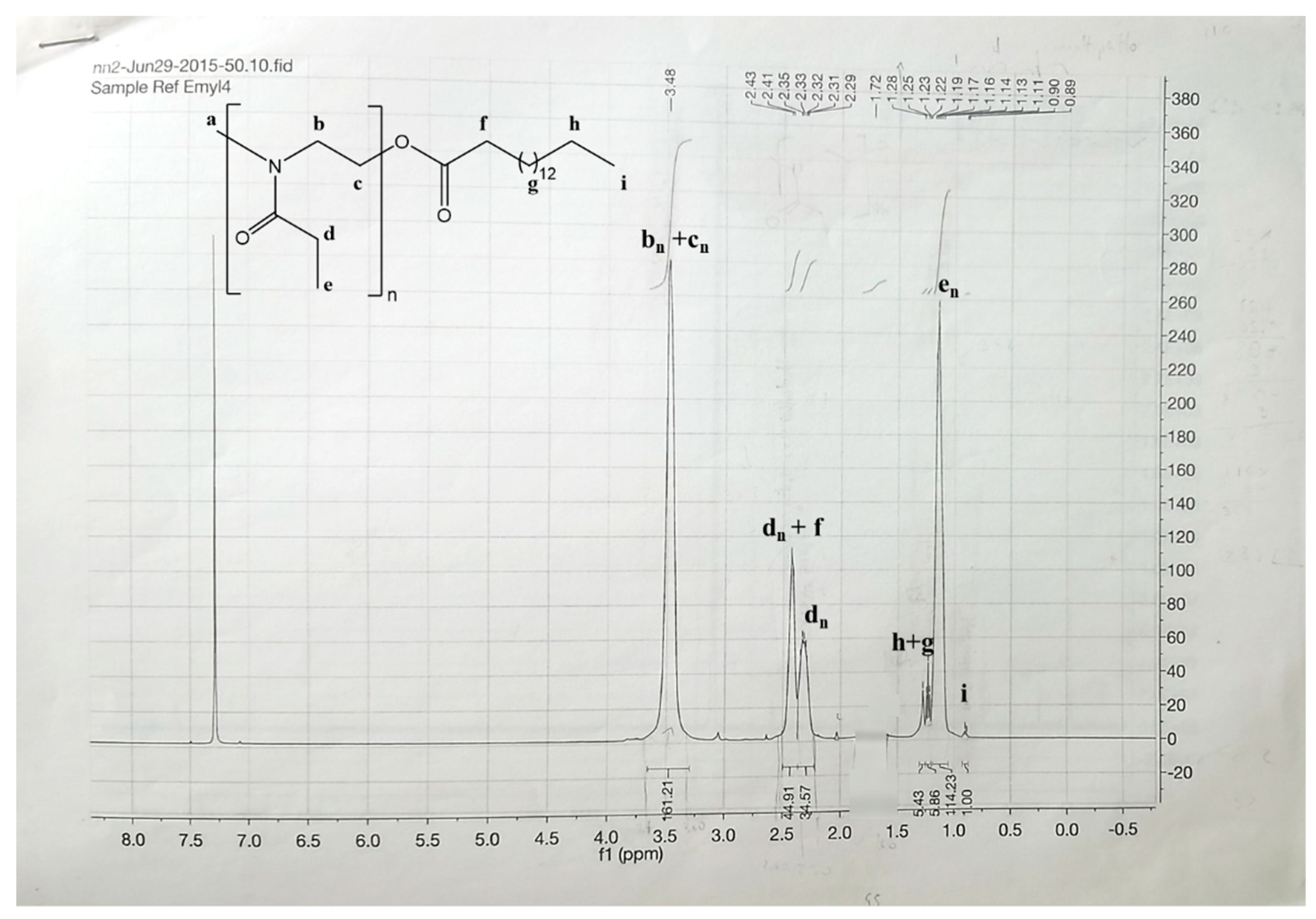
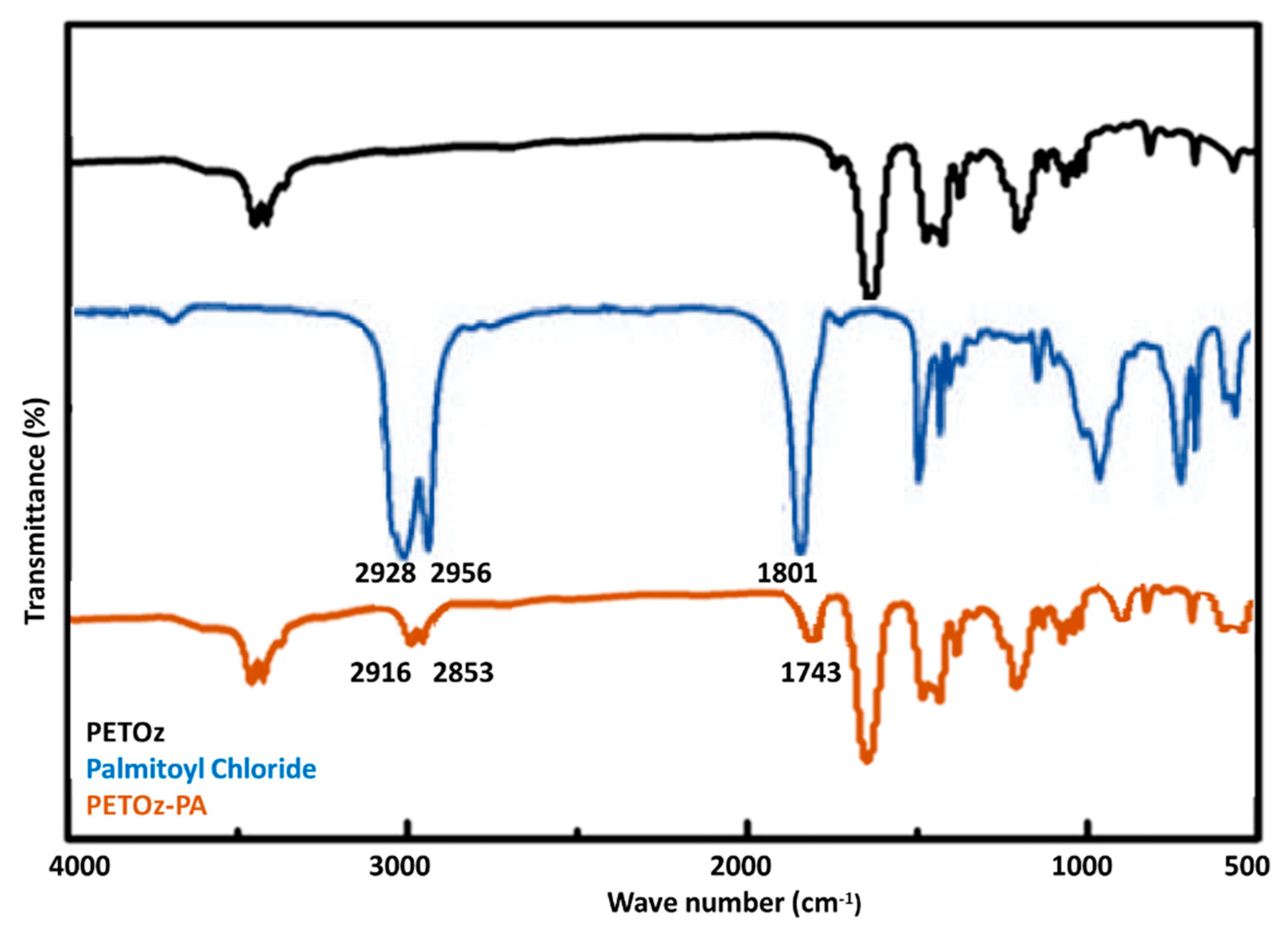
3.1. Synthesis and Characterization of PEtOz-PA
3.2. Particle Size, Zeta Potential, and Stability of PetOzPA (C) (1:20; 1:10, and 1:5)
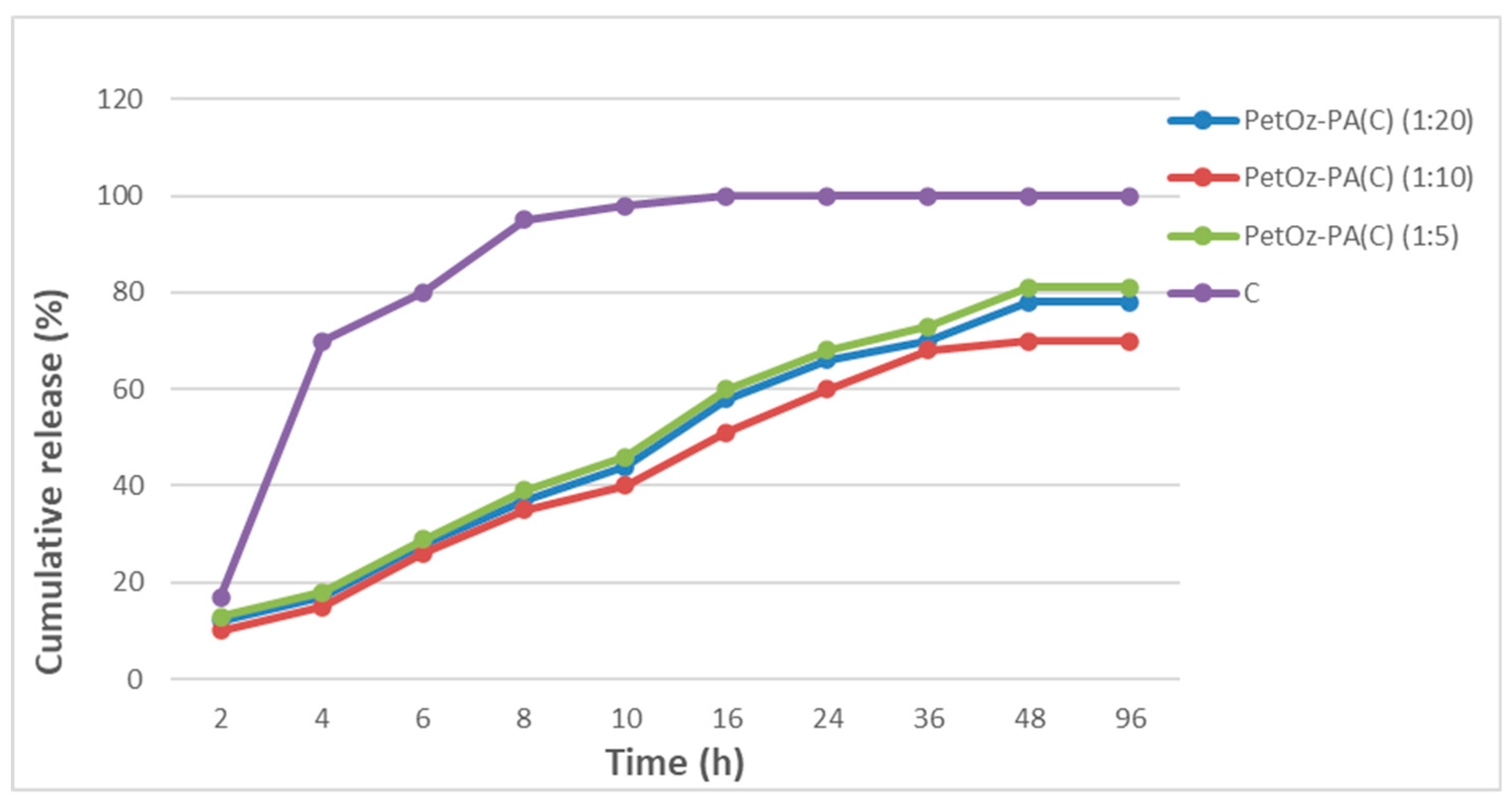
3.3. Entrapment Efficiencies, Drug Loading, and In Vitro Release of Curcumin
3.4. Critical Micelle Concentration (CMC)
3.5. DPPH Scavenging Activity
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Poly(2-ethyl-2-oxazoline) Palmitoyl ester (PEtOz-PA)
4.3. Preparation of PEtOz-PA and PEtOz-PA(C) Polymeric Micelles
4.4. Physicochemical Characterization of Curcumin-Loaded Polymeric Micelles
- [C]E is the concentration of curcumin detected spectroscopically within the nanocarriers after 0.22 µm filtration to remove unencapsulated material, and
- [C]S is the concentration of curcumin originally added to the formulation.
- W1 = total weight of entrapped curcumin in PetOz-PA.
- W0 = total weight of solid lipid, surfactant and curcumin in PetOz-PA.
