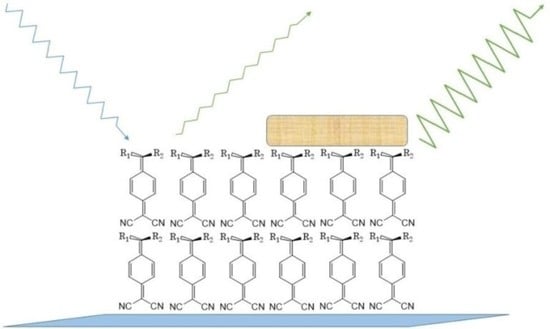
Modulated Fluorescence in LB Films Based on DADQs—A Potential Sensing Surface?
Abstract
:1. Introduction
2. Results
2.1. LB Film Isotherms and LB Film Deposition
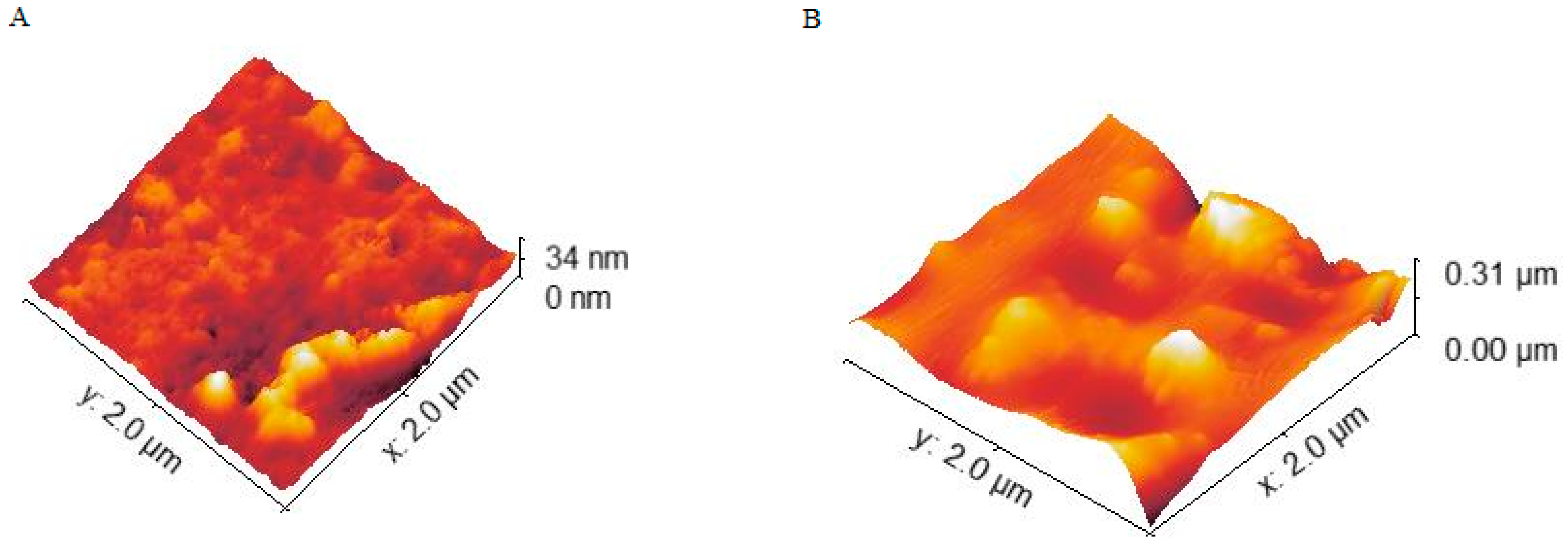
2.2. Atomic Force Microscopy
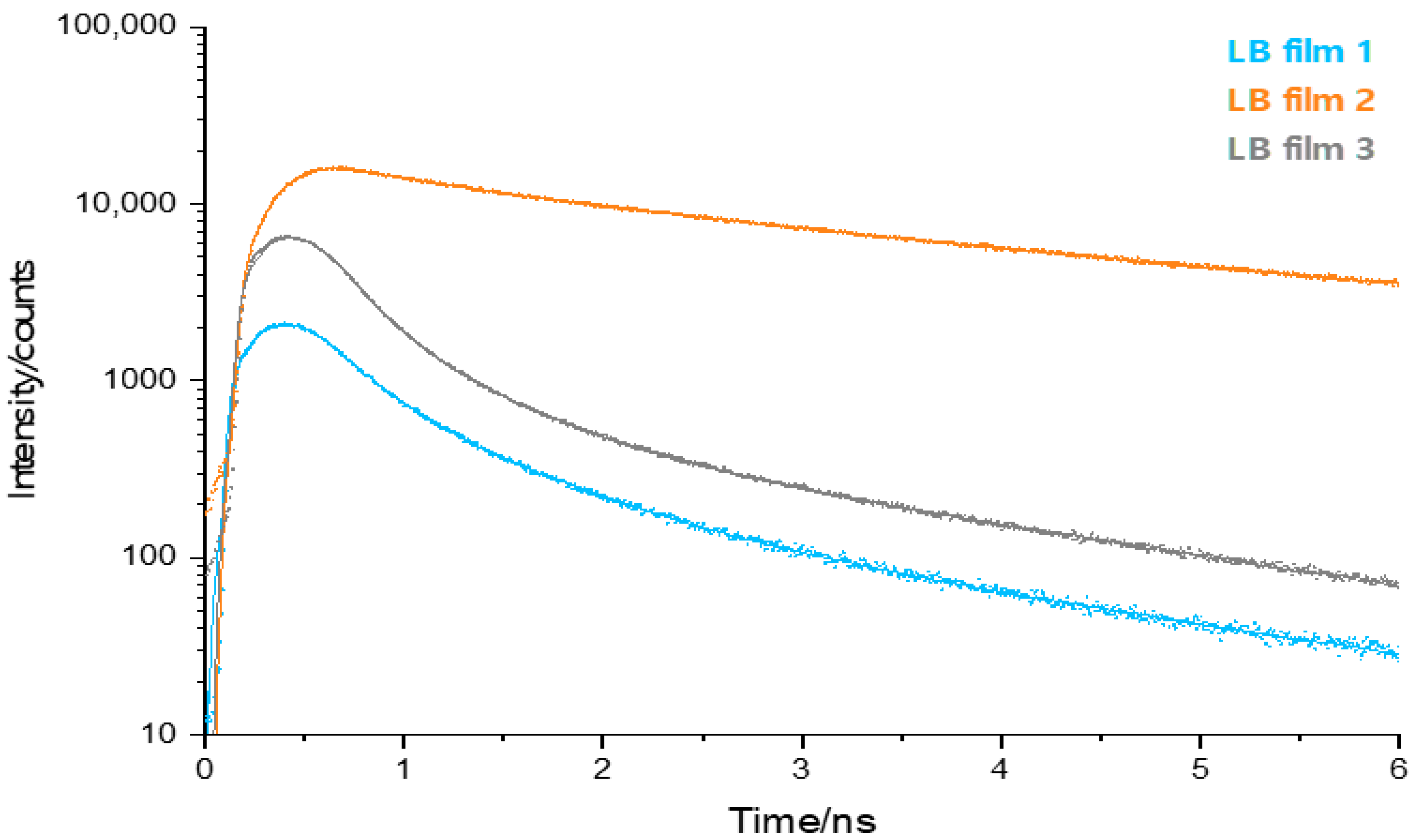
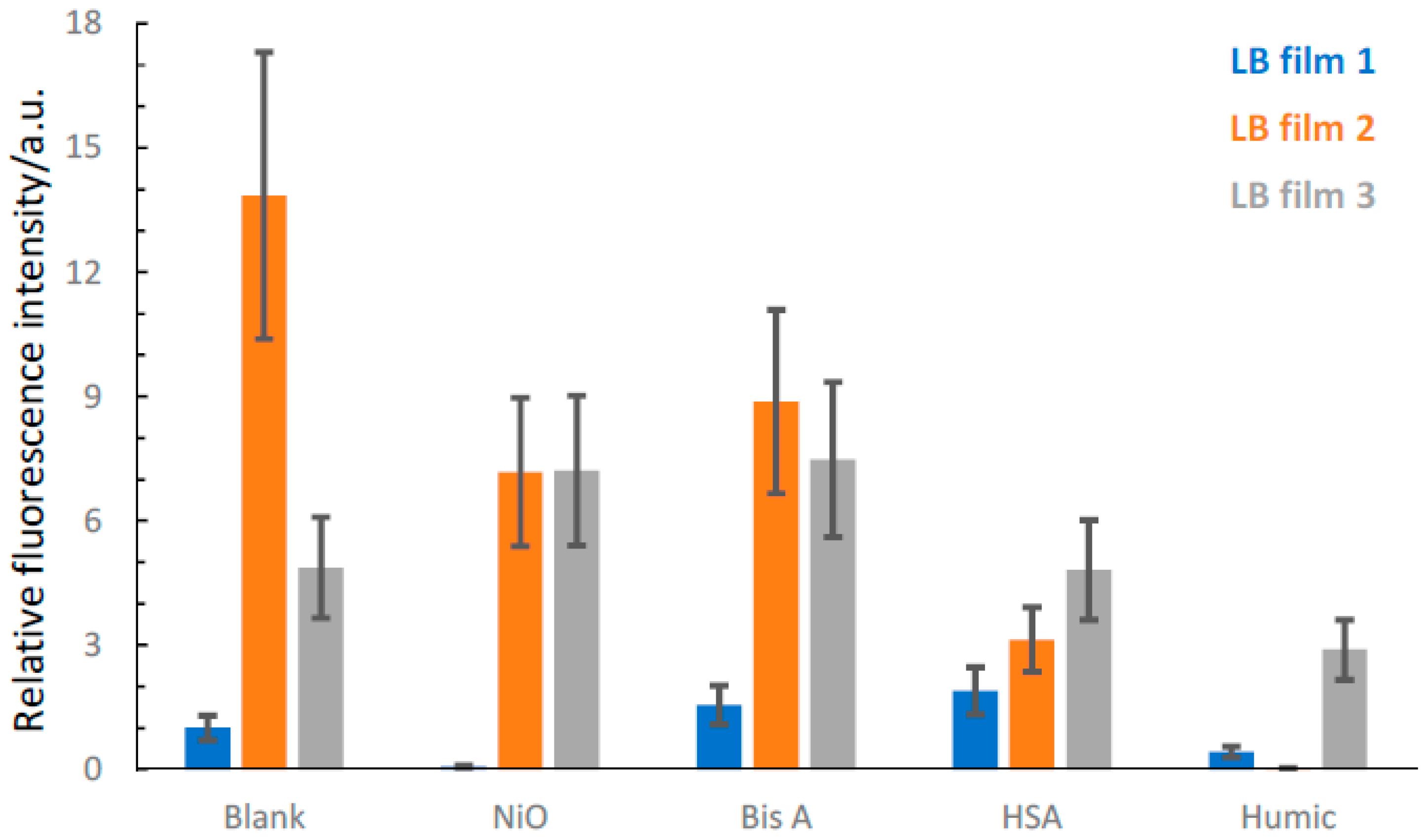
2.3. Time-Resolved Fluorescence Microscopy
3. Discussion
4. Materials and Methods
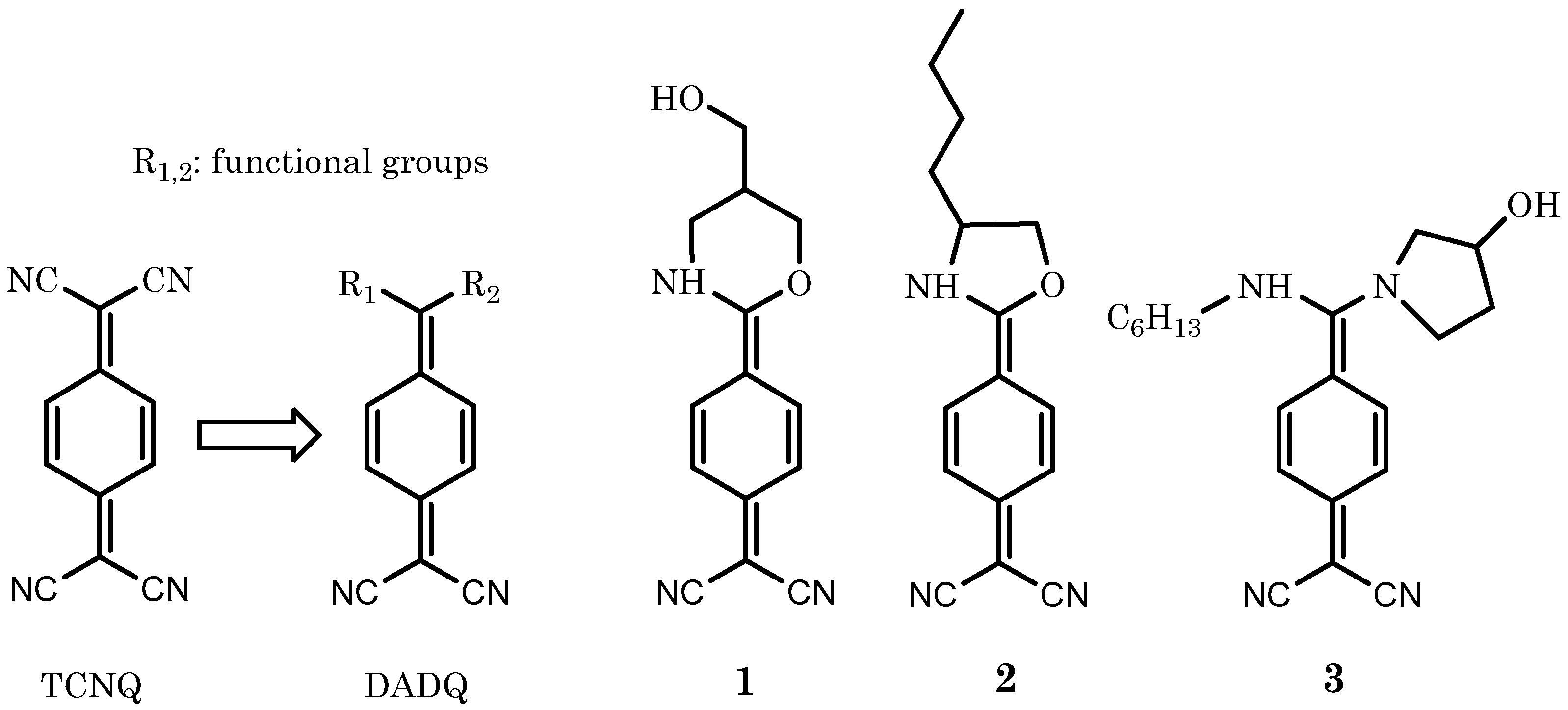
4.1. Materials
4.2. LB Film Fabrication
4.3. Time-Resolved Fluorescence Microscopy
4.4. Atomic Force Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gur, B.; Meral, K. Characterization of merocyanine 540-octadecylamine thin films fabricated by Langmuir-Blodgett and Spin-Coating techniques. J. Mol. Struc. 2019, 1197, 227–234. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, B.; Zhu, Q.; Duan, C. Characterization and Application of PVDF and Its Copolymer Films Prepared by Spin-Coating and Langmuir–Blodgett Method. Polymers 2019, 11, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Zotti, M.; Corvi, G.; Gatto, E.; Di Napoli, B.; Mazzuca, C.; Palleschi, A.; Placidi, E.; Biondi, B.; Crisma, M.; Formaggio, F.; et al. Controlling the