Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research
Abstract
:1. Introduction
2. Curcumin Scaffold as a Tool for Understanding AD
3. Curcumin Scaffold as a Tool for AD Diagnosis
3.1. Fluorescent Probe for Detecting Insoluble Aβ
3.2. Fluorescent Probe for Detecting Soluble Aβ
3.3. Fluorescent Probe for Detecting Tau Protein
3.4. Fluorescent Probe for Detecting ROS
3.5. PET Tracers
3.6. NIRF Probe for Monitoring Therapy
4. Curcumin Scaffold as a Tool for AD Therapy
4.1. Aβ Inhibition
4.2. Tau Inhibition
4.3. Metal Ion Binding
4.4. ROS Scavenging and Neuroprotective Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, A.; Anwar, S.; Mohammad, T.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Hasan, G.M.; Islam, A.; Hassan, M.I. MARK4 Inhibited by AChE Inhibitors, Donepezil and Rivastigmine Tartrate: Insights into Alzheimer’s Disease Therapy. Biomolecules 2020, 10, 789. [Google Scholar] [CrossRef]
- Shamsi, A.; Mohammad, T.; Anwar, S.; Alajmi, M.F.; Hussain, A.; Hassan, M.I.; Ahmad, F.; Islam, A. Probing the interaction of Rivastigmine Tartrate, an important Alzheimer’s drug, with serum albumin: Attempting treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2020, 148, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Shahwan, M.; Khan, M.S.; Husain, F.M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rehman, M.T.; Hassan, M.I.; Islam, A. Elucidating the Interaction of Human Ferritin with Quercetin and Naringenin: Implication of Natural Products in Neurodegenerative Diseases: Molecular Docking and Dynamics Simulation Insight. ACS Omega 2021, 6, 7922–7930. [Google Scholar] [CrossRef]
- Hamley, I.W. The amyloid beta peptide: A chemist’s perspective. Role in Alzheimer’s and fibrillization. Chem. Rev. 2012, 112, 5147–5192. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Gotz, J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.K.; Choi, S.H.; Washicosky, K.J.; Eimer, W.A.; Tucker, S.; Ghofrani, J.; Lefkowitz, A.; McColl, G.; Goldstein, L.E.; Tanzi, R.E.; et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 340ra372. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, K.; Liu, F.; Gong, C.X. Alzheimer disease therapeutics: Focus on the disease and not just plaques and tangles. Biochem. Pharmacol. 2014, 88, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Sisodia, S.S.; St George-Hyslop, P.H. gamma-Secretase, Notch, Abeta and Alzheimer’s disease: Where do the presenilins fit in? Nat. Rev. Neurosci. 2002, 3, 281–290. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hureau, C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 1: An overview. Coord. Chem. Rev. 2012, 256, 2164–2174. [Google Scholar] [CrossRef]
