Effects of Anthraquinones on Immune Responses and Inflammatory Diseases
Abstract
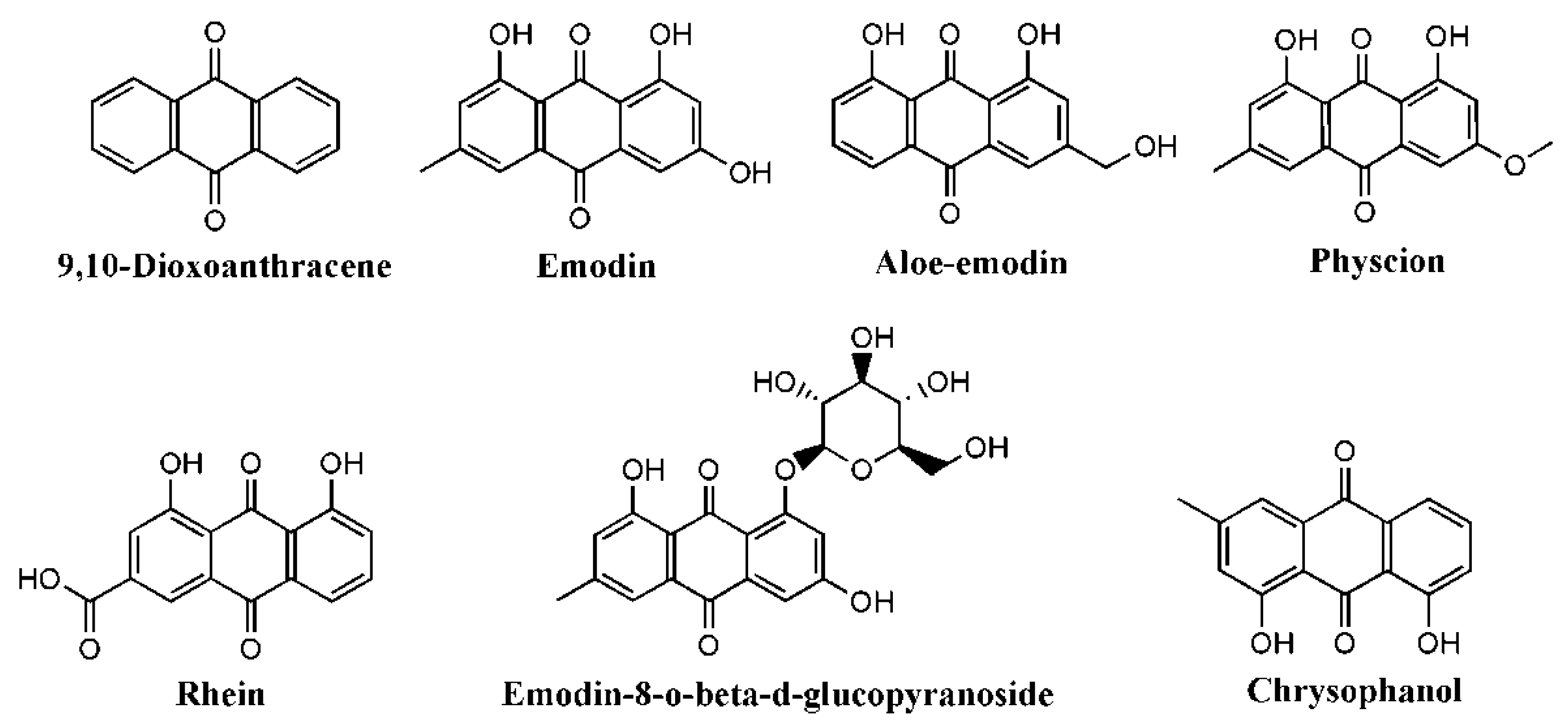
:1. Introduction
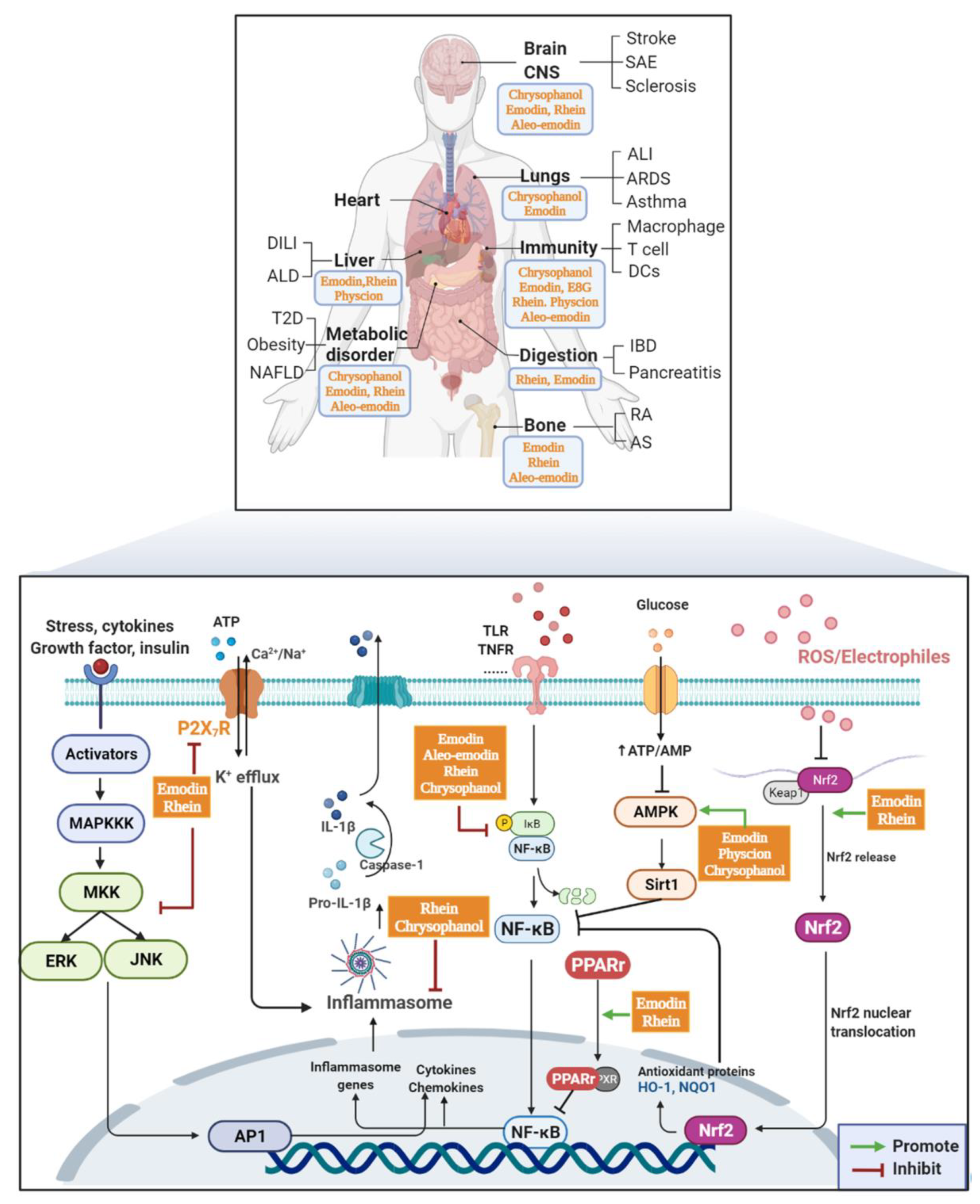
2. AQs Effects
2.1. Immune System
2.2. Digestive Diseases
2.2.1. IBDs
2.2.2. Pancreatitis
2.3. Inflammatory Diseases of the Respiratory System
2.4. Bone Tissue
2.5. Metabolic Disease
2.6. Cerebral Vascular System and Central Nervous System
2.7. Liver
2.8. Kidney
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Rhein | DILI | MTX | Wistar rats | ig. 20, 50 and 100 mg/kg | ALT, AST, morphological damage↓ | NF-κB↓ Nrf2-HO1↑ | [146] |
| Rhein | In vitro | MTX | L02 | 5, 10, 20 μM | TNFα, caspase-3↓ | ||
| Rhein | In vitro | Uric acid | TCMK-1 | 10, 20 and 40 μg/mL | TNFα, IL-1β, and IL-6↓ Apoptosis↓ | miR-150-5p/STAT1 | [156] |
| Emodin | DILI | APAP | C57B/6 mice | ig. 10, 30 mg/kg | ALT, AST↓, tissue damage↓ Antioxidant enzyme↑ | AMPK-Hippo/Yap↑ | [148] |
| Emodin | In vitro | APAP | HepG2 | 3–30 μM | Cell death↓, ROS↓, Mitochondrial dysfunction↓ | ||
| Emodin | In vitro | Glucose | SV40-MES13 | 20, 40 μm | MDA, ROS↓, SOD↑ TNFα, IL-1β, IL-6↓ FN, collogen I↓ | Circ_0000064/miR-30c-5p/Lmp7 | [153] |
| Emodin | In vitro | LPS | NRK-52E | 20, 40 μm | TNFα, IL-1β, and IL-6↓ | TLR2-NF-κB | [154] |
| Physcion | ALD | Ethanol | C57BL/6 | ig. 250, 500 μg kg | Fat vacuole accumulation NLRP3 inflammasome↓ | BMAL1 AMPK/PPARα | [150] |
| Physcion | In vitro | Ethanol | HepG2 | 0.125, 0.25 μm | IL-1β and Caspase-1↓ Lipid Accumulation↓ |
3. Discussion and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADRs | adverse drug reactions |
| AKI | acute kidney injury |
| ALD | alcoholic liver disease |
| ALI | acute lung injury |
| AMPK | AMP-activated protein kinase |
| AP | acute pancreatitis |
| APAP | acetaminophen |
| APCs | antigen-presenting cells |
| AQs | anthraquinones |
| ARDS | acute respiratory distress syndrome |
| Arg-1 | arginase-1 |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| cAMP | cyclic adenosine monophosphate |
| CCL17 | C-C chemokine ligand 17 |
| CIA | collagen-induced arthritis |
| CKD | chronic kidney disease |
| COPD | chronic obstructive pulmonary disease |
| DCs | dendritic cells |
| DEP | diesel exhaust particles |
| DILI | drug-induced liver injury |
| DM | diabetes mellitus |
| DN | diabetic nephropathy |
| DSS | dextran sodium sulfate |
| EAE | experimental autoimmune encephalomyelitis |
| ECM | extracellular matrix |
| EMT | epithelial-Mesenchymal Transition |
| GSIS | glucose-stimulated insulin secretion |
| GSK3β | glycogen synthase kinase-3beta |
| HDAC | histone deacetylase |
| HFD | high-fat diet |
| HMGB1 | high mobility group box-1 |
| IBDs | inflammatory bowel diseases |
| ICAM-1 | intercellular adhesion molecule 1 |
| IL | interleukin |
| IκB | inhibitor of NF-κB |
| JNK | c-Jun N-terminal kinase |
| LC3 | light chain 3 |
| Lmp7 | large multifunctional protease 7 |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinases |
| MCAO | middle cerebral artery occlusion |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MHC II | major histocompatibility complex class II |
| MLCK | myosin light chain kinase |
| MMPs | matrix metalloproteinases |
| mTOR | mechanistic target of rapamycin |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NF-κB | nuclear factor kappa B |
| NIK | NF-κB-inducing kinase |
| NLRP3 | NACHT, LRR, and PYD domains-containing protein 3 |
| OA | osteoarthritis |
| OGD/R | oxygen and glucose deprivation reperfusion |
| OVA | ovalbumin |
| PGE2 | prostaglandin E2 |
| PI3K | phosphoinositide 3-kinase |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| RA | rheumatoid arthritis |
| ROCK | Rho-associated coiled-coil containing kinases |
| ROS | reactive oxygen species |
| SAE | sepsis-associated encephalopathy |
| SAP | severe acute pancreatitis |
| SOD | superoxide dismutase |
| T2D | type 2 diabetes |
| TGFβ | transforming growth factor-beta |
| Th | T helper |
| TLRs | Toll-like receptors |
| tMCAO | transient middle cerebral artery occlusion |
| TNFα | Tumour necrosis factor alpha |
| Tr1 | type 1 Regulatory T |
| TrkB | tropomysin related kinase B |
| UC | ulcerative colitis |
| VCAM1 | vascular cell adhesion molecule 1 |
| VDAC1 | voltage-dependent anion channel 1 |
| ZO-1 | Zona occludens-1 |
| α-SMA | α-smooth muscle actin |
References
- Malik, E.M.; Müller, C.E. Anthraquinones as Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Hulst, M.B.; Grocholski, T.; Neefjes, J.J.C.; van Wezel, G.P.; Metsä-Ketelä, M. Anthracyclines: Biosynthesis, engineering and clinical applications. Nat. Prod. Rep. 2022, 39, 814–841. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, J.-G. Health functions and structure–activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Zhuang, T.; Gu, X.; Zhou, N.; Ding, L.; Yang, L.; Zhou, M. Hepatoprotection and hepatotoxicity of Chinese herb Rhubarb (Dahuang): How to properly control the “General (Jiang Jun)” in Chinese medical herb. Biomed. Pharmacother. 2020, 127, 110224. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, Q.; Wu, F.; Liu, S. Research progress on chemical constituents and pharmacological effects of medicinal plants in genus rheum. Chin. J. New Drugs 2022, 31, 555–566. [Google Scholar]