Formation of Peptide Films: Fully Developed Helical Peptides are Required to Obtain a Homogenous Coating over a Large Area. Chem. Plus. Chem. 2019, 84, 1688–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieling, T.; Christoffers, J.; Brand, I. Langmuir−Blodgett Monolayers of Partially Fluorinated Ionic Liquids as Two-Dimensional, More Sustainable Functional Materials and Coatings. ACS Sustain. Chem. Eng. 2019, 7, 11593–11602. [Google Scholar] [CrossRef]
- Saha, S.; Chowdhury, J. Sustained and improved enzymatic activity of trypsin immobilized in the Langmuir Blodgett film of DPPC: A rapid enzyme sensor for the detection of Azocasein. Mater. Chem. Phys. 2020, 243, 12264. [Google Scholar] [CrossRef]
- Wang, X.; Yin, F.; Tu, Y. A uric acid biosensor based on Langmuir-blodgett film as an enzyme-immobilizing matrix. Anal. Lett. 2010, 43, 1507–1515. [Google Scholar]
- Sriyudthsak, M.; Yamagishi, H.; Moriizumi, T. Enzyme-immobilized Langmuir-blodgett film for A biosensor. Thin Solid Film. 1988, 160, 463–469. [Google Scholar] [CrossRef]
- Murray, D.J.; Kim, J.H.; Grzincic, E.M.; Kim, S.C.; Abate, A.R.; Zuckermann, R.N. Uniform, Large-Area, Highly Ordered Peptoid Monolayer and Bilayer Films for Sensing Applications. Langmuir 2019, 35, 13671–13680. [Google Scholar] [CrossRef]
