Supramolecular Diiodine-Bromostannate(IV) Complexes: Narrow Bandgap Semiconductors
Abstract
:1. Introduction
2. Experimental Part
2.1. Synthesis of 1
2.2. Synthesis of 2
2.3. Synthesis of 3
2.4. X-ray Diffractometry
2.5. Raman Spectroscopy
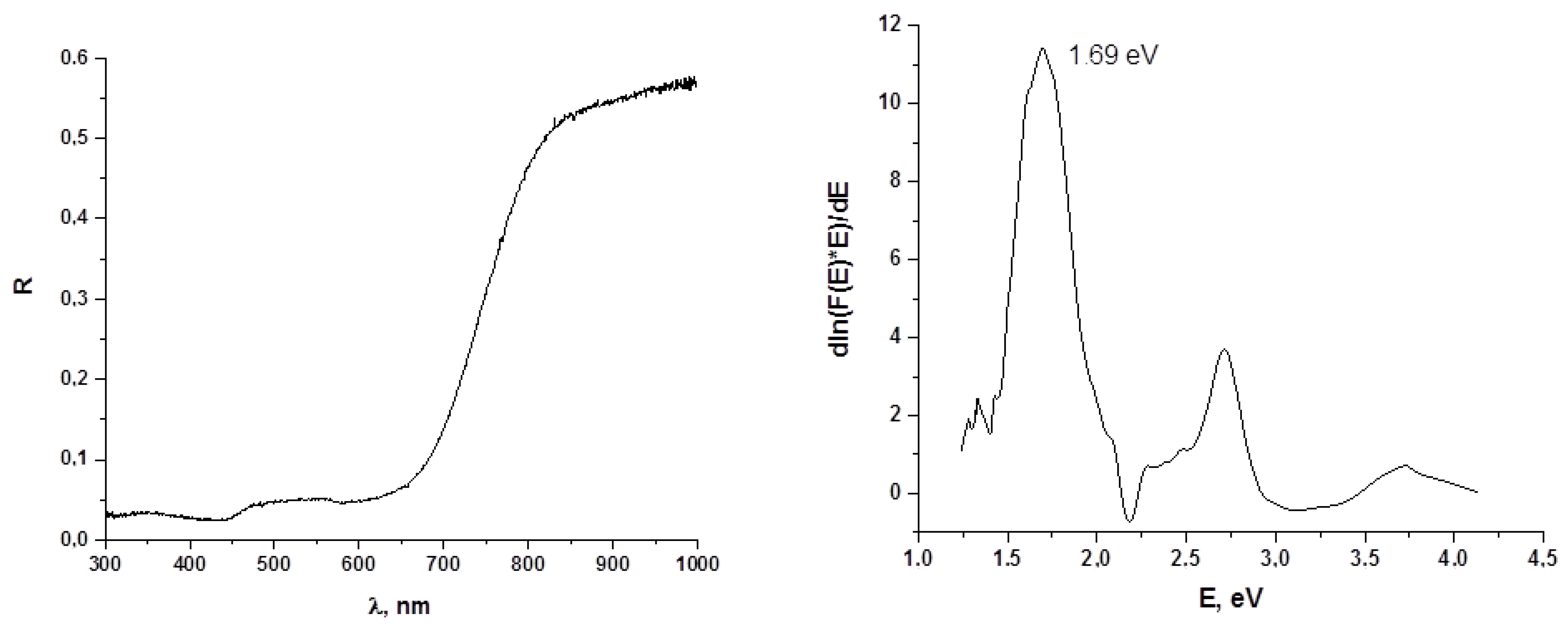
2.6. Diffuse Reflectance Spectroscopy
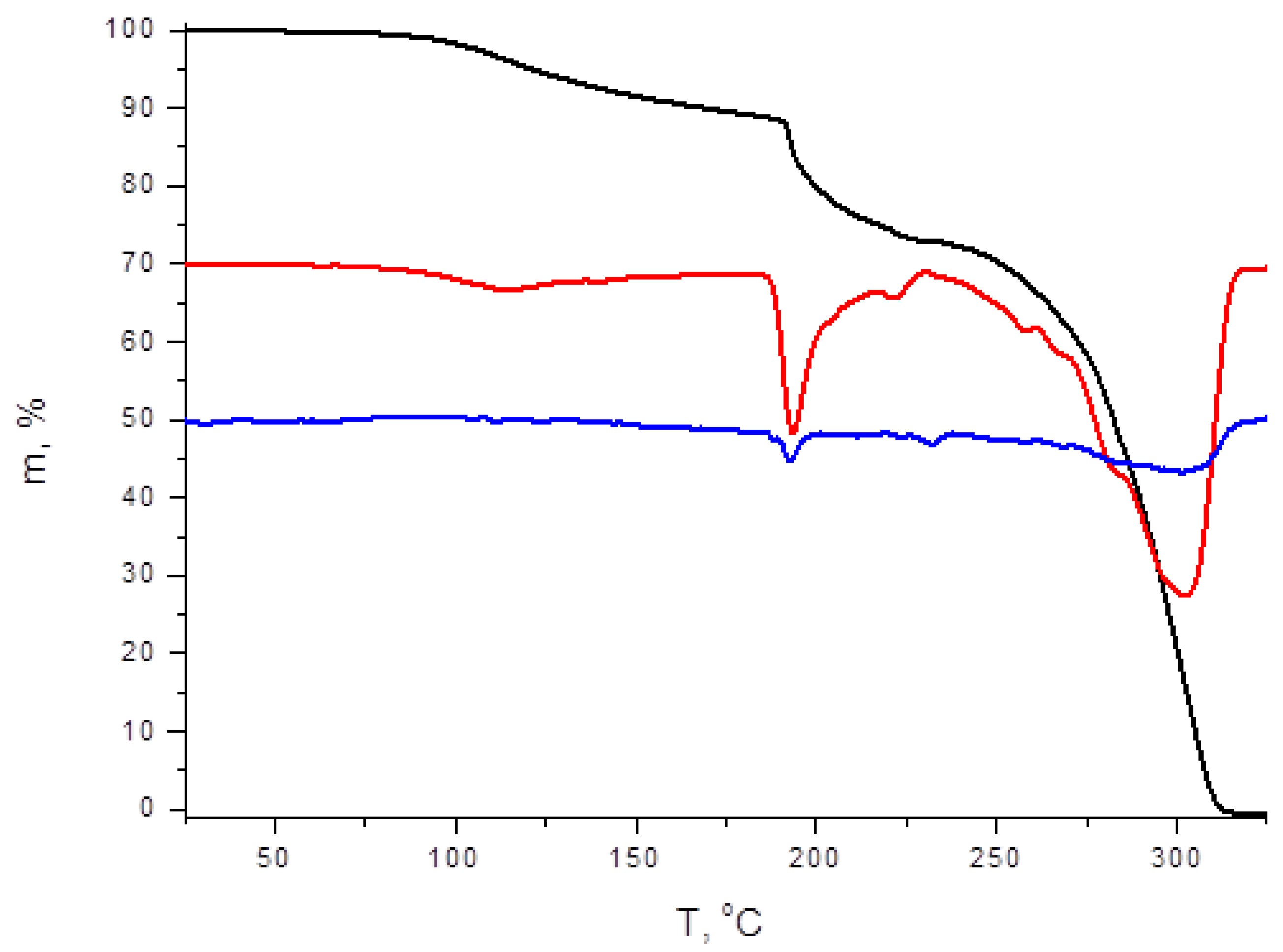
2.7. Thermogravimetric Analysis (TGA)
2.8. Powder X-ray Diffractometry (PXRD)
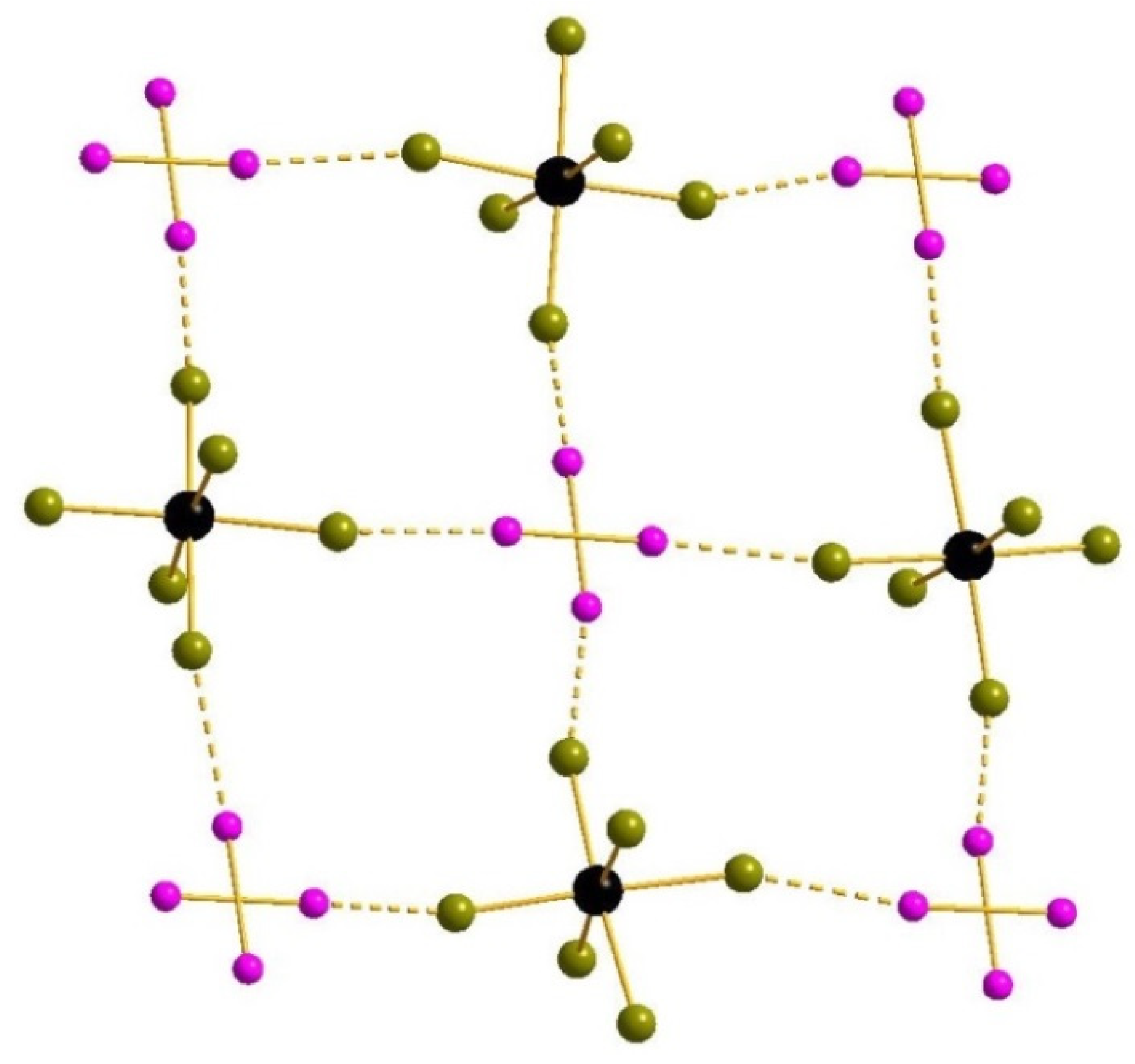
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Przesławski, J.; Piecha-Bisiorek, A.; Jakubas, R. Specific heat anomaly in ferroelectric: Bis(imidazolium) pentachloroantimonate(III) (C3N2H5)2[SbCl5]. J. Mol. Struct. 2016, 1110, 97–101. [Google Scholar] [CrossRef]
- Mencel, K.; Kinzhybalo, V.; Jakubas, R.; Zarȩba, J.K.; Szklarz, P.; Durlak, P.; Drozd, M.; Piecha-Bisiorek, A. 0D Bismuth (III)-Based Hybrid Ferroelectric: Tris (acetamidinium) Hexabromobismuthate (III). Chem. Mater. 2021, 33, 8591–8601. [Google Scholar] [CrossRef]
- Piecha, A.; Białońska, A.; Jakubas, R. Structure and ferroelectric properties of [C3N2H5]5[Bi2Br11]. J. Phys. Condens. Matter 2008, 20, 325224. [Google Scholar] [CrossRef]
- Owczarek, M.; Szklarz, P.; Jakubas, R. Towards ferroelectricity-inducing chains of halogenoantimonates(iii) and halogenobismuthates(iii). RSC Adv. 2021, 11, 17574–17586. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, P.; Jakubas, R.; Medycki, W.; Gągor, A.; Cichos, J.; Karbowiak, M.; Bator, G. (C3N2H5)3Sb2I9 and (C3N2H5)3Bi2I9: Ferroelastic lead-free hybrid perovskite-like materials as potential semiconducting absorbers. Dalt. Trans. 2022, 51, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-W.; Yue, C.-Y.; Feng, L.-J.; Han, Y.-F.; Meng, R.-R.; Yang, J.-T.; Ding, H.; Gao, C.-S.; Wang, C.-Y. Syntheses, crystal structures and photocatalytic properties of four hybrid iodoargentates with zero- and two-dimensional structures. CrystEngComm 2015, 18, 427–436. [Google Scholar] [CrossRef]