- Feehan, K.T.; Gilroy, D.W. Is Resolution the End of Inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Li, D.; Hou, H. Novel anthraquinone compounds as anticancer agents and their potential mechanism. Futur. Med. Chem. 2020, 12, 627–644. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, X. Epigenetic Remodeling in Innate Immunity and Inflammation. Annu. Rev. Immunol. 2021, 39, 279–311. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Vannella, K.M.; Wynn, T.A. Mechanisms of Organ Injury and Repair by Macrophages. Annu. Rev. Physiol. 2017, 79, 593–617. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147. [Google Scholar] [CrossRef] [Green Version]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Wei, Y.; Wei, X. Epigenetic regulation of macrophages: From homeostasis maintenance to host defense. Cell. Mol. Immunol. 2020, 17, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, W.; Hao, D.; He, B.; Kong, L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019, 70, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. NF-κB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Zhang, H.; Meng, X.; Wang, F.; Wang, P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. J. Ethnopharmacol. 2014, 153, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bobiński, R.; Dutka, M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm. Res. 2019, 68, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- de Souza Basso, B.; Haute, G.V.; Ortega-Ribera, M.; Luft, C.; Antunes, G.L.; Bastos, M.S.; Carlessi, L.P.; Levorse, V.G.; Cassel, E.; Donadio, M.V.F.; et al. Methoxyeugenol deactivates hepatic stellate cells and attenuates liver fibrosis and inflammation through a PPAR-ɣ and NF-kB mechanism. J. Ethnopharmacol. 2021, 280, 114433. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, W.; Feng, S.J.; Yu, H.P. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int. Immunopharmacol. 2016, 34, 16–24. [Google Scholar] [CrossRef]
- Wen, Q.; Miao, J.; Lau, N.; Zhang, C.; Ye, P.; Du, S.; Mei, L.; Weng, H.; Xu, Q.; Liu, X.; et al. Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264.7 cells: Targeting the PPAR-γ signal pathway. Can. J. Physiol. Pharmacol. 2020, 98, 357–365. [Google Scholar] [CrossRef]
- Wen, Q.; Mei, L.; Ye, S.; Liu, X.; Xu, Q.; Miao, J.; Du, S.; Chen, D.; Li, C.; Li, H. Chrysophanol demonstrates anti-inflammatory properties in LPS-primed RAW 264.7 macrophages through activating PPAR-γ. Int. Immunopharmacol. 2018, 56, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, Y.; Wen, C.; Wang, S.; Ruan, Q.; Li, Z.; Xu, J.; Xu, Z.; Deng, J. Anti-Inflammatory Efficacy of Fabricated Rhein Micelles. J. Biomed. Nanotechnol. 2020, 16, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; Gu, Y.; Shen, W.; Zhang, L.; Qian, F.; Zhao, Y.; Li, H.; Zhang, T.; Lu, W. Rheinic acid ameliorates radiation-induced acute enteritis in rats through PPAR-γ/NF-κB. Genes Genom. 2019, 41, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa-Ishimoto, Y.; Hwang, S.; Cadwell, K. Autophagy and Inflammation. Annu. Rev. Immunol. 2018, 36, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Lu, J.-H. Autophagy and Macrophage Functions: Inflammatory Response and Phagocytosis. Cells 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.J.; Tan, B.; Jiang, L.; Wu, Z.H.; Yu, H.J.; Li, X.Q.; Yang, A.D. Emodin Inhibits Lipopolysaccharide-Induced Inflammation by Activating Autophagy in RAW 264.7 Cells. Chin. J. Integr. Med. 2021, 27, 345–352. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Tang, H.; Liang, Y.; Wu, J.; Yang, Q.; Zeng, L.; Ma, Z. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des. Dev. Therm. 2017, 11, 1663–1671. [Google Scholar] [CrossRef] [Green Version]
- Markwardt, F. Human P2X7 receptors—Properties of single ATP-gated ion channels. Biochem. Pharmacol. 2020, 187, 114307. [Google Scholar] [CrossRef]
- Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2021, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xing, F.; Zhu, G.; Xu, G.; Li, C.; Qu, J.; Lee, I.; Pan, L. Rhein antagonizes P2X7 receptor in rat peritoneal macrophages. Sci. Rep. 2015, 5, 14012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Wang, Y.; Wang, X.; Li, J.; Hu, F. Emodin inhibits ATP-induced IL-1β secretion, ROS production and phagocytosis attenuation in rat peritoneal macrophages via antagonizing P2X₇ receptor. Pharm. Biol. 2014, 52, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, X.; Fang, L.; Liu, F.; Cai, R.; Peng, C.; Qi, Y. Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages. Free Radic. Biol. Med. 2014, 72, 104–112. [Google Scholar] [CrossRef]