- Xu, Y.; Hao, Q.; Mandler, D. Electrochemical detection of dopamine by a calixarene-cellulose acetate mixed Langmuir-Blodgett monolayer. Anal. Chim. Acta 2018, 1042, 29–36. [Google Scholar] [CrossRef]
- Gabaji, M.; Médard, J.; Hemmerle, A.; Pinson, J.; Michel, J.P. From Langmuir−Blodgett to Grafted Films. Langmuir 2020, 36, 2534–2542. [Google Scholar] [CrossRef]
- Bodik, M.; Maxian, O.; Hagara, J.; Nadazdy, P.; Jergel, M.; Majkova, E.; Siffalovic, P. Langmuir−Scheaffer Technique as a Method for Controlled Alignment of 1D Materials. Langmuir 2020, 36, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yamanaka, M.; Kanno, S.; Mizushima, S.; Tsuchiyagaito, J.; Kondo, K.; Kondo, T.; Iwasawa, D.; Komiya, H.; Saso, A.; et al. Europium amphiphilic naphthalene based complex for the enhancement of linearly polarized luminescence in Langmuir–Blodgett films. New J. Chem. 2019, 43, 6472–6479. [Google Scholar]
- Shang, C.; Wang, L.; An, Y.; Chen, P.; Chang, X.; Qi, Y.; Kang, R.; Fang, Y. Langmuir–Blodgett films of perylene bisimide derivatives and fluorescent recognition of diamines. Phys. Chem. Chem. Phys. 2017, 19, 23898–23904. [Google Scholar] [CrossRef]