- Lei, X.-W.; Yue, C.-Y.; Zhao, J.-Q.; Han, Y.-F.; Yang, J.-T.; Meng, R.-R.; Gao, C.-S.; Ding, H.; Wang, C.-Y.; Chen, W.-D. Low-Dimensional Hybrid Cuprous Halides Directed by Transition Metal Complex: Syntheses, Crystal Structures, and Photocatalytic Properties. Cryst. Growth Des. 2015, 15, 5416–5426. [Google Scholar] [CrossRef]
- Lei, X.-W.; Yue, C.-Y.; Wei, J.-C.; Li, R.-Q.; Mi, F.-Q.; Li, Y.; Gao, L.; Liu, Q.-X. Novel 3D Semiconducting Open-Frameworks based on Cuprous Bromides with Visible Light Driven Photocatalytic Properties. Chem. A Eur. J. 2017, 23, 14547–14553. [Google Scholar] [CrossRef]
- Feng, L.-J.; Zhao, Y.-Y.; Song, R.-Y.; Lei, X.-W. Three homologous 1D lead halide perovskites with broadband white-light emissions. Inorg. Chem. Commun. 2021, 136, 109146. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Shi, H.-S.; Zeng, L.-R.; Ge, H.; Hou, Y.-H.; Wu, X.-M.; Yue, C.-Y.; Lei, X.-W. Highly emissive zero-dimensional antimony halide for anti-counterfeiting and confidential information encryption-decryption. Chem. Eng. J. 2021, 431, 134336. [Google Scholar] [CrossRef]
- Ahern, J.C.; Nicholas, A.; Kelly, A.W.; Chan, B.; Pike, R.D.; Patterson, H.H. A terbium chlorobismuthate(III) double salt: Synthesis, structure, and photophysical properties. Inorganica Chim. Acta 2018, 478, 71–76. [Google Scholar] [CrossRef]
- Kelly, A.W.; Nicholas, A.; Ahern, J.; Chan, B.; Patterson, H.H.; Pike, R. Alkali metal bismuth(III) chloride double salts. J. Alloys Compd. 2016, 670, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Heine, J.; Wehner, T.; Bertermann, R.; Steffen, A.; Müller-Buschbaum, K. 2∞[Bi2Cl6(pyz)4]: A 2D-pyrazine coordination polymer as soft host lattice for the luminescence of the lanthanide ions Sm3+, Eu3+, Tb3+, and Dy3+. Inorg. Chem. 2014, 53, 7197–7203. [Google Scholar] [CrossRef]