- Iwanowycz, S.; Wang, J.; Altomare, D.; Hui, Y.; Fan, D. Emodin Bidirectionally Modulates Macrophage Polarization and Epigenetically Regulates Macrophage Memory. J. Biol. Chem. 2016, 291, 11491–11503. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, H.J.; Nguyen, T.T.H.; Kim, S.C.; Ree, J.; Choi, T.G.; Sohng, J.K.; Park, Y.I. Emodin 8-O-glucoside primes macrophages more strongly than emodin aglycone via activation of phagocytic activity and TLR-2/MAPK/NF-κB signalling pathway. Int. Immunopharmacol. 2020, 88, 106936. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Kim, S.-J.; Yee, S.-T. Physcion-Matured Dendritic Cells Induce the Differentiation of Th1 Cells. Int. J. Mol. Sci. 2020, 21, 1753. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Ye, Z.; Li, N.; Jin, F.; Yan, J.; Wu, K. Effect of emodin on T cell subsets in NOD mice with NaI-induced experimental autoimmune thyroiditis. Mol. Med. Rep. 2018, 18, 4303–4312. [Google Scholar] [CrossRef] [Green Version]
- Qu, K.; Shen, N.Y.; Xu, X.S.; Su, H.B.; Wei, J.C.; Tai, M.H.; Meng, F.D.; Zhou, L.; Zhang, Y.L.; Liu, C. Emodin induces human T cell apoptosis in vitro by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacol. Sin. 2013, 34, 1217–1228. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.-F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Shale, M.; Schiering, C.; Powrie, F. CD4(+) T-cell subsets in intestinal inflammation. Immunol. Rev. 2013, 252, 164–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoytema van Konijnenburg, D.P.; Reis, B.S.; Pedicord, V.A.; Farache, J.; Victora, G.D.; Mucida, D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 2017, 171, 783–794.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tang, B.; Wang, F.C.; Tang, L.; Lei, Y.Y.; Luo, Y.; Huang, S.J.; Yang, M.; Wu, L.Y.; Wang, W.; et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225–5241. [Google Scholar] [CrossRef]
- Yan, J.B.; Luo, M.M.; Chen, Z.Y.; He, B.H. The Function and Role of the Th17/Treg Cell Balance in Inflammatory Bowel Disease. J. Immunol. Res. 2020, 2020, 8813558. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhong, J.; Bian, Y.; Fan, Y.; Chen, Q.; Liu, P.; Liu, Z. Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs. Life Sci. 2019, 216, 168–175. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhong, J.; Bian, Y.; Fan, Y.; Chen, Q.; Liu, P.; Liu, Z. Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int. Immunopharmacol. 2019, 71, 321–327. [Google Scholar] [CrossRef]
- Strati, F.; Pujolassos, M.; Burrello, C.; Giuffrè, M.R.; Lattanzi, G.; Caprioli, F.; Troisi, J.; Facciotti, F. Antibiotic-associated dysbiosis affects the ability of the gut microbiota to control intestinal inflammation upon fecal microbiota transplantation in experimental colitis models. Microbiome 2021, 9, 39. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated with Response to Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, W.; Bousquenaud, M.; Cattin, S.; Zaric, J.; Sun, L.K.; Rüegg, C. Emodin Inhibits Inflammation, Carcinogenesis, and Cancer Progression in the AOM/DSS Model of Colitis-Associated Intestinal Tumorigenesis. Front. Oncol. 2020, 10, 564674. [Google Scholar] [CrossRef]
- Chen, Y.K.; Xu, Y.K.; Zhang, H.; Yin, J.T.; Fan, X.; Liu, D.D.; Fu, H.Y.; Wan, B. Emodin alleviates jejunum injury in rats with sepsis by inhibiting inflammation response. Biomed. Pharmacother. 2016, 84, 1001–1007. [Google Scholar] [CrossRef]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef]
- Garg, P.K.; Singh, V.P. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology 2019, 156, 2008–2023. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Cirillo, C.; Capasso, R. Constipation and Botanical Medicines: An Overview. Phytother. Res. 2015, 29, 1488–1493. [Google Scholar] [CrossRef]
- Cao, Y.J.; Pu, Z.J.; Tang, Y.P.; Shen, J.; Chen, Y.Y.; Kang, A.; Zhou, G.S.; Duan, J.A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Li, P.; Zhang, T. Rhubarb combined with trypsin inhibitor for severe acute pancreatitis: A systematic review and meta-analysis. Phytother. Res. 2018, 32, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Huang, X.; Li, H.; Xiong, Y. Add-on effect of crude rhubarb to somatostatin for acute pancreatitis: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2016, 194, 495–505. [Google Scholar] [CrossRef]
- Chen, X.; Yang, K.; Jing, G.; Yang, J.; Li, K. Meta-Analysis of Efficacy of Rhubarb Combined with Early Enteral Nutrition for the Treatment of Severe Acute Pancreatitis. JPEN J. Parenter. Enter. Nutr. 2020, 44, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Xiong, W.; Liu, L.; Xiang, J.; Tang, M.; Yuan, Z. Protective Effects of Rhubarb in Rats with Acute Pancreatitis and the Role of Its Active Compound Rhein on Mitochondria of Exocrine Cells. Evid.-Based Complement. Altern. Med. 2018, 2018, 7321352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Wang, J.Q.; Wu, L.; Zhang, F.; Chen, Z.P.; Li, W.D.; Cai, H.; Liu, X. Emodin attenuates cell injury and inflammation in pancreatic acinar AR42J cells. J. Asian Nat. Prod. Res. 2019, 21, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ni, Y.; Zhou, Q.; Liu, H.; Xiang, H.; Sui, H.; Shang, D. Emodin Attenuates Severe Acute Pancreatitis via Antioxidant and Anti-inflammatory Activity. Inflammation 2019, 42, 2129–2138. [Google Scholar] [CrossRef]