- Rajesh, K.; Rajendra, K.; Radhkrishnan, T.P. Fluorescent enhancement in in Langmuir-Blodgett films—Role of amphiphile structure, orientation and assembley. J. Phys. Chem. B 2010, 114, 849–856. [Google Scholar] [CrossRef]
- Zheng, Y.; Orbulescu, J.; Ji, X.; Andreopoulos, F.M.; Pham, S.M.; Leblanc, R.M. Development of Fluorescent Film Sensors for the Detection of Divalent Copper. J. Am. Chem. Soc. 2003, 125, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Balaswamy, B.; Maganti, L.; Sharma, S.; Radhakrishnan, T.P. Mechanical Control of Molecular Aggregation and Fluorescence Switching/Enhancement in an Ultrathin Film. Langmuir 2012, 28, 17313–17321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Liu, J.; Xiong, Y.; Li, Y.; Li, H. Fluorescent sensing of free bilirubin at nanomolar level using a Langmuir–Blodgett film of glucuronic acid–functionalized gold nanoclusters. Anal. Bioanalyt. Chem. 2021, 413, 7009–7019. [Google Scholar] [CrossRef]
- Van der Auweraer, M.; Verschuere, B.; De Schryver, F.C. Absorption and Fluorescence Properties of Rhodamine B Derivatives Forming Langmuir-Blodgett Films. Langmuir 1988, 4, 583–588. [Google Scholar] [CrossRef]