- Dehnhardt, N.; Klement, P.; Chatterjee, S.; Heine, J. Divergent Optical Properties in an Isomorphous Family of Multinary Iodido Pentelates. Inorg. Chem. 2019, 58, 10983–10990. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.-Q.; Yin, X.; Xiao, P.-C.; Gao, Y.-J.; Wu, X.-M.; Yue, C.-Y.; Lei, X.-W. Bulk Mn2+ Doped 1D Hybrid Lead Halide Perovskite with Highly Efficient, Tunable and Stable Broadband Light Emissions. Chem. A Eur. J. 2022, 28, e202103043. [Google Scholar] [CrossRef]
- Lin, R.-G.; Xu, G.; Lu, G.; Wang, M.-S.; Li, P.-X.; Guo, G.-C. Photochromic hybrid containing in situ-generated benzyl viologen and novel trinuclear [Bi3Cl14]5−: Improved photoresponsive behavior by the π··· π interactions and size effect of inorganic oligomer. Inorg. Chem. 2014, 53, 5538–5545. [Google Scholar] [CrossRef]
- Toma, O.; Mercier, N.; Botta, C. N-Methyl-4, 4′-bipyridinium and N-Methyl-N′-oxide-4, 4′-bipyridinium Bismuth Complexes–Photochromism and Photoluminescence in the Solid State. Eur. J. Inorg. Chem. 2013, 2013, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Grishko, A.Y.; Zharenova, E.A.; Goodilina, E.A.; Tarasov, A.B. Solvent-free deposition of hybrid halide perovskites onto thin films of copper iodide p-type conductor. Mendeleev Commun. 2021, 31, 163–165. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Fateev, S.A.; Petrov, A.A.; Goodilin, E.A.; Tarasov, A.B. Theoretical assessment of thermodynamic stability of 2D octane-1,8-diammonium lead halide perovskites. Mendeleev Commun. 2020, 30, 279–281. [Google Scholar] [CrossRef]
- Petrov, A.A.; Fateev, S.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zvereva, I.A.; Petrov, A.V.; Goodilin, E.A.; Tarasov, A.B. Methylammonium polyiodides: Remarkable phase diversity of the simplest and low-melting alkylammonium polyiodide system. J. Phys. Chem. Lett. 2019, 10, 5776–5780. [Google Scholar] [CrossRef]