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.L.; Dang, H.Z.; Fan, H.; Chen, X.P.; Rao, Y.X.; Ren, Y.; Yang, J.D.; Shi, J.; Wang, P.W.; Tian, J.Z. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 734–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-beta Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, H.F. The beta-Amyloid Hypothesis in Alzheimer’s Disease: Seeing Is Believing. ACS Med. Chem. Lett. 2012, 3, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, T.; Shakeri, A.; Rao, P.P. Amyloid cascade in Alzheimer’s disease: Recent advances in medicinal chemistry. Eur J. Med. Chem. 2016, 113, 258–272. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-beta oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Zhang, H.; Therriault, J.; Kang, M.S.; Ng, K.P.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S.; the Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Elovsson, G.; Bergkvist, L.; Brorsson, A.C. Exploring Abeta Proteotoxicity and Therapeutic Candidates Using Drosophila melanogaster. Int. J. Mol. Sci. 2021, 22. [Google Scholar]
- Gray, E.G.; Paula-Barbosa, M.; Roher, A. Alzheimer’s disease: Paired helical filaments and cytomembranes. Neuropathol. Appl. Neurobiol. 1987, 13, 91–110. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New perspectives and challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 2015, 6, 6176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, L. Retraction: Chrysin attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial inflammation. RSC Adv. 2021, 11, 4173. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Pratico, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Huang, Y.; Erdmann, N.; Peng, H.; Zhao, Y.; Zheng, J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol. Immunol. 2005, 2, 113–122. [Google Scholar]
- Xie, H.; Hou, S.; Jiang, J.; Sekutowicz, M.; Kelly, J.; Bacskai, B.J. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 7904–7909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, N.; Kurokawa, T.; Nonami, T.; Tumurkhuu, G.; Koide, N.; Yokochi, T. Hydrogen peroxide induces the production of tumor necrosis factor-alpha in RAW 264.7 macrophage cells via activation of p 38 and stress-activated protein kinase. Innate Immun. 2008, 14, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Park, Y.M.; Kim, G.Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010, 10, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Nikmahzar, E.; Sayyahi, A. Vitamin E therapy prevents the accumulation of congophilic amyloid plaques and neurofibrillary tangles in the hippocampus in a rat model of Alzheimer’s disease. Iran. J. Basic Med. Sci. 2020, 23, 86–92. [Google Scholar] [PubMed]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Walsh, D.M.; Hartley, D.M.; Kusumoto, Y.; Fezoui, Y.; Condron, M.M.; Lomakin, A.; Benedek, G.B.; Selkoe, D.J.; Teplow, D.B. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999, 274, 25945–25952. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.D.; Malagodi, A.J.; Chi, E.Y.; Evans, D.G. Computational Study of the Driving Forces and Dynamics of Curcumin Binding to Amyloid-beta Protofibrils. J. Phys. Chem. B 2019, 123, 551–560. [Google Scholar] [CrossRef]
- Zhao, L.N.; Long, H.; Mu, Y.; Chew, L.Y. The toxicity of amyloid beta oligomers. Int J. Mol. Sci. 2012, 13, 7303–7327. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.N.; Chiu, S.W.; Benoit, J.; Chew, L.Y.; Mu, Y.G. The Effect of Curcumin on the Stability of A beta Dimers. J. Phys. Chem. B 2012, 116, 7428–7435. [Google Scholar] [CrossRef]
- Reinke, A.A.; Gestwicki, J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Biol. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, D.; Kato, T.; Taguchi, H.; Shirai, N.; Hirao, K.; Sogabe, T.; Tomiyama, T.; Gamo, K.; Hirahara, Y.; Kitada, M.; et al. Keto form of curcumin derivatives strongly binds to Abeta oligomers but not fibrils. Biomaterials 2021, 270, 120686. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, A.C.; Lokesh, B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell Biochem. 1994, 137, 1–8. [Google Scholar] [CrossRef]
- Khorasani, M.Y.; Langari, H.; Sany, S.B.T.; Rezayi, M.; Sahebkar, A. The role of curcumin and its derivatives in sensory applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109792. [Google Scholar] [CrossRef]
- Mutsuga, M.; Chambers, J.K.; Uchida, K.; Tei, M.; Makibuchi, T.; Mizorogi, T.; Takashima, A.; Nakayama, H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer’s brain. J. Vet. Med. Sci. 2012, 74, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.S.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Guo, T.L.; Chojnacki, J.; Lee, H.G.; Wang, X.; Siedlak, S.L.; Rao, W.; Zhu, X.; Zhang, S. Bivalent ligand containing curcumin and cholesterol as fluorescence probe for Abeta plaques in Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 141–146. [Google Scholar] [CrossRef]
- Yanagisawa, D.; Amatsubo, T.; Morikawa, S.; Taguchi, H.; Urushitani, M.; Shirai, N.; Hirao, K.; Shiino, A.; Inubushi, T.; Tooyama, I. In vivo detection of amyloid beta deposition using (1)(9)F magnetic resonance imaging with a (1)(9)F-containing curcumin derivative in a mouse model of Alzheimer’s disease. Neuroscience 2011, 184, 120–127. [Google Scholar] [CrossRef]