- Zhang, Q.; Tao, X.; Xia, S.; Qu, J.; Song, H.; Liu, J.; Li, H.; Shang, D. Emodin attenuated severe acute pancreatitis via the P2X ligand-gated ion channel 7/NOD-like receptor protein 3 signaling pathway. Oncol. Rep. 2019, 41, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, L.; Fan, L.; Wang, L.; Zhou, Y.; Qin, D.; Sun, Q.; Wu, J.; Cao, S. Free Total Rhubarb Anthraquinones Protect Intestinal Injury via Regulation of the Intestinal Immune Response in a Rat Model of Severe Acute Pancreatitis. Front. Pharmacol. 2018, 9, 75. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Wu, H.Y.; Xia, J.; Zhang, H.B.; Liu, M.W.; Qian, C.Y. Effects of emodin on intestinal mucosal barrier by the upregulation of miR-218a-5p expression in rats with acute necrotizing pancreatitis. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420941765. [Google Scholar] [CrossRef]
- Banno, A.; Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. Bidirectional interaction of airway epithelial remodeling and inflammation in asthma. Clin. Sci. 2020, 134, 1063–1079. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.P.; Park, S.Y.; Moon, K.A.; Ha, E.H.; Woo, Y.D.; Chung, D.H.; Kwon, H.S.; Kim, T.B.; Park, H.S.; Moon, H.B.; et al. Macrophage-derived progranulin promotes allergen-induced airway inflammation. Allergy 2020, 75, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Steelant, B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020, 145, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Li, X.; Shan, C.; Wu, Z.; Yu, H.; Yang, A.; Tan, B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm. Res. 2020, 69, 365–373. [Google Scholar] [CrossRef]
- Liu, B.; Cheng, Y.; Wu, Y.; Zheng, X.; Li, X.; Yang, G.; He, T.; Li, S.; Shen, F. Emodin improves alveolar hypercoagulation and inhibits pulmonary inflammation in LPS-provoked ARDS in mice via NF-κB inactivation. Int. Immunopharmacol. 2020, 88, 107020. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respir. Physiol. Neurobiol. 2015, 215, 51–57. [Google Scholar] [CrossRef]
- Wen, Q.; Lau, N.; Weng, H.; Ye, P.; Du, S.; Li, C.; Lv, J.; Li, H. Chrysophanol Exerts Anti-inflammatory Activity by Targeting Histone Deacetylase 3 Through the High Mobility Group Protein 1-Nuclear Transcription Factor-Kappa B Signaling Pathway in vivo and in vitro. Front. Bioeng. Biotechnol. 2020, 8, 623866. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Shrestha Palikhe, N.; Wu, Y.; Konrad, E.; Gandhi, V.D.; Rowe, B.H.; Vliagoftis, H.; Cameron, L. Th2 cell markers in peripheral blood increase during an acute asthma exacerbation. Allergy 2021, 76, 281–290. [Google Scholar] [CrossRef]
- Simpson, J.L.; Grissell, T.V.; Douwes, J.; Scott, R.J.; Boyle, M.J.; Gibson, P.G. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax 2007, 62, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1β-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Hua, S.; Liu, F.; Wang, M. Emodin Alleviates the Airway Inflammation of Cough Variant Asthma in Mice by Regulating the Notch Pathway. Med. Sci. Monit. 2019, 25, 5621–5629. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, X.G.; Li, Y.H.; Jia, X.; Zhang, S.J.; Gao, Y.S.; Liu, M.; Wu, R.H. Protective effect of emodin against airway inflammation in the ovalbumin-induced mouse model. Chin. J. Integr. Med. 2015, 21, 431–437. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Yu, S.; Lv, W.; Guan, Z.; Sun, M.; Wang, J. Chrysophanol attenuates airway inflammation and remodeling through nuclear factor-kappa B signaling pathway in asthma. Phytother. Res. 2019, 33, 2702–2713. [Google Scholar] [CrossRef]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Takagaki, Y.; Lee, S.M.; Dongqing, Z.; Kitada, M.; Kanasaki, K.; Koya, D. Endothelial autophagy deficiency induces IL6-dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 2020, 16, 1905–1914. [Google Scholar] [CrossRef]
- Peng, L.; Wen, L.; Shi, Q.F.; Gao, F.; Huang, B.; Meng, J.; Hu, C.P.; Wang, C.M. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020, 11, 978. [Google Scholar] [CrossRef]
- Tian, S.-L.; Yang, Y.; Liu, X.-L.; Xu, Q.-B. Emodin Attenuates Bleomycin-Induced Pulmonary Fibrosis via Anti-Inflammatory and Anti-Oxidative Activities in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Shao, L.; Nie, X.; Yan, H.; Li, C.; Yeo, A.J.; Lavin, M.F.; Xia, Q.; Shao, H.; Yu, G.; et al. Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int. Immunopharmacol. 2021, 91, 107277. [Google Scholar] [CrossRef]
- Spel, L.; Martinon, F. Inflammasomes contributing to inflammation in arthritis. Immunol. Rev. 2020, 294, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Chang, W.C.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Rhein, An Anthraquinone Drug, Suppresses the NLRP3 Inflammasome and Macrophage Activation in Urate Crystal-Induced Gouty Inflammation. Am. J. Chin. Med. 2019, 47, 135–151. [Google Scholar] [CrossRef]
- Hu, F.; Zhu, D.; Pei, W.; Lee, I.; Zhang, X.; Pan, L.; Xu, J. Rhein inhibits ATP-triggered inflammatory responses in rheumatoid rat fibroblast-like synoviocytes. Int. Immunopharmacol. 2019, 75, 105780. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Li, L.; Yang, S.; Du, G.; Lu, Y. Anti-inflammatory Effects and Mechanisms of Rhein, an Anthraquinone Compound, and Its Applications in Treating Arthritis: A Review. Nat. Prod. Bioprospect. 2020, 10, 445–452. [Google Scholar] [CrossRef]