- Acker, D.S.; Harder, R.J.; Hertler, W.R.; Mahler, W.; Melby, L.R.; Benson, R.E.; Mochel, W.E. 7,7,8,8-Tetracyanoquinodimetane and its Electrically Conducting Anion-Radical Derivatives. J. Am. Chem. Soc. 1960, 82, 6408–6409. [Google Scholar] [CrossRef]
- Hertler, W.R.; Hartzler, H.D.; Acker, D.S.; Benson, R.E. Substituted Quinodimethans. III. Displacement Reactions of 7,7,8,8-Tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3387–3393. [Google Scholar] [CrossRef]
- Szablewski, M.; Thomas, P.R.; Thornton, A.; Bloor, D.; Cross, G.H.; Cole, J.M.; Howard, J.A.K.; Malagoli, M.; Meyers, F.; Brédas, J.-L.; et al. Highly Dipolar, Optically Nonlinear Adducts of Tetracyano-p-quinodimethane: Synthesis, Physical Characterization, and Theoretical Aspects. J. Am. Chem. Soc. 1997, 119, 3144–3154. [Google Scholar] [CrossRef] [Green Version]
- Bloor, D.; Kagawa, Y.; Szablewski, M.; Ravi, M.; Clark, S.J.; Cross, G.H.; Pålsson, L.-O.; Beeby, A.; Parmer, C.; Rumbles, G. Matrix dependence of light emission from TCNQ adducts. J. Mater. Chem. 2001, 11, 3053–3062. [Google Scholar] [CrossRef]
- Ashwell, G.J.; Dawnay, E.J.C.; Kuczynski, A.P.; Szablewski, M.; Sandy, I.M.; Bryce, M.R.; Grainger, A.M.; Hasan, M. Langmuir–Blodgett alignment of zwitterionic optically non-linear D–π–A materials. J. Chem. Soc. Faraday Trans. 1990, 86, 1117–1121. [Google Scholar] [CrossRef]