4.5. In Vitro Release of Curcumin
4.6. Critical Micelle Concentration
4.7. DPPH Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Hruby, M.; Filippov, S.K.; Panek, J.; Novakova, M.; Mackova, H.; Kucka, J.; Vetvicka, D.; Ulbrich, K. Polyoxazoline thermoresponsive micelles as radionuclide delivery systems. Macromol. Biosci. 2010, 10, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Wilson, P.; Chun Keb, P.; Davis, T.P.; Kempe, K. Poly(2-oxazoline)-based micro- and nanoparticles: A review. Eur. Polym. J. 2017, 88, 486–515. [Google Scholar] [CrossRef]
- Sedlacek, O.; Monnery, B.D.; Filippov, S.K.; Hoogenboom, R.; Hruby, M. Poly(2-Oxazoline)s—Are They More Advantageous for Biomedical Applications Than Other Polymers? Macromol. Rapid Commun. 2012, 33, 1648–1662. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, H.; Zhou, L.; Hu, F.; Xie, S.; Jiang, L. Novel poly(2-oxazoline)s with pendant l-prolinamide moieties as efficient organocatalysts for direct asymmetric aldol reaction. Catal. Sci. Technol. 2016, 6, 6739–6749. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H.; Bailon, P.; Won, C.Y. PEG-modified biopharmaceuticals. Expert Opin. Drug Deliv. 2009, 6, 1. [Google Scholar]
- Mittal, K.L. Micellization, Solubilization, and Microemulsions; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Hiemenz, P.C. Principles of Colloid and Surface Chemistry; Marcell Dekker, Inc.: New York, NY, USA, 1986. [Google Scholar]
- Mero, A.; Pasut, G.; Via, L.D.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. nthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to PEG-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef]
- Woodle, M.C.; Engbers, C.M.; Zalipsky, S. New Amphipatic Polymer-Lipid Conjugates Forming Long-Circulating Reticuloendothelial System-Evading Liposomes. Bioconjugate Chem. 1994, 5, 493–496. [Google Scholar] [CrossRef]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of Blood Clearance Rates and Biodistribution of Poly(2-oxazoline)-Grafted Liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef]
- Nuyken, O.; Persigehl, P.; Weberskirch, R. Amphiphilic poly(oxazoline)s—Synthesis and application for micellar catalysis. Macromol. Symp. 2002, 177, 163–174. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, Y.; Lu, J.; Pinon, V.; Weck, M. Shell Cross-Linked Micelle-Based Nanoreactors for the Substrate-Selective Hydrolytic Kinetic Resolution of Epoxides. J. Am. Chem. Soc. 2011, 133, 14260–14263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pinon, V.; Weck, M. Poly(norbornene) block copolymer -based shell cross-linked micelles with Co(III)—salen cores. Polym. Chem. 2011, 2, 1964–1975. [Google Scholar] [CrossRef]
- Reiss-Husson, F.; Luzzati, V. The Structure of the Micellar Solutions of Some Amphiphilic Compounds in Pure Water as Determined by Absolute Small-Angle X-Ray Scattering Techniques. J. Phys. Chem. 1964, 68, 3504–3511. [Google Scholar] [CrossRef]
- Young, C.Y.; Missel, P.J.; Mazer, N.A.; Benedek, G.B.; Carey, M.C. Deduction of micellar shape from angular dissymmetry measurements of light scattered from aqueous sodium dodecyl-sulfate solutions at high sodium-chloride concentrations. J. Phys. Chem. 1978, 82, 1375–1378. [Google Scholar] [CrossRef]
- Rohde, A.; Sackmann, E. Quasielastic light-scattering studies of micellar sodium dodecyl sulfate solutions at the low concentration limit. J. Colloid Interface Sci. 1979, 70, 494–505. [Google Scholar] [CrossRef]
- Missel, P.J.; Mazer, N.A.; Benedek, G.B.; Young, C.Y.; Carey, M.C. Thermodynamic analysis of the growth of sodium dodecyl sulfate micelles. J. Phys. Chem. 1980, 84, 1044–1057. [Google Scholar] [CrossRef]
- Wiesbrock, F.; Hoogenboom, R.; Leenen, M.A.M.; Meier, M.A.R.; Schubert, U.S. Microwave-Assisted Synthesis of a 42-Membered Library of Diblock Copoly(2-oxazoline)s and Chain-Extended Homo Poly(2-oxazoline)s and Their Thermal Characterization. Macromolecules 2005, 38, 5025–5034. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Paonessa, R.; Nardi, M.; Procopio, A. Catalyst-free tosylation of lipophilic alcohols in water. RSC Adv. 2013, 3, 2548–2552. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. A Bifuctional Heterogeneous Catalyst Erbium-Based: A Cooperative Route Towards C-C Bond Formation. Molecules 2014, 19, 10218–10229. [Google Scholar] [CrossRef] [Green Version]