- Ran, C.; Xu, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Shao, C.; Ye, X.; Di, X.; Li, D.; Zhao, H.; Zhang, B.; Chen, G.; Liu, H.-K.; Qian, Y. In Vivo Brain Imaging of Amyloid-β Aggregates in Alzheimer’s Disease with a Near-Infrared Fluorescent Probe. ACS Sens. 2021, 6, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer’s disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tian, Y.; Zhang, C.; Tian, X.; Ross, A.W.; Moir, R.D.; Sun, H.; Tanzi, R.E.; Moore, A.; Ran, C. Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 9734–9739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, J.; Liu, H.; Yang, J.; Du, L.; Feng, H.; Tian, Y.; Cao, J.; Ran, C. Tuning the stereo-hindrance of a curcumin scaffold for the selective imaging of the soluble forms of amyloid beta species. Chem. Sci. 2017, 8, 7710–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, D.; Wen, X.; Wang, Y.; Sun, Y.; An, R.; Zhou, Y.; Ye, D.; Liu, H. Engineering of donor-acceptor-donor curcumin analogues as near-infrared fluorescent probes for in vivo imaging of amyloid-beta species. Theranostics 2022, 12, 3178–3195. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Li, Y.; Xu, Y.; Ran, C. Near-infrared Fluorescence Ocular Imaging (NIRFOI) of Alzheimer’s Disease. Mol. Imaging Biol. 2019, 21, 35–43. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, F.; Li, X.; Ran, C.; Xu, Y.; Li, Y. Highly specific detection of Abeta oligomers in early Alzheimer’s disease by a near-infrared fluorescent probe with a “V-shaped” spatial conformation. Chem. Commun. 2020, 56, 583–586. [Google Scholar] [CrossRef]
- Zeng, F.; Yang, J.; Li, X.; Peng, K.; Ran, C.; Xu, Y.; Li, Y. Versatile near-infrared fluorescent probe for in vivo detection of Abeta oligomers. Bioorg. Med. Chem. 2020, 28, 115559. [Google Scholar] [CrossRef]
- Zhang, X.L.; Tian, Y.L.; Yuan, P.; Li, Y.Y.; Yaseen, M.A.; Grutzendler, J.; Moore, A.; Ran, C.Z. A bifunctional curcumin analogue for two-photon imaging and inhibiting crosslinking of amyloid beta in Alzheimer’s disease. Chem. Commun. 2014, 50, 11550–11553. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Zeng, F.; Sun, G.; Peng, K.; Li, X.; Yu, H.; Cheng, C.; Xu, Y.; Yang, J. Curcumin Complex Analogues as Near-Infrared Fluorescent Probes for Monitoring all Aβ Species in the Early Alzheimer’s Disease Model. ACS Chem. Neurosci. 2021, 12, 3683–3689. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Liang, S.H.; Xu, Y.G.; Moore, A.; Ran, C.Z. Imaging hydrogen peroxide in Alzheimer’s disease via cascade signal amplification. Sci Rep. 