- Elnaggar, M.M.; Frolova, L.A.; Gordeeva, A.M.; Ustinova, M.I.; Laurenzen, H.; Akkuratov, A.V.; Nikitenko, S.L.; Solov’eva, E.A.; Luchkin, S.Y.; Fedotov, Y.S.; et al. Improving stability of perovskite solar cells using fullerene-polymer composite electron transport layer. Synth. Met. 2022, 286, 117028. [Google Scholar] [CrossRef]
- Udalova, N.N.; Fateev, S.A.; Nemygina, E.M.; Zanetta, A.; Grancini, G.; Goodilin, E.A.; Tarasov, A.B. Nonmonotonic Photostability of BA2MAn−1PbnI3n+1 Homologous Layered Perovskites. ACS Appl. Mater. Interfaces 2022, 14, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Gutsev, L.G.; Ramachandran, B.R.; Dremova, N.N.; Aldoshin, S.M.; Troshin, P.A. Exploring CsPbI3–FAI alloys: Introducing low-dimensional Cs2FAPb2I7 absorber for efficient and stable perovskite solar cells. Chem. Eng. J. 2021, 426, 131754. [Google Scholar] [CrossRef]
- Petrov, A.A.; Ordinartsev, A.A.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Solubility of Hybrid Halide Perovskites in DMF and DMSO. Molecules 2021, 26, 7541. [Google Scholar] [CrossRef] [PubMed]
- Mangrulkar, M.; Luchkin, S.Y.; Akbulatov, A.F.; Zhidkov, I.; Kurmaev, E.Z.; Troshin, P.A.; Stevenson, K.J. Rationalizing the effect of overstoichiometric PbI2 on the stability of perovskite solar cells in the context of precursor solution formulation. Synth. Met. 2021, 278, 116823. [Google Scholar] [CrossRef]
- Alidaei, M.; Ahmadi, V.; Mousavi, S.M.; Roghabadi, F.A. Stability improvement of perovskite solar cell using photoswitchable and moisture resistant dual-function interfacial layer. J. Alloys Compd. 2022, 903, 163891. [Google Scholar] [CrossRef]
- Oniy Aghmiuni, K.; Arabpour Roghabadi, F.; Rezvani, H.; Alidaei, M.; Falahi, M.; Pashaei Soorbaghi, F.; Ahmadi, V. The Future of Hybrid and Inorganic Perovskite Materials: Technology Forecasting. Energy Technol. 2021, 9, 2100376. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond methylammonium lead iodide: Prospects for the emergent field of ns2 containing solar absorbers. Chem. Commun. 2016, 53, 20–44. [Google Scholar] [CrossRef] [Green Version]