- Almezgagi, M.; Zhang, Y.; Hezam, K.; Shamsan, E.; Gamah, M.; Al-Shaebi, F.; Abbas, A.B.; Shoaib, M.; Saif, B.; Han, Y.; et al. Diacerein: Recent insight into pharmacological activities and molecular pathways. Biomed. Pharmacother. 2020, 131, 110594. [Google Scholar] [CrossRef]
- Hwang, J.K.; Noh, E.M.; Moon, S.J.; Kim, J.M.; Kwon, K.B.; Park, B.H.; You, Y.O.; Hwang, B.M.; Kim, H.J.; Kim, B.S.; et al. Emodin suppresses inflammatory responses and joint destruction in collagen-induced arthritic mice. Rheumatology 2013, 52, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Ha, M.K.; Song, Y.H.; Jeong, S.J.; Lee, H.J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.E.; Kim, S.H. Emodin inhibits proinflammatory responses and inactivates histone deacetylase 1 in hypoxic rheumatoid synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Wen, B.; Zhang, Q.; Shao, P.P.; Gu, W.; Qu, K.; Shi, Y.; Wang, B. Emodin induces apoptosis and autophagy of fibroblasts obtained from patient with ankylosing spondylitis. Drug Des. Dev. Therm. 2019, 13, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Kshirsagar, A.D.; Panchal, P.V.; Harle, U.N.; Nanda, R.K.; Shaikh, H.M. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents. Int. J. Inflamm. 2014, 2014, 690596. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Ying, W.; Fu, W.; Lee, Y.S.; Olefsky, J.M. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat. Rev. Endocrinol. 2020, 16, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2020, 320, C375–C391. [Google Scholar] [CrossRef]
- Jia, X.; Iwanowycz, S.; Wang, J.; Saaoud, F.; Yu, F.; Wang, Y.; Hu, J.; Chatterjee, S.; Wang, Q.; Fan, D. Emodin attenuates systemic and liver inflammation in hyperlipidemic mice administrated with lipopolysaccharides. Exp. Biol. Med. 2014, 239, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Yu, N.; Peng, J.; Zhao, Y.; Zhang, L.; Wang, X.; Xu, X.; Zhou, J.; Wang, F. Emodin inhibits lipid accumulation and inflammation in adipose tissue of high-fat diet-fed mice by inducing M2 polarization of adipose tissue macrophages. FASEB J. 2021, 35, e21730. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, Y.; Fu, Y.; Wang, R.; Zhang, H.; Li, M.; Li, Z.; Zhang, Y.; Xuan, L.; Li, X.; et al. Emodin improves glucose metabolism by targeting microRNA-20b in insulin-resistant skeletal muscle. Phytomedicine 2019, 59, 152758. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Lv, P.; Yang, J.; Deng, Y.; Xu, J.; Zhu, R.; Zhang, D.; Yang, Y. Emodin up-regulates glucose metabolism; decreases lipolysis, and attenuates inflammation in vitro. J. Diabetes 2015, 7, 360–368. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, H.; Wang, L.; Zhao, X. Chrysophanol ameliorates high-fat diet-induced obesity and inflammation in neonatal rats. Pharmazie 2018, 73, 228–233. [Google Scholar]
- Chen, Y.; Feng, B.; Yuan, Y.; Hu, J.; Zhao, W.; Jiang, H.; Li, W.; Fan, Z.; Du, Z. Aloe Emodin Reduces Cardiac Inflammation Induced by a High-Fat Diet through the TLR4 Signaling Pathway. Mediat. Inflamm. 2020, 2020, 6318520. [Google Scholar] [CrossRef]
- Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Aliyu, A.B.; Isah, M.B. Antidiabetic potential of anthraquinones: A review. Phytother. Res. 2020, 34, 486–504. [Google Scholar] [CrossRef]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune responses to stroke: Mechanisms, modulation, and therapeutic potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.N.; Parry-Jones, A.R.; Allan, S.M. Interleukin-1 and acute brain injury. Front. Cell. Neurosci. 2015, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, X.; Liu, X.; Wang, H.; Xue, J.; Yu, J.; Kang, N.; Wang, X. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. Mediat. Inflamm. 2014, 2014, 370530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fang, Y.; Li, J.; Duan, Y.; Zhao, H.; Gao, L.; Luo, Y. Neuroprotective effects of Chrysophanol against inflammation in middle cerebral artery occlusion mice. Neurosci. Lett. 2016, 630, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity; oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Xian, M.; Cai, J.; Zheng, K.; Liu, Q.; Liu, Y.; Lin, H.; Liang, S.; Wang, S. Aloe-emodin prevents nerve injury and neuroinflammation caused by ischemic stroke via the PI3K/AKT/mTOR and NF-κB pathway. Food Funct. 2021, 12, 8056–8067. [Google Scholar] [CrossRef]
- Li, R.; Liu, W.; Ou, L.; Gao, F.; Li, M.; Wang, L.; Wei, P.; Miao, F. Emodin Alleviates Hydrogen Peroxide-Induced Inflammation and Oxidative Stress via Mitochondrial Dysfunction by Inhibiting the PI3K/mTOR/GSK3β Pathway in Neuroblastoma SH-SY5Y Cells. BioMed Res. Int. 2020, 2020, 1562915. [Google Scholar] [CrossRef]
- Ren, C.; Yao, R.Q.; Zhang, H.; Feng, Y.W.; Yao, Y.M. Sepsis-associated encephalopathy: A vicious cycle of immunosuppression. J. Neuroinflammation 2020, 17, 14. [Google Scholar] [CrossRef]