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, X.; Yuan, P.; Yang, J.; Xu, Y.; Grutzendler, J.; Shao, Y.; Moore, A.; Ran, C. Oxalate-curcumin-based probe for micro- and macroimaging of reactive oxygen species in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 12384–12389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Im, Y.H.; Ahn, J.; Yang, J.; Choi, J.Y.; Lee, K.H.; Kim, B.T.; Choe, Y.S. Synthesis and in vivo characterization of (18)F-labeled difluoroboron-curcumin derivative for beta-amyloid plaque imaging. Sci. Rep. 2019, 9, 6747. [Google Scholar] [CrossRef] [Green Version]
- Ryu, E.K.; Choe, Y.S.; Lee, K.H.; Choi, Y.; Kim, B.T. Curcumin and dehydrozingerone derivatives: Synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, R.; Fu, H.; Yang, J.; Kumar, M.; Lu, J.; Xu, Y.; Liang, S.H.; Cui, M.; Ran, C. Half-curcumin analogues as PET imaging probes for amyloid beta species. Chem. Commun. 2019, 55, 3630–3633. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, F.; Ge, Y.; Peng, K.; Li, X.; Li, Y.; Xu, Y. Development of Near-Infrared Fluorescent Probes for Use in Alzheimer’s Disease Diagnosis. Bioconjug. Chem. 2020, 31, 2–15. [Google Scholar] [CrossRef]
- Gomperts, S.N.; Johnson, K.A.; Growdon, J. Reply: Beyond the limits of detection: Failure of PiB imaging to capture true Abeta burden. Mov. Disord. 2013, 28, 407. [Google Scholar] [CrossRef] [Green Version]
- Teoh, C.L.; Su, D.; Sahu, S.; Yun, S.W.; Drummond, E.; Prelli, F.; Lim, S.; Cho, S.; Ham, S.; Wisniewski, T.; et al. Chemical Fluorescent Probe for Detection of Abeta Oligomers. J. Am. Chem. Soc. 2015, 137, 13503–13509. [Google Scholar] [CrossRef] [Green Version]
- Ran, K.; Yang, J.; Nair, A.V.; Zhu, B.; Ran, C. CRANAD-28: A Robust Fluorescent Compound for Visualization of Amyloid Beta Plaques. Molecules 2020, 25, 863. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Kim, M.K.; Seo, Y.; Ha, T.; Yoo, K.; Hyeon, S.J.; Hwang, Y.J.; Lee, J.; Ryu, H.; Choo, H.; et al. A Difluoroboron beta-Diketonate Probe Shows “Turn-on” Near-Infrared Fluorescence Specific for Tau Fibrils. ACS Chem. Neurosci. 2017, 8, 2124–2131. [Google Scholar] [CrossRef]
- Park, K.S.; Seo, Y.; Kim, M.K.; Kim, K.; Kim, Y.K.; Choo, H.; Chong, Y. A curcumin-based molecular probe for near-infrared fluorescence imaging of tau fibrils in Alzheimer’s disease. Org. Biomol. Chem. 2015, 13, 11194–11199. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Mastrangelo, M.A.; Oddo, S.; LaFerla, F.M.; Federoff, H.J.; Bowers, W.J. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J. Neuroinflammation 2005, 2, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, G.; Kralisch, S.; Lipfert, J.; Weise, S.; Krause, K.; Jessnitzer, B.; Lossner, U.; Bluher, M.; Stumvoll, M.; Fasshauer, M. Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J. Cell. Biochem. 