- Ashwell, G.J.; Sambles, J.R.; Martin, A.S.; Parker, W.G.; Szablewski, M. Rectifying characteristics of Mg|(C16H33-Q3CNQ LB film)|Pt structures. J. Chem. Soc. Chem. Commun. 1990, 19, 1374–1376. [Google Scholar] [CrossRef]
- Campo, J.; Wenseleers, W.; Goovaerts, E.; Szablewski, M.; Cross, G.H. Accurate Determination and Modeling of the Dispersion of the First Hyperpolarizability of an Efficient Zwitterionic Nonlinear Optical Chromophore by Tunable Wavelength Hyper-Rayleigh Scattering. J. Phys. Chem. 2008, 112, 287–296. [Google Scholar] [CrossRef]
- Szablewski, M.; Fox, M.A.; Dias, F.B.; Namiah, H.; Snedden, E.W.; King, S.M.; Dai, D.; Pålsson, L.-O. Ultrafast Dynamics and Computational Studies on Diaminodicyanoquinodimethanes (DADQs). J. Phys. Chem. B 2014, 118, 6815–6828. [Google Scholar] [CrossRef] [Green Version]
- Srujana, P.; Gera, T.; Radhakrishnan, T.P. Fluorescence enhancement in crystals tuned by a molecular torsion angle: A model to analyze structural impact. J. Mater. Chem. C 2016, 4, 6510–6515. [Google Scholar] [CrossRef]
- Sillen, A.; Engelborghs, Y. The Correct Use of “Average” Fluorescence Parameters. Photochem. Photobiol. 1998, 67, 475–486. [Google Scholar]