- Gao, L.L.; Wang, Z.H.; Mu, Y.H.; Liu, Z.L.; Pang, L. Emodin Promotes Autophagy and Prevents Apoptosis in Sepsis-Associated Encephalopathy through Activating BDNF/TrkB Signaling. Pathobiology 2021, 89, 135–145. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, G.; Zhang, L.; Tang, L. Neuroprotective effect of emodin on acute brain injury in sepsis mice. Med. J. Chin. People’s Lib. 2019, 44, 13–19. [Google Scholar]
- Olek, M.J. Multiple Sclerosis. Ann. Intern. Med. 2021, 174, ITC81–ITC96. [Google Scholar] [CrossRef]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold Spring Harb. Perspect. Med. 2018, 8, a028977. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Yuan, Y.; Wang, W. Effect of active components of rhubarb on expression of related gene protein and inflammatory factors in experimental autoimmune encephalomyelitis. Chin. J. Immunol. 2018, 34, 1501–1505. [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An update. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Surani, S.; Udeani, G.; Mathew, S.; John, S.; Sajan, S.; Mishra, J. Drug-induced liver injury and prospect of cytokine based therapy; A focus on IL-2 based therapies. Life Sci. 2021, 278, 119544. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef]
- Bu, T.; Wang, C.; Meng, Q.; Huo, X.; Sun, H.; Sun, P.; Zheng, S.; Ma, X.; Liu, Z.; Liu, K. Hepatoprotective effect of rhein against methotrexate-induced liver toxicity. Eur. J. Pharmacol. 2018, 834, 266–273. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Toxicity: Novel Insights Into Mechanisms and Future Perspectives. Gene Expr. 2018, 18, 19–30. [Google Scholar] [CrossRef]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Emodin in Rheum undulatum inhibits oxidative stress in the liver via AMPK with Hippo/Yap signalling pathway. Pharm. Biol. 2020, 58, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Yao, Y.; Zuo, A.; Deng, Q.; Liu, S.; Zhan, T.; Wang, M.; Xu, H.; Ma, J.; Zhao, Y. Physcion Protects Against Ethanol-Induced Liver Injury by Reprogramming of Circadian Clock. Front. Pharmacol. 2020, 11, 573074. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol. Therm. 2016, 12, S41–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 2017, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Han, Y.; Shen, C.; Luo, H.; Wang, Z. Emodin alleviates high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation of mesangial cells by the circ_0000064/miR-30c-5p/Lmp7 axis. J. Recept. Signal. Transduct. 2021, 42, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, W.; Yang, J.; Zhong, J.; Zhang, L.; Zheng, J.; Liu, H.; Zhang, Q.; Ouyang, X.; Lei, L.; et al. Attenuation of Inflammation by Emodin in Lipopolysaccharide-induced Acute Kidney Injury via Inhibition of Toll-like Receptor 2 Signal Pathway. Iran. J. Kidney Dis. 2015, 9, 202–208. [Google Scholar]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, Z.; Wu, H.; Wang, D. Rhein attenuates renal inflammatory injury of uric acid nephropathy via lincRNA-Cox2/miR-150–5p/STAT1 axis. Int. Immunopharmacol. 2020, 85, 106620. [Google Scholar] [CrossRef]
- Tu, C.; Niu, M.; Li, C.; Liu, Z.; He, Q.; Li, R.; Zhang, Y.; Xiao, X.; Wang, J. Network pharmacology oriented study reveals inflammatory state-dependent dietary supplement hepatotoxicity responses in normal and diseased rats. Food Funct. 2019, 10, 3477–3490. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Zuo, Z. Overview of Pharmacokinetics and Liver Toxicities of Radix Polygoni Multiflori. Toxins 2020, 12, 729. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, L.J.; Huang, T.; Ying, J.Q.; Li, J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 2020, 18, 425–435. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Qu, L.; He, Y.; Routledge, M.N.; Yun Gong, Y.; Qiao, B. Identification of rhein as the metabolite responsible for toxicity of rhubarb anthraquinones. Food Chem. 2020, 331, 127363. [Google Scholar] [CrossRef]
- Liu, Y.; Mapa, M.S.T.; Sprando, R.L. Liver toxicity of anthraquinones: A combined in vitro cytotoxicity and in silico reverse dosimetry evaluation. Food Chem. Toxicol. 2020, 140, 111313. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-R.; Liu, X.-F.; Feng, S.-X.; Shu, S.-N.; Wang, P.-Y.; Zhang, N.; Li, J.-S.; Qu, L.-B. Pharmacodynamics of Five Anthraquinones (Aloe-emodin, Emodin, Rhein, Chysophanol, and Physcion) and Reciprocal Pharmacokinetic Interaction in Rats with Cerebral Ischemia. Molecules 2019, 24, 1898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fu, Y.; Li, L.; Liu, Y.; Zhang, C.; Yu, D.; Ma, Y.; Xiao, Y. Pharmacokinetic comparisons of major bioactive components after oral administration of raw and steamed rhubarb by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2019, 171, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, S.X.; Zhang, H.J.; Zhang, N.; Liu, X.F.; Wan, Y.; Zhou, Y.X.; Li, J.S. Pharmacokinetics, tissue distribution and excretion of five rhubarb anthraquinones in rats after oral administration of effective fraction of anthraquinones from rheum officinale. Xenobiotica 2021, 51, 916–925. [Google Scholar] [CrossRef]
- Shia, C.-S.; Tsai, S.-Y.; Lin, J.-C.; Li, M.-L.; Ko, M.-H.; Chao, P.-D.L.; Huang, Y.-C.; Hou, Y.-C. Steady-state pharmacokinetics and tissue distribution of anthraquinones of Rhei Rhizoma in rats. J. Ethnopharmacol. 2011, 137, 1388–1394. [Google Scholar] [CrossRef]