2009, 108, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- May, P.C.; Dean, R.A.; Lowe, S.L.; Martenyi, F.; Sheehan, S.M.; Boggs, L.N.; Monk, S.A.; Mathes, B.M.; Mergott, D.J.; Watson, B.M.; et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J. Neurosci. 2011, 31, 16507–16516. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.H.; Manczak, M.; Yin, X.L.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-beta in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef]
- Geng, J.; Zhao, C.; Ren, J.; Qu, X. Alzheimer’s disease amyloid beta converting left-handed Z-DNA back to right-handed B-form. Chem. Commun. 2010, 46, 7187–7189. [Google Scholar] [CrossRef]
- Atamna, H.; Boyle, K. Amyloid-beta peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Okumura, N.; Nishimura, Y.; Kitagishi, Y.; Matsuda, S. Turmeric and curcumin suppress presenilin 1 protein expression in Jurkat cells. Exp. Ther. Med. 2011, 2, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zheng, N.; Zhou, H.B.; Huang, J. Curcumin inhibits BACE1 expression through the interaction between ERbeta and NFkappaB signaling pathway in SH-SY5Y cells. Mol. Cell Biochem. 2020, 463, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kruger, L.; Mandelkow, E.M. Tau neurotoxicity and rescue in animal models of human Tauopathies. Curr. Opin. Neurobiol. 2016, 36, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Tran, N.; Geekiyanage, H.; Liu, L.; Chan, C. Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons. Neurosci. Lett. 2013, 554, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.; Netzer, W.J.; Tanemura, K.; Li, F.; Hartl, F.U.; Takashima, A.; Gouras, G.K.; Greengard, P.; Xu, H. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA 2003, 100, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, X.; Wang, C.; Teng, Z.; Li, Y. Curcumin Decreases Hyperphosphorylation of Tau by Down-Regulating Caveolin-1/GSK-3beta in N2a/APP695swe Cells and APP/PS1 Double Transgenic Alzheimer’s Disease Mice. Am. J. Chin. Med. 2017, 45, 1667–1682. [Google Scholar] [CrossRef]
- Kell, D.B. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch. Toxicol. 2010, 84, 825–889. [Google Scholar]
- Liu, G.; Huang, W.; Moir, R.D.; Vanderburg, C.R.; Lai, B.; Peng, Z.; Tanzi, R.E.; Rogers, J.T.; Huang, X. Metal exposure and Alzheimer’s pathogenesis. J. Struct. Biol. 2006, 155, 45–51. [Google Scholar] [CrossRef]