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A Mesoporous Er(III)-MCM-41 Catalyst for the Cyanosilylation of Aldehydes and Ketones under Solvent-free Conditions. ChemSusChem 2008, 1, 916–919. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Olivito, F.; Costanzo, P.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. Efficient synthesis of organic thioacetate in water. Org. Biomol. Chem. 2018, 16, 7753–7759. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable Deep Eutectic Solvent for Selective and Scalable Conversion of Furfural into Cyclopentenone Derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Hosseini, A.; Hosseinzadeh, H. Antidotal or protective effects of Curcuma longa (turmeric) and its active ingredient, curcumin, against natural and chemical toxicities: A review. Biomed. Pharmacother. 2018, 99, 411–421. [Google Scholar] [CrossRef]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The spicy modulator of breast carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- dos Santos, S.; Edronho, B.; Santos, T.; Antunes, F.E. Amphiphilic molecules in drug delivery systems. In Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalised Treatment; Coelho, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–85. [Google Scholar]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly (DL-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–9866. [Google Scholar] [CrossRef] [Green Version]
- Gülçın, İ.; Oktay, M.; Kıreçcı, E.; Küfrevıoglu, Ö.İ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
| Storage Condition | PetOz-PA(C) (1:20) | PetOz-PA(C) (1:10) | PetOz-PA(C) (1:5) |
|---|---|---|---|
| Day 1 (Initial) | |||
| Particle size (nm) | 200.1 ± 1.350 | 171.7 ± 1.015 | 157.5 ± 1.328 |
| PDI | 0.080 ± 0.017 | 0.119 ± 0.017 | 0.145 ± 0.014 |
| Zeta potential (mV) | −3.79 ± 0.361 | −5.31 ± 3.64 | −13.1 ± 5.26 |
| Day 30 at 4 °C | |||
| Particle size (nm) | 225.3 ± 1.210 | 185.7 ± 1.051 | 173.1 ± 1.218 |
| PDI | 0.110 ± 0.012 | 0.123 ± 0.011 | 0.151 ± 0.014 |
| Zeta potential (mV) | −3.38 ± 0.121 | −5.01 ± 3.12 | −13.01 ± 4.26 |
| Day 30 at 25 °C | |||
| Particle size (nm) | 425.3 ± 1.110 | 377.7 ± 1.111 | 371.1 ± 1.254 |
| PDI | 0.180 ± 0.012 | 0.123 ± 0.011 | 0.221 ± 0.014 |
| Zeta potential (mV) | −3.34 ± 0.154 | −5.71 ± 3.12 | −13.01 ± 4.26 |
| Day 60 at 4 °C | |||
| Particle size (nm) | 227.1 ± 1.050 | 187.7 ± 1.017 | 177.5 ± 1.328 |
| PDI | 0.098 ± 0.017 | 0.117 ± 0.043 | 0.145 ± 0.014 |
| Zeta potential (mV) | −3.39 ± 0.121 | −4.81 ± 2.14 | −12.89 ± 3.16 |
| Day 60 at 25 °C | |||
| Particle size (nm) | 453.2 ± 1.016 | 388.5 ± 1.034 | 388.1 ± 1.126 |
| PDI | 0.188 ± 0.017 | 0.117 ± 0.043 | 0.225 ± 0.011 |
| Zeta potential (mV) | −3.99 ± 0.761 | −4.98 ± 2.54 | −12.89 ± 3.16 |
| Entrapment Efficiency (Mean %, ±SD) | Drug Loading (Mean %, ±SD) | |
|---|---|---|
| PetOz-PA(C) (1:20) | 83.6 ± 0.43 | 3.8 ± 0.03 |
| PetOz-PA(C) (1:10) | 96.6 ± 0.15 | 4.6 ± 0.04 |
| PetOz-PA(C) (1:5) | 68.5 ± 021 | 5.9 ± 0.03 |
| Entry | Compound | IC50 ± SDa (µM) |
|---|---|---|
| 1 | PetOz-PA(C) (1:20) | 78.39 ± 1.312 |
| 2 | PetOz-PA(C) (1:10) | 49.493 ± 1.404 |
| 3 | PetOz-PA(C) (1:5) | 41.611 ± 0.303 |
| 4 | C | 3.827 ± 0.055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardi, M.; Sarubbi, E.; Somavarapu, S. Eco-Friendly Synthesis of PEtOz-PA: A Promising Polymer for the Formulation of Curcumin-Loaded Micelles. Molecules 2022, 27, 3788. https://doi.org/10.3390/molecules27123788
Nardi M, Sarubbi E, Somavarapu S. Eco-Friendly Synthesis of PEtOz-PA: A Promising Polymer for the Formulation of Curcumin-Loaded Micelles. Molecules. 2022; 27(12):3788. https://doi.org/10.3390/molecules27123788
Chicago/Turabian StyleNardi, Monica, Emiliana Sarubbi, and Satyanarayana Somavarapu. 2022. "Eco-Friendly Synthesis of PEtOz-PA: A Promising Polymer for the Formulation of Curcumin-Loaded Micelles" Molecules 27, no. 12: 3788. https://doi.org/10.3390/molecules27123788
APA StyleNardi, M., Sarubbi, E., & Somavarapu, S. (2022). Eco-Friendly Synthesis of PEtOz-PA: A Promising Polymer for the Formulation of Curcumin-Loaded Micelles. Molecules, 27(12), 3788. https://doi.org/10.3390/molecules27123788