- Li, P.; Lu, Q.; Jiang, W.; Pei, X.; Sun, Y.; Hao, H.; Hao, K. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed. Pharmacother. 2017, 91, 425–435. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Zhu, T.T.; Wang, X.L.; Qin, K.M.; Pei, K.; Cai, B.C. UHPLC-MS/MS method for the simultaneous quantitation of five anthraquinones and gallic acid in rat plasma after oral administration of prepared rhubarb decoction and its application to a pharmacokinetic study in normal and acute blood stasis rats. J. Sep. Sci. 2017, 40, 2382–2389. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Li, C.; Yang, N.; Yu, H.; Zhou, W.; Chen, S.; Yang, S.; Li, Y. Study on Hepatotoxicity of Rhubarb Based on Metabolomics and Network Pharmacology. Drug Des. Devel. Therm. 2021, 15, 1883–1902. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Huyiligeqi Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Tang, H.; Song, J.; Long, J.; Zhang, L.; Li, X. Chrysophanol: A review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 2019, 71, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Aloe-emodin | In vitro | LPS | RAW264.7 | 10–20 μM | NO, IL-6, and IL-1β↓ | NF-κB↓ | [25] |
| Emodin | In vitro | LPS | RAW264.7 | 25 μM | ICAM-1, MCP-1 and TNFα↓ | NF-κB↓; PPARγ↑ | [29] |
| Emodin | In vitro | LPS | RAW264.7 | 0–50 μM | TNFα IL-1β and IL-6↓ | NF-κB↓; LC3B II/I (autophagy)↑ | [37] |
| Emodin | In vitro | LPS/IL-4 | Primary macrophages | 0–50 μM | Phagocytosis↓; NO↓; Migration↓ | NF-κB/IRF5/STAT1↓(LPS) IRF4/STAT6↓(IL-4) | [45] |
| Emodin | In vitro | ATP | peritoneal macrophages | 0.1–10 μM | cytosolic Ca2+↓; phagocytosis↓ ROS, IL-1β↓ | P2X7↓ | [43] |
| Emodin | Autoimmune thyroiditis | NaI | non-obese diabetic mice | ig. 15, 75 or 150 mg/kg | serum TgAb↓ serum IFN-γ↓ | CD3+CD4+ T cell↓ | [48] |
| Emodin | In vitro | _ | Primary human T cells | 1–100 μM | Apoptosis↑; Ca2+ ROS, MDA↑; SOD↓ Caspase 3, 8, 9↑ | Endoplasmic reticulum stress↑ Mitochondrial dysfunction | [49] |
| Emodin 8-O-glucoside | In vitro | LPS | RAW264.7 THP-1 | 20 μM | TNFα, IL-6, NO↑ Phagocytosis↑ | TLR-2/MAPK/NF-κB ↑ | [46] |
| Rhein | In vitro | LPS | RAW264.7 | 60–140 μM | iNOS and TNFα↓ | NF-κB↓; PPARγ↑ | [30] |
| Rhein micelles | In vitro | LPS | RAW264.7 | 40 μM | iNOS, TNFα, IL-1β, and IL-6↓ COX-2, PGE2, NO↓ | NF-κB↓ | [32] |
| Rhein | In vitro | LPS | RAW264.7 | 0–35 μM | NO, IL-6↓ IL-1, HMGB1↑ | NF-κB↓ IKKβ↓, Caspase 1↑ | [44] |
| Rhein | In vitro | LPS + ATP | RAW264.7 | 1–20 μM | TNFα, IL-1β, and IL-6↓ COX-2↓ | NF-κB↓ NALP3 inflammasome↓ | [39] |
| Rhein | In vitro | ATP | peritoneal macrophages | 0–10 μM | cytosolic Ca2+↓; Phagocytosis↓ ROS, IL-1β↓ | P2X7↓ | [42] |
| Chrysophanol | In vitro | LPS | RAW264.7 | 15 μM | iNOS, TNFα and IL-1β↓ | NF-κB↓; PPARγ↑ | [31] |
| Physcion | In vitro | _ | Primary dendritic cells | 1–100 μM | DCs maturation↑ Th1 differentiation↑ | CD40, CD80, CD86, and MHC II↑; IL-12p70↑ | [47] |
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Rhein | Intestinal barrier injury | LPS ip. | SD rats | ig. 66.7 mg/kg/day | intestinal damage↓ TNFα, IL-1 and IL-6↓ GSH-Px, HO-1↑ | MAPK↓ Nrf2↑ | [58] |
| Rhein | In vitro | TNFα | IEC-6 | 0–4 μM | TNFα, IL-1 and IL-6↓; ZO-1↑ | MLCK and NF-κB↓ | [59] |
| Rhein | Ulcerative colitis | DSS | C57BL/6J mice | ig. 50, 100 mg/kg/day, ig. | Histological changes↓ Th1, Th17↓ uric acid levels↓ | Probiotic Lactobacillus↑ | [63] |
| Rhein | Acute enteritis | Radiation | SD rats | ig. 90 μg/kg | NO, TNFα IL-1β and IL-6↓ MDA↓, SOD and GSH↑ Cleaved caspase-3, PARP↓ | NF-κB ↓; PPARγ↑ | [33] |
| Emodin | Colitis-associated tumorigenesis | AOM + DSS | BALB/c mice | ig. 50 mg/kg/day | Week 3: adenoma↓ Inflammatory cells infiltration↓ TNFα, IL-1α/β and IL6↓; Week 14: Dysplastic lesions↓ | _ | [64] |
| Emodin | Sepsis-induced jejunum injury | Cecal ligation and puncture | Wistar rats | ip. 10 mg/kg/day | Intestinal mucosal damage↓ TNFα, IL-6 and PCT↓ Apoptosis↓ | JAK1/STAT3↑ | [65] |
| Emodin | In vitro | Cerulein/LPS | AR42J | 10–40 μM | Mitochondrial damage↓; ROS↓; TNFα, IL-6↓ | ASK1/TRAF2 (JNK)↓ p38 MAPK↓ | [75] |
| Emodin | SAP | Taurocholate | SD rats | ig. 60 mg/kg | TNFα, IL-6↓ neutrophils derived ROS↓ | VDAC1↓ NLRP3 inflammasome↓ | [76] |
| Emodin | SAP | Taurocholate | SD rats | ig. 30, 60 mg/kg | Pathological changes Amylase, Lipase TNFα, IL-6, MPO↓ | P2X7↓ NLRP3 inflammasome↓ | [77] |
| Emodin | SAP | Taurocholate | SD rats | ig. 50 mg/kg | Intestine mucosal barrier↑ Occludin, ZO-1, E-cadherin↓ Intestinal cell apoptosis ↓ | Notch1, RhoA/ROCK↓ miR-218a-5p↓ | [79] |
| Rhein | In vitro | Cerulein | AR42J | 1 μM | Mitochondrial swelling and spinal fracture↓ | PI3K/AKT/mTOR↓ | [74] |
| Total Rhubarb anthraquinones | SAP | Taurocholate | SD rats | ig. 36, 72 mg/kg | Endotoxin, TNFα, IL-1β↓ Intestinal mucosal barrier↑ NO, MPO↓; Tregs, Th1/Th2↓ | NLRP3 inflammasome↓ | [78] |
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Emodin | ALI | LPS | Wistar rats | ig. 20, 40 mg/kg | Pathological changes↓; Infiltrated inflammatory cells↓; TNFα, IL-1β, and IL-6↓ | mTOR/HIF-1α/VEGF↓ NF-κB↓ | [85] |