- Usoltsev, A.N.; Elshobaki, M.; Adonin, S.A.; Frolova, L.A.; Derzhavskaya, T.; Abramov, P.A.; Anokhin, D.V.; Korolkov, I.V.; Luchkin, S.Y.; Dremova, N.N.; et al. Polymeric iodobismuthates [Bi3I10] and [BiI4] with N-heterocyclic cations: Promising perovskite-like photoactive materials for electronic devices. J. Mater. Chem. A 2019, 7, 5957–5966. [Google Scholar] [CrossRef]
- Han, L.; Wang, P.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Huang, B. Zero-dimensional hydrazine iodobismuthate as a lead-free perovskite-like light absorber in a self-powered photodetector. J. Alloys Compd. 2021, 893, 162347. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, F.; Meng, Y.; Bu, X.; Kang, X.; Quan, Q.; Wang, W.; Yip, S.P.; Liu, C.; Ho, J.C. Solution-processed lead-free double perovskite microplatelets with enhanced photoresponse and thermal stability. Sci. China Mater. 2022, 65, 1313–1319. [Google Scholar] [CrossRef]
- Shuang, Z.; Zhou, H.; Wu, D.; Zhang, X.; Xiao, B.; Ma, G.; Zhang, J.; Wang, H. Low-temperature process for self-powered lead-free Cs2AgBiBr6 perovskite photodetector with high detectivity. Chem. Eng. J. 2022, 433, 134544. [Google Scholar] [CrossRef]
- Li, L.; Ye, G.; Luo, T.; Chen, X.; Zhang, G.; Wu, H.; Yang, L.; Zhang, W.; Chang, H. Centimeter-Sized Stable Zero-Dimensional Cs3Bi2I9 Single Crystal for Mid-Infrared Lead-Free Perovskite Photodetector. J. Phys. Chem. C 2022, 126, 3646–3652. [Google Scholar] [CrossRef]
- Mei, J.; Liu, M.; Vivo, P.; Pecunia, V. Two-Dimensional Antimony-Based Perovskite-Inspired Materials for High-Performance Self-Powered Photodetectors. Adv. Funct. Mater. 2021, 31, 2106295. [Google Scholar] [CrossRef]