- Hestand, N.J.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef]
- Pearson, C.; Moore, A.J.; Gibson, E.; Bryce, M.R.; Petty, M.C. A field effect transistor based on Langmuir-Blodgett films of an Ni(dmit)2 charge transfer complex. Thin Solid Film. 1994, 244, 932–935. [Google Scholar] [CrossRef]
- Rune, A.; Hartvig, R.A.; van de Weert, M.; Østergaard, J.; Jorgensen, L.; Jensen, H. Protein Adsorption at Charged Surfaces: The Role of Electrostatic Interactions and Interfacial Charge Regulation. Langmuir 2011, 27, 2634–2643. [Google Scholar]
- Makoto, I.; Akihiro, M.; Tokuji, M. Non-specific and Specific Adsorption of Proteins on Langmuir–Blodgett Films of Amino Acid Derivative Polymers. Bull. Chem. Soc. Jpn. 2001, 74, 1355–1359. [Google Scholar]
- Sah, B.K.; Kundu, S. Behaviour of protein (BSA)-lipid (DMPA) mixed monolayer on the spreading order of the individual component. Chem. Phys. Lip. 2019, 225, 104810. [Google Scholar] [CrossRef] [PubMed]
- Chae, I.; Ngo, D.; Chen, Z.; Kwansa, A.L.; Chen, X.; Meddeb, A.B.; Podraza, N.J.; Yingling, Y.G.; Ounaies, Z.; Kim, S.H. Anisotropic Optical and Frictional Properties of Langmuir-Blodgett Film Consisting of Uniaxially-Aligned Rod-Shaped Cellulose Nanocrystals. Adv. Mater. Interfaces 2020, 7, 1902169. [Google Scholar] [CrossRef]