| Emodin | ARDS | LPS | C57BL/6J mice | ip. 5, 10, 20 mg/kg | Lung injury, inflammatory infiltration↓ Pulmonary TF, PAI-1, Collagen I, III↓ Pulmonary IL-8, IL- 1β, TNFα↓ | NF-κB↓ | [86] |
| Emodin | Lung function decreases | DEP air pollution | BALB/C mice | ip. 4 mg/kg | Lung function↑ Lipid peroxidation, ROS, GSH↓ | _ | [87] |
| Emodin | Asthma | Ovalbumin | BALB/c mice | ip. 15, 30, 60 mg/kg | Airway resistance↓ Pulmonary tissues injury↓ IL-5 and IL-17↓; IFNγ↑ | Notch1-3↓ | [94] |
| Emodin | Asthma | Ovalbumin | C57BL/6J mice | ip. 10, 20 mg/kg | Macrophages and eosinophils↓ IL-4, 5, 13, 17, NO and IFNγ↓ | _ | [95] |
| Emodin | pulmonary fibrosis | Bleomycin | SD rats | ig. 20 mg/kg | Lung structural damage↓; Collagen deposition↓ Inflammatory cell infiltration↓ Pro-inflammatory cytokines↓ | NF-κB↓ Nrf2↑ | [100] |
| Emodin | In vitro | TGFβ1 | A549 | 60 μM | EMT↓ | ||
| Emodin | Silicosis | Silica | C57BL/6J mice | ig. 25, 50 mg/kg | alveolitis and fibrosis↓ Smad3↓ | NF-κB↓ TGF-β1/Smad3↓ | [101] |
| Chrysophanol | ALI | LPS | BALB/c mice | ip. 7.5, 15, and 30 mg/kg) | Lung injury↓ TNFα, IL-1β, IL-16, HMGB-1↓ | HMGB1/NF-κB↓ | [88] |
| Chrysophanol | Asthma | Ovalbumin | BALB/c mice | ip. 0.1, 1, 10 mg/kg | TNFα,IL-4, IL-5, IL-13↓ Airway remodeling↓ | NF-κB↓ | [96] |
| Chrysophanol | In vitro | TNFα | BEAS-2B | 2, 20 μM | Proliferation↓, p-IκB↓ |
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Rhein | In vitro | PMA/urate | THP-1 | 1–10 μg/mL | IL-1β, TNFα, Caspase1↓ | NLRP3 inflammasome↓ | [106] |
| Rhein | In vitro | ATP | Synoviocytes of CIA rats | 0.1–10 μM | Ca2+↓; ROS↓ MMP-9, COX-2 and IL-6↓ | P2 × 4R | [107] |
| Emodin | RA | Collagen | DBA/1 mice | ip. 10 mg/kg | Synovial inflammation↓ Joint destruction↓, MMP-1, -3↓ | NF-κB↓ | [110] |
| Emodin | In vitro | LPS | Synoviocytes of RA patients | 0.1–10 μM | TNFα, IL-6 and IL-8↓ COX-2, VEGF, HIF-1a↓ MMP-1, MMP-13↓ | HDAC1↓ | [111] |
| Emodin | In vitro | _ | Fibroblasts of AS patients | 10 µM | Caspase-3, -9↑ Atg12, Atg5, and Beclin 1↑ | autophagy↑ | [112] |
| Aloe-emodin | RA | Complete Freund’s Adjuvant | Wistar rats | 50 and 75 mg/kg | paw edema volume↓ Arthritis score↓ WBC count ↓ LPO, NO↓; GSH, CAT, SOD↑ | — | [113] |
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Emodin | NAFLD | HFD + LPS | LDLR−/− mice | ip. 40 mg/kg | TNFα, IL-1β, IL-6, IFNγ, G-CSF, GM-CSF, MCP-1, RANTES↓; Liver leukocyte infiltration↓; Liver function↑ | Erk1/2 and p38↓ | [119] |
| Emodin | Obesity | HFD | C57BL/6 mice | ig. 80 mg/kg | glucose and insulin↓ brown AT (BAT) mass↓ systematic inflammation↓ | M2 macrophage↑ TREM2↑ | [120] |
| Emodin | T2D | HFD + streptozotocin | T2D | Hyperglycemia, dyslipidemia↓ | miR-20b/SMAD 7 | [121] | |
| Emodin | In vitro | Palmitic acid | L6 myoblasts | 5–20 μM | Glucose consumption↑ | ||
| Emodin | In vitro | _ | C2C12/3T3-L1 | 6.25–50 μM | Glucose uptake, consumption↑ Glycolysis↑; lipolysis↓ | NF-κB↓ | [122] |
| Chrysophanol | Obesity | HFD | SD rats | ip. 10 mg/kg | Body weight, blood glucose↓ TG↓, HDL-C↑ IL-6, IL-1β↓; IL-10↑ | AMPK/Sirt1↑ | [123] |
| Aloe-emodin | Obesity | HFD | Wistar rats | ig. 100 mg/kg | TNFα, IL-1β, IL-6↓ VCAM1, ICAM-1↓ | TLR4/NF-κB↓ | [124] |
| Compound | Disease/Injury | Stimuli | Cell/Animal | Doses | Effects | Mechanism | Ref |
|---|---|---|---|---|---|---|---|
| Chrysophanol | Stroke | MCAO | CD1 mice | ip. 0.1,1, 10 mg/kg | Neurons Caspase-1, IL-1β↓ Neurological deficit↓, Brain Edema↓ | NALP3↓ | [131] |
| Chrysophanol | Stroke | MCAO | C57BL mice | ip. 0.1,1, 10 mg/kg | Survival rates↑, apoptosis↓ Neurological function↑ Pro-inflammatory cytokines↓ | NF-κB↓ | [132] |
| Aloe-emodin | Stroke | MCAO | SD rats | ig. 25, 50, 100 mg/kg | Neurological disorder↓ Infarct size↓ TNFα, MDA↓; SOD↑ | PI3K/AKT/mTOR↓ NF-κB↓ | [134] |
| Aloe-emodin | in vitro | OGD/R LPS | SH-SY5Y BV2 | 0–10 µM | |||
| Emodin | in vitro | H2O2 | SH-SY5Y | 10–100 µM | Viability↑, Apoptosis, LDH↑ | PI3K/mTOR/GSK3β | [135] |
| Emodin | SAE | Cecal ligation and puncture (CLP) | BALB/C mice | i.p. 20 mg/kg | Neurons apoptosis↓ Cognitive dysfunction ↓ Pathological injury↓ | BDNF/TrkB↑ Autophagy↑ | [137] |
| Emodin | sepsis-realted brain injury | LPS | BALB/c mice | ip. 20 mg/kg | Serum S100β, IL-6, TNFα, NSE↓ Brain AchE, LA↓ | _ | [138] |
| Rhein | Multiple sclerosis | EAE | Mice | ip. 5, 10, 20 mg/kg | Brain IL-2↓, Foxp3↑ | Treg differentiation | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, D.; Li, H.; Zhou, S.; Zhong, H.; Pu, W. Effects of Anthraquinones on Immune Responses and Inflammatory Diseases. Molecules 2022, 27, 3831. https://doi.org/10.3390/molecules27123831
Xin D, Li H, Zhou S, Zhong H, Pu W. Effects of Anthraquinones on Immune Responses and Inflammatory Diseases. Molecules. 2022; 27(12):3831. https://doi.org/10.3390/molecules27123831
Chicago/Turabian StyleXin, Dandan, Huhu Li, Shiyue Zhou, Hao Zhong, and Weiling Pu. 2022. "Effects of Anthraquinones on Immune Responses and Inflammatory Diseases" Molecules 27, no. 12: 3831. https://doi.org/10.3390/molecules27123831