- Wu, L.-M.; Wu, X.-T.; Chen, L. Structural overview and structure–property relationships of iodoplumbate and iodobismuthate. Coord. Chem. Rev. 2009, 253, 2787–2804. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Yelavik, N.A.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Role of I2 Molecules and Weak Interactions in Supramolecular Assembling of Pseudo-Three-Dimensional Hybrid Bismuth Polyiodides: Synthesis, Structure, and Optical Properties of Phenylenediammonium Polyiodobismuthate(III). Cryst. Growth Des. 2018, 18, 2572–2578. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From isolated anions to polymer structures through linking with i2: Synthesis, structure, and properties of two complex bismuth(III) iodine iodides. Inorg. Chem. 2018, 57, 4077–4087. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Plyusnin, P.E.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Diiodine—Iodobismuthate(III) Hybrids Cat3[Bi2I9](I2)3: Syntheses, Stability, and Optical Properties. Inorg. Chem. 2020, 59, 17320–17325. [Google Scholar] [CrossRef]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.S.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.J.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem. A Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef]
- Novikov, A.V.; Usoltsev, A.N.; Adonin, S.A.; Bardin, A.A.; Samsonenko, D.G.; Shilov, G.V.; Sokolov, M.N.; Stevenson, K.J.; Aldoshin, S.M.; Fedin, V.P.; et al. Tellurium complex polyhalides: Narrow bandgap photoactive materials for electronic applications. J. Mater. Chem. A 2020, 8, 21988–21992. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondi, A. van der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals radü for the whole main group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- And Two-Dimensional Iodine-Rich Iodobismuthate(III) Complexes: Structure, Optical Properties, and Features of Halogen Bonding in the Solid State. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]
- Tudela, D.; Khan, M.A. Tin-bromine bond lengths and Mössbauer quadrupole splittings of tin(IV) bromide complexes. Crystal structure of pyridinium tetrabromodiphenylstannate(IV). J. Chem. Soc. Dalt. Trans. 1991, 1003–1006. [Google Scholar] [CrossRef]