- Lopes de Andrade, V.; Marreilha Dos Santos, A.P.; Aschner, M. Neurotoxicity of Metal Mixtures. Adv. Neurotoxicol. 2021, 5, 329–364. [Google Scholar] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P.; Drago, D.; Bolognin, S.; Sensi, S.L. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol. Sci. 2009, 30, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes-A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Patro, B.S.; Rele, S.; Chintalwar, G.J.; Chattopadhyay, S.; Adhikari, S.; Mukherjee, T. Protective activities of some phenolic 1,3-diketones against lipid peroxidation: Possible involvement of the 1,3-diketone moiety. Chembiochem 2002, 3, 364–370. [Google Scholar] [CrossRef]
- Momeni, H.R.; Eskandari, N. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Exp. Toxicol. Pathol. 2017, 69, 93–97. [Google Scholar] [CrossRef]
- Picciano, A.L.; Vaden, T.D. Complexation between Cu(II) and curcumin in the presence of two different segments of amyloid beta. Biophys. Chem. 2013, 184, 62–67. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Frautschy, S.A.; Hu, W.; Kim, P.; Miller, S.A.; Chu, T.; Harris-White, M.E.; Cole, G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging 2001, 22, 993–1005. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Park, S.Y.; Kim, J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA (1-42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Chin, D.; Huebbe, P.; Pallauf, K.; Rimbach, G. Neuroprotective properties of curcumin in Alzheimer’s disease--merits and limitations. Curr. Med. Chem. 2013, 20, 3955–3985. [Google Scholar] [CrossRef]
- Belkacemi, A.; Doggui, S.; Dao, L.; Ramassamy, C. Challenges associated with curcumin therapy in Alzheimer disease. Expert Rev. Mol. Med. 2011, 13, e34. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and Its Derivatives as Theranostic Agents in Alzheimer’s Disease: The Implication of Nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef]

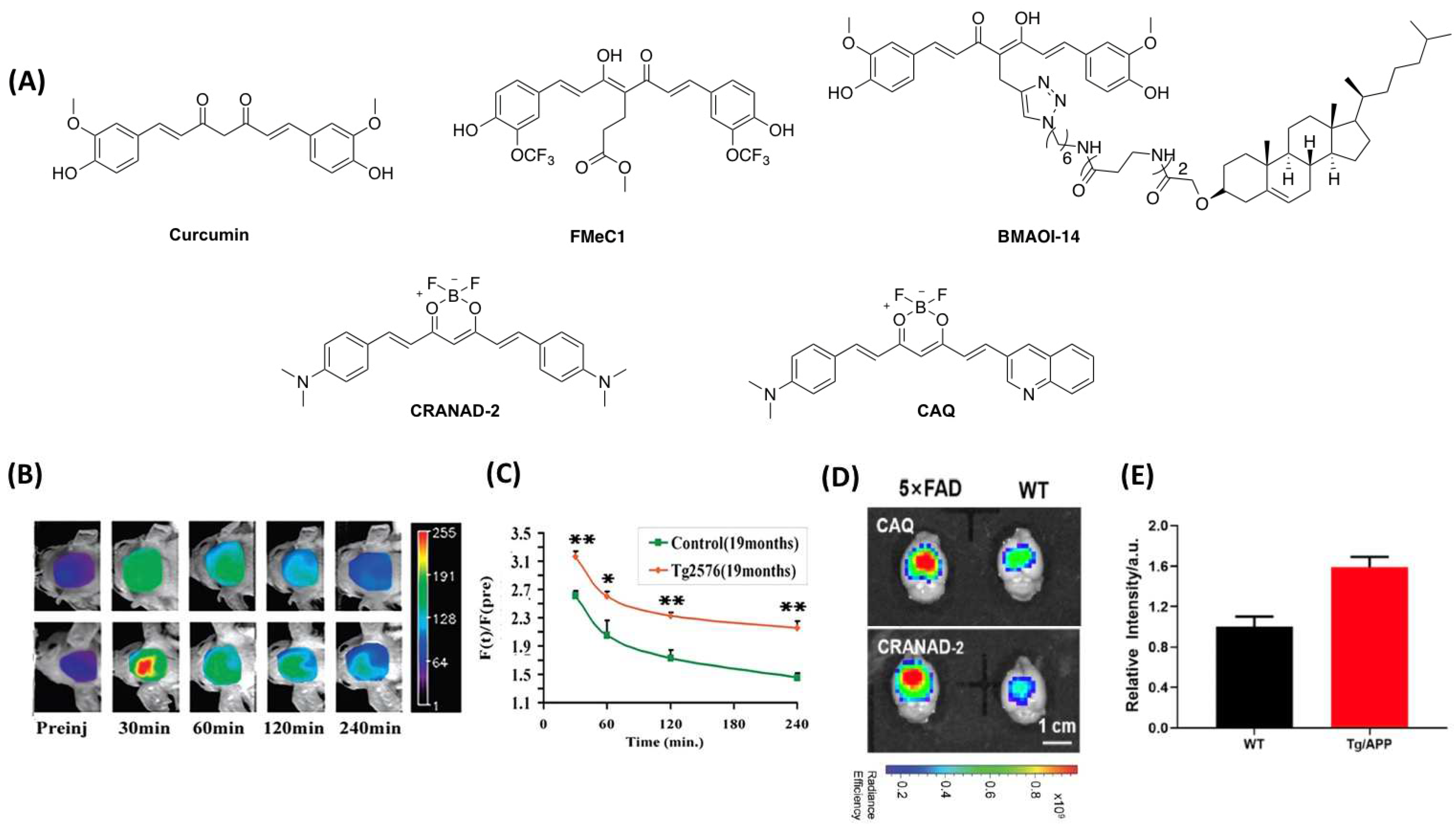

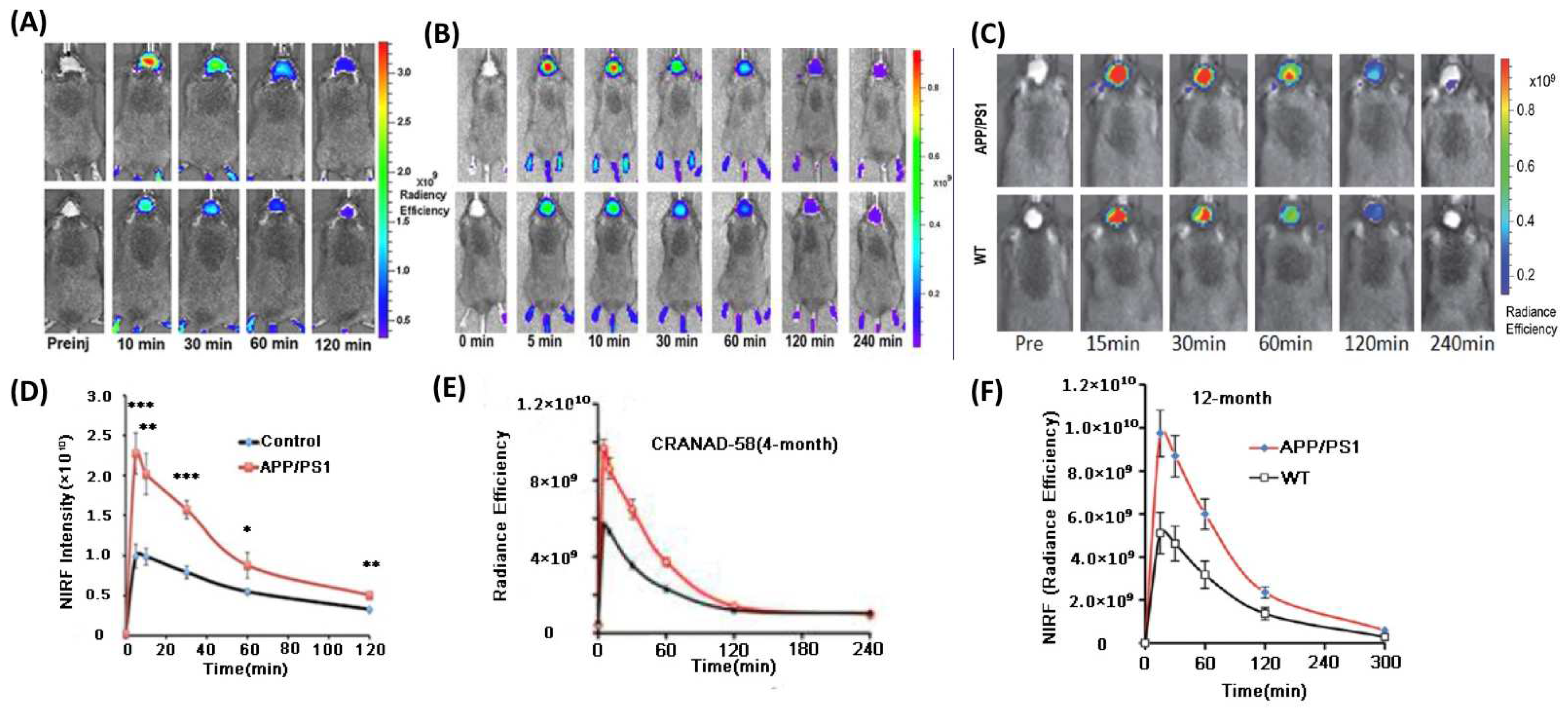
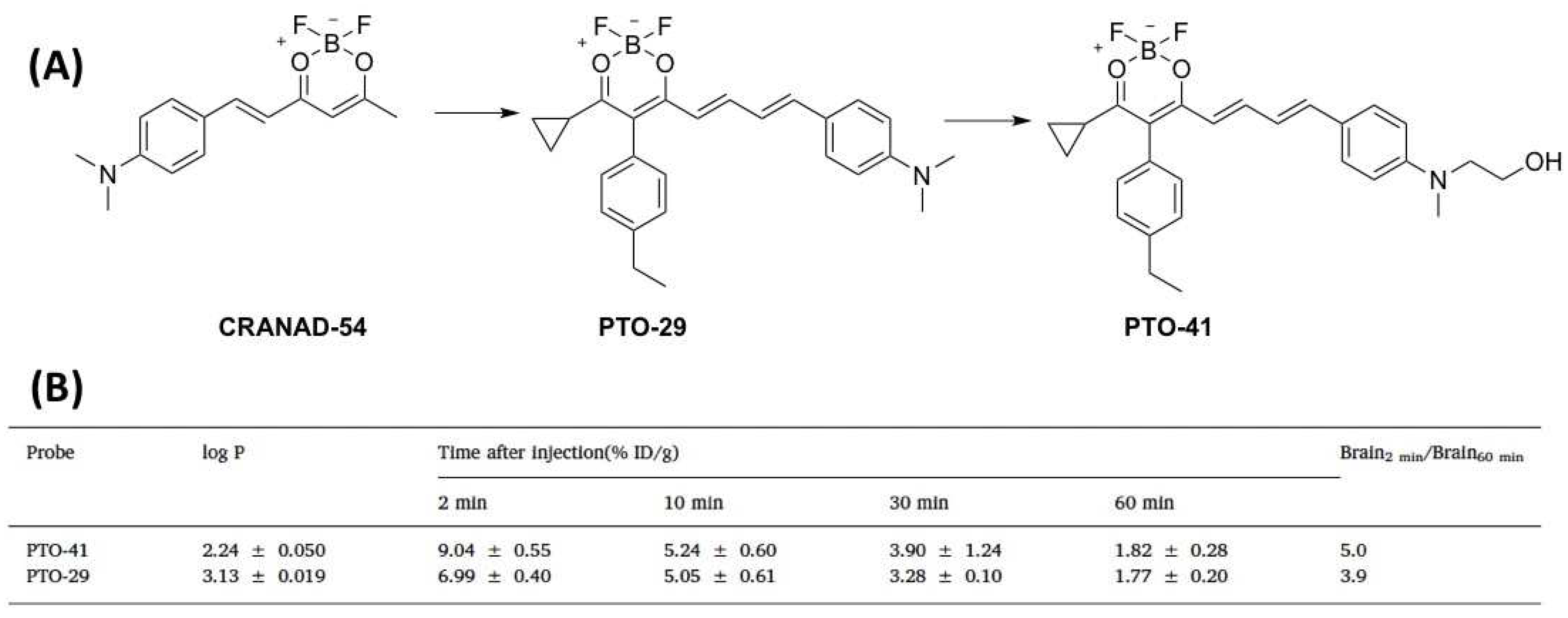
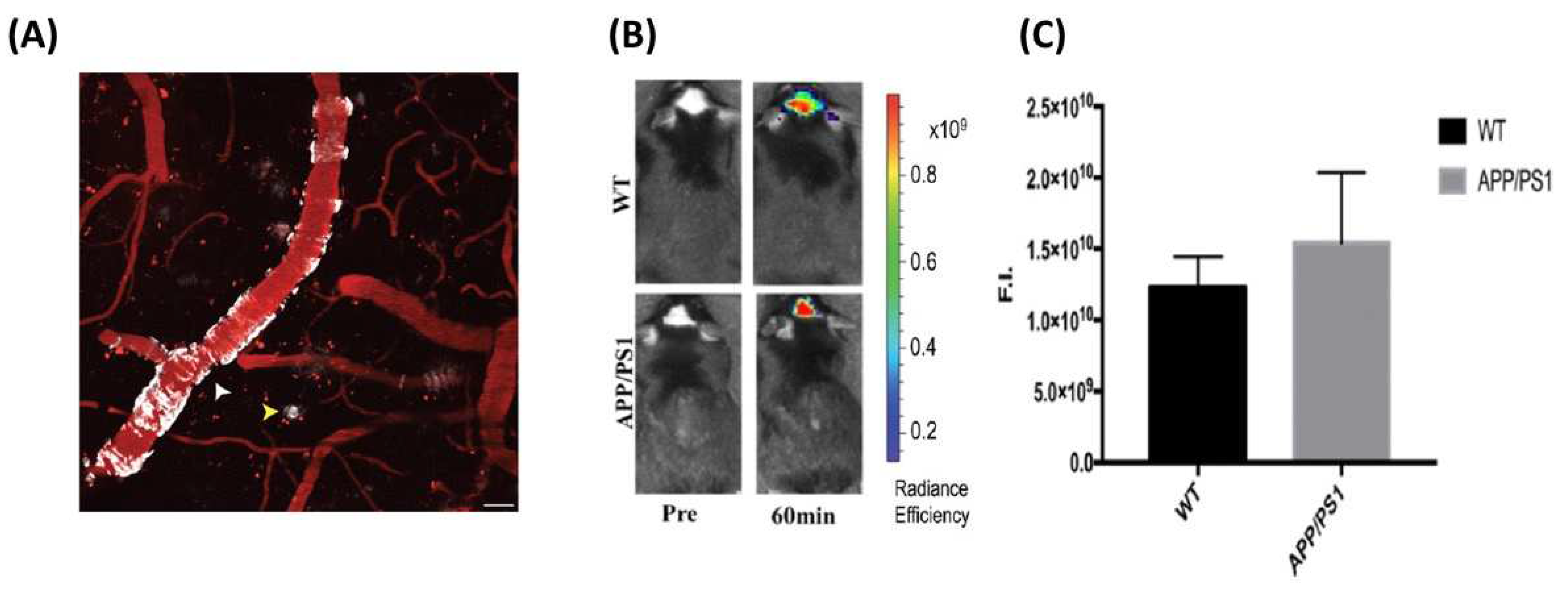

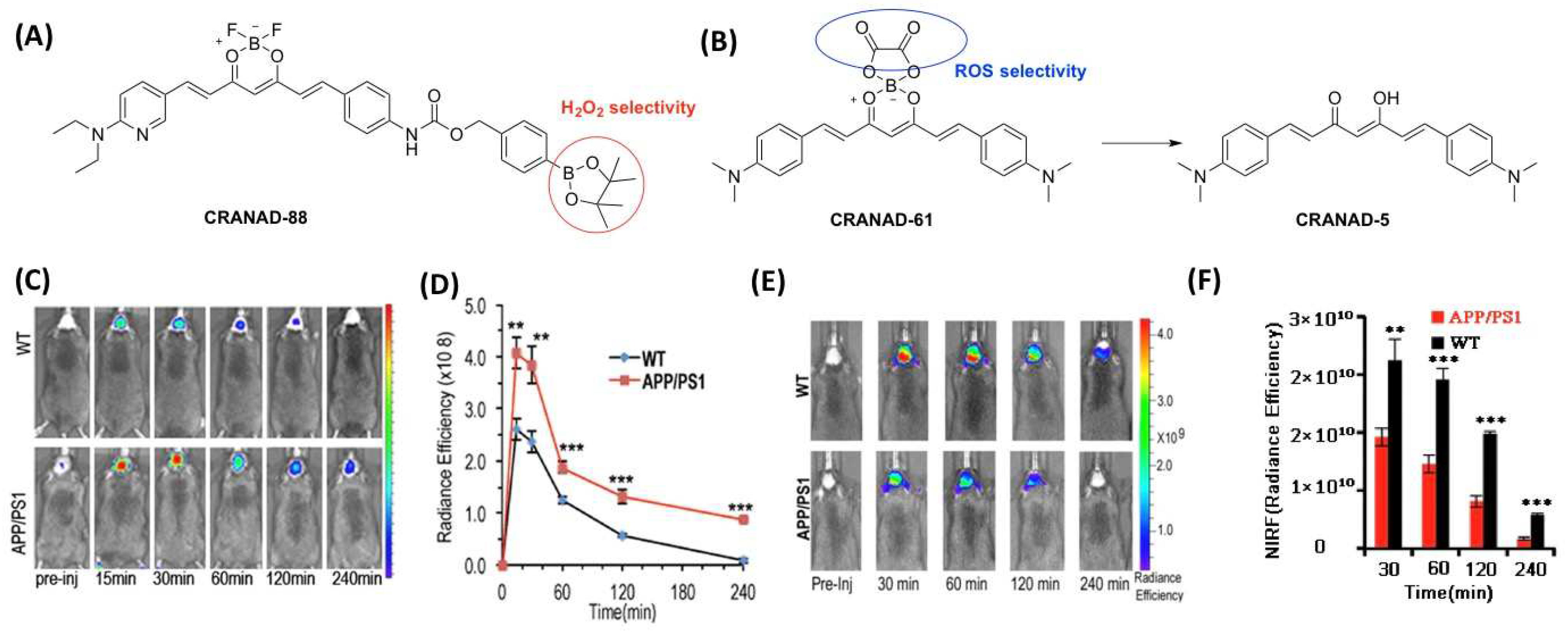
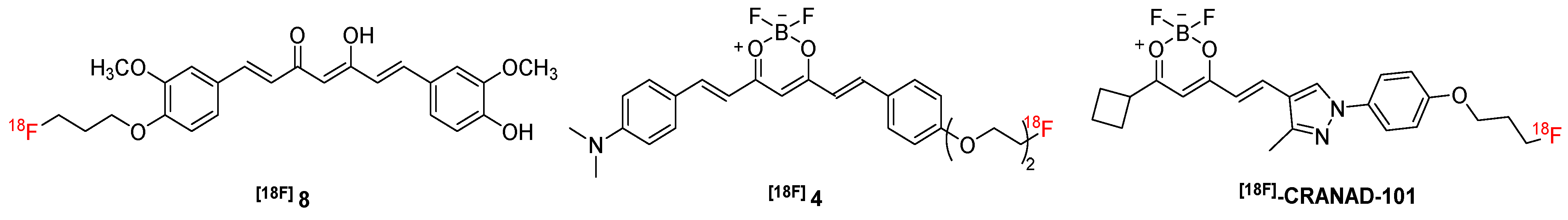

| Name | Mw | Ex/Em (nm) | Kd (nM) | Φ | Selectivity | APP Mouse Month | Ref. |
|---|---|---|---|---|---|---|---|
| Curcumin | 368.39 | 510/560 | n.d. | n.d. | n.d. | 22 | [47] |
| BMAOI-14 | 1117.48 | 445/480~700 | 2030 b 2170 c | n.d. | insoluble Aβ | 2, 7 | [48] |
| FMeC1 | 562.42 | 440/556 | n.d. | n.d | insoluble Aβ | 12 | [49] |
| CRANAD-1 | 416.18 | 540/640 | n.d. | n.d. | n.d. | 19 | [50] |
| CRANAD-2 | 410.27 | 640/715 | 38.69 d | n.d. | Aβ | 19 | [50] |
| CAQ | 418.25 | 614/726 | 78.89 | 1.1% | insoluble Aβ | 6, 10 | [51] |
| CRANAD-17 | 456.26 | n.d. | n.d. | n.d. | Aβ | n.d. | [52] |
| CRANAD-54 | 279.09 | n.d. | n.d. | n.d. | Aβ | n.d. | [52] |
| CRANAD-58 | 439.31 | 630/750 | 105.8 a 45.8 b | n.d. | Aβ | 4, 18 | [52] |
| CRANAD-3 | 468.25 | 535/730 | 24 a 23 b 27 c | n.d. | Aβ | 4 | [53] |
| CRANAD-65 | 602.53 | n.d. | n.d. | 3% | soluble Aβ | n.d. | [54] |
| CRANAD-75 | 686.70 | n.d. | n.d. | 4% | soluble Aβ | n.d. | [54] |
| CRANAD-102 | 630.59 | 580/810 | 203.4 a 722.8 b 7.5 c | 1.8% | soluble Aβ | 4, 5, 12, 14 | [54] |
| Probe 1 | 491.26 | 619/675 | 4.00 b 35.66 c 15.38 d | 13.2% | Aβ | 4–14 | [55] |
| Probe 2 | 462.35 | 620/700 | 8.64 b 67.83 c 28.02 d | 26.3% | Aβ | 4–14 | [55] |
| Probe 3 | 474.36 | 621/705 | 10.64 b 36.16 c 13.78 d | n.d. | Aβ | 4–14 | [55] |
| Probe 4 | 488.39 | 623/708 | 31.66 b 186.8 c 39.93 d | n.d. | Aβ | 4–14 | [55] |
| Probe 5 | 490.40 | 622/706 | 15.57 b 72.57 c 54.15 d | n.d. | Aβ | 4–14 | [55] |
| Probe 6 | 492.37 | 605/705 | 3.01 b 25.62 c 13.51 d | n.d. | Aβ | 4–14 | [55] |
| Probe 7 | 512.41 | 610/718 | 64.20 b 166.8 c 142.1 d | n.d. | Aβ | 4–14 | [55] |
| Probe 8 | 476.37 | 622/700 | 10.14 b 118.6 c 76.34 d | n.d. | Aβ | 4–14 | [55] |
| Probe 9 | 542.43 | 620/697 | 11.16 b 36.59 c 14.57 d | 20.31% | Aβ | 4–14 | [55] |
| CRANAD-30 | 476.33 | 572/727 | n.d. | n.d. | Retina Aβ | 14 | [56] |
| PTO-29 | 331.17 | 570/680 | 248(AβO) | 15% | AβOs | 4 | [57] |
| PTO-41 | 361.20 | 538/690 | 349 c | 26% | AβOs | 4 | [58] |
| CRANAD-28 | 484.31 | 498/578 | 68.8 a 159.7 b 85.7 c | 32% | Aβ | 9 | [59] |
| CRANAD-44 | 332.12 | 490/550 | n.d. | 29% | Aβ | 9 | [59] |
| AD-1 | 526.49 | 550/704 | 1040 b 769.4 c | 38% | Aβ | 4, 14 | [60] |
| CRANAD-88 | 671.36 | 580/690 | n.d. | n.d. | H2O2 | 11, 15 | [61] |
| CRANAD-5 | 362.47 | n.d. | 10 | n.d. | Aβ | n.d. | [62] |
| CRANAD-61 | 460.29 | 675/760 | 38.1(Aβs) | 0.55% | ROS, Aβ | 4, 12, 18 | [62] |
| [18F]8 | 427.46 | n.d. | 0.07 | n.d. | Aβ | n.d. | [63] |
| [18F]4 | 472.30 | 550/650 | 19.66 | n.d. | Aβ | 18 | [64] |
| [18F]-CRANAD-101 | 417.23 | 420/550 | 11.64 b 592.3 c | n.d. | Aβ | 5, 14 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zeng, F.; Luo, Y.; Zheng, C.; Ran, C.; Yang, J. Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research. Molecules 2022, 27, 3879. https://doi.org/10.3390/molecules27123879
Yang H, Zeng F, Luo Y, Zheng C, Ran C, Yang J. Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research. Molecules. 2022; 27(12):3879. https://doi.org/10.3390/molecules27123879
Chicago/Turabian StyleYang, Haijun, Fantian Zeng, Yunchun Luo, Chao Zheng, Chongzhao Ran, and Jian Yang. 2022. "Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research" Molecules 27, no. 12: 3879. https://doi.org/10.3390/molecules27123879
APA StyleYang, H., Zeng, F., Luo, Y., Zheng, C., Ran, C., & Yang, J. (2022). Curcumin Scaffold as a Multifunctional Tool for Alzheimer’s Disease Research. Molecules, 27(12), 3879. https://doi.org/10.3390/molecules27123879