- Korobeynikov, N.A.; Usoltsev, A.N.; Abramov, P.A.; Novikov, A.S.; Sokolov, M.N.; Adonin, S.A. Bromine-rich tin(IV) halide complexes: Experimental and theoretical examination of Br···Br noncovalent interactions in crystalline state. Polyhedron 2022, 222, 115912. [Google Scholar] [CrossRef]
- Goforth, A.M.; Tershansy, M.A.; Smith, M.D.; Peterson, L.; Kelley, J.G.; DeBenedetti, W.J.I.; zur Loye, H.-C. Structural Diversity and Thermochromic Properties of Iodobismuthate Materials Containing d-Metal Coordination Cations: Observation of a High Symmetry [Bi3I11]2− Anion and of Isolated I− Anions. J. Am. Chem. Soc. 2011, 133, 603–612. [Google Scholar] [CrossRef]
- Shayapov, V.R.; Usoltsev, A.N.; Adonin, S.A.; Sokolov, M.N.; Samsonenko, D.G.; Fedin, V.P. Thermochromism of bromotellurates(iv): Experimental insights. New J. Chem. 2019, 43, 3927–3930. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Kolesov, B.A.; Novikov, A.S.; Samsonenko, D.G.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. Rule, Not Exclusion: Formation of Dichlorine-Containing Supramolecular Complexes with Chlorometalates(IV). Inorg. Chem. 2021, 60, 4171–4177. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobeynikov, N.A.; Usoltsev, A.N.; Abramov, P.A.; Sokolov, M.N.; Adonin, S.A. Supramolecular Diiodine-Bromostannate(IV) Complexes: Narrow Bandgap Semiconductors. Molecules 2022, 27, 3859. https://doi.org/10.3390/molecules27123859
Korobeynikov NA, Usoltsev AN, Abramov PA, Sokolov MN, Adonin SA. Supramolecular Diiodine-Bromostannate(IV) Complexes: Narrow Bandgap Semiconductors. Molecules. 2022; 27(12):3859. https://doi.org/10.3390/molecules27123859
Chicago/Turabian StyleKorobeynikov, Nikita A., Andrey N. Usoltsev, Pavel A. Abramov, Maxim N. Sokolov, and Sergey A. Adonin. 2022. "Supramolecular Diiodine-Bromostannate(IV) Complexes: Narrow Bandgap Semiconductors" Molecules 27, no. 12: 3859. https://doi.org/10.3390/molecules27123859