- Furini, L.N.; Martin, C.S.; Camacho, S.A.; Rubira, R.J.G.; Fernandes, J.D.; Silva, E.A.; Gomes, T.C.; Stunges, G.M.; Constantino, C.J.L.; Alessio, P. Electrochemical properties of nickel phthalocyanine: The effect of thin film morphology tuned by deposition techniques. Thin Solid Film. 2020, 699, 137897. [Google Scholar] [CrossRef]
- Pal, R.; Barker, A.C.J.; Hummel, D.; Pålsson, L.-O. In Vitro and in Cellulo Sensing of Transition Metals Using Time-Resolved Fluorescence Spectroscopy and Microscopy. J. Fluoresc. 2019, 29, 255–263. [Google Scholar] [CrossRef] [Green Version]
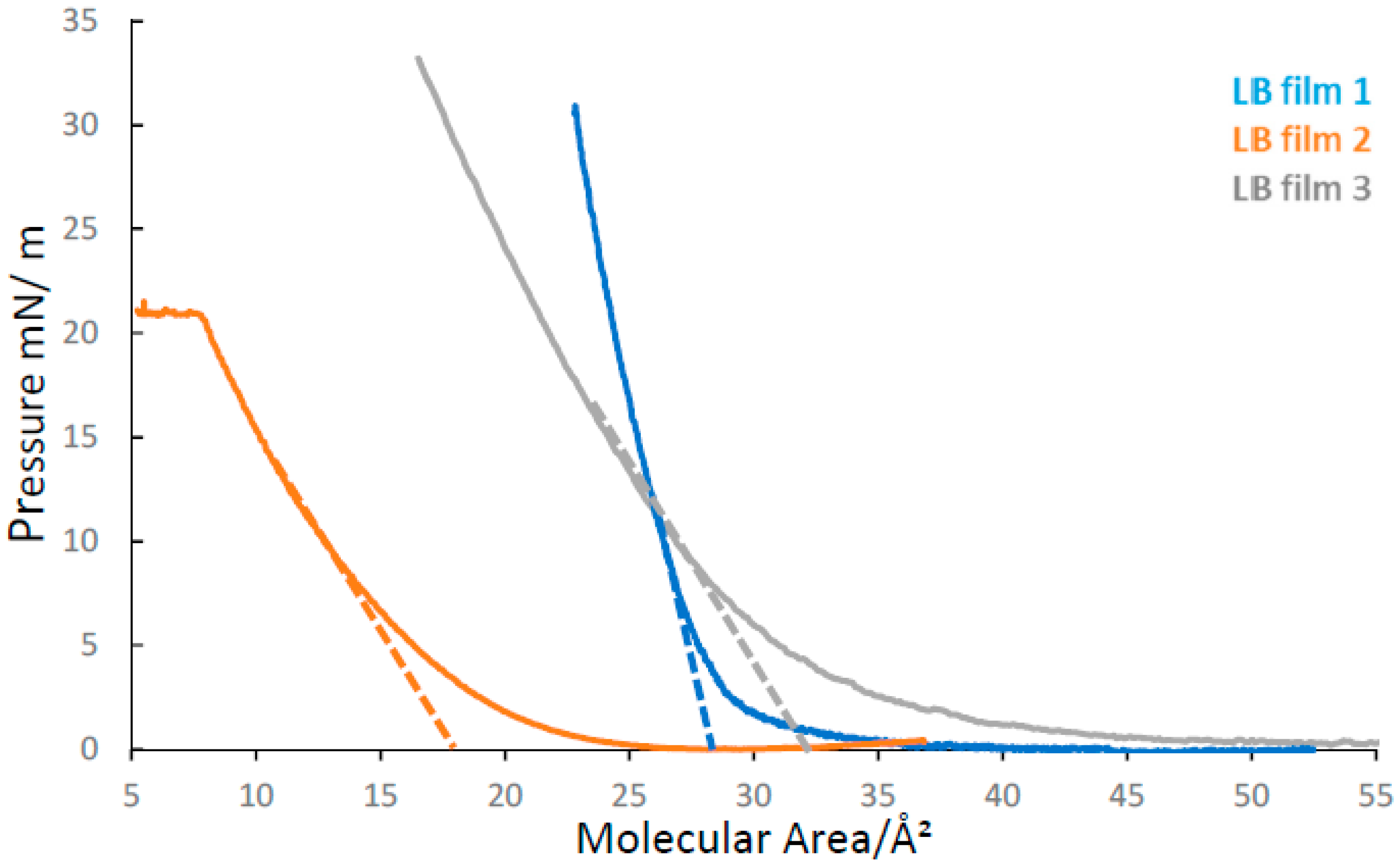


| DADQ LB Film | Target Surface Pressure/mN × m−1 | Molecular Head Area /Å2 | Transfer Ratio /a.u. | Compressibility /mN × m−1 |
|---|---|---|---|---|
| 1 | 30 | 28.0 ± 3.0 | 1.03 ± 0.04 | 7.14 ± 0.82 |
| 2 | 18 | 18.0 ± 3.0 | 1.23 ± 0.04 | 38.15 ± 6.48 |
| 3 | 34 | 32.0 ± 4.0 | 0.97 ± 0.05 | 15.62 ± 2.02 |
| DADQ LB Film | 1 | 2 | 3 |
|---|---|---|---|
| NiO | No change in average lifetime. Decreased intensity. Anomaly? | Shorter average lifetime. Decreased intensity. Consistent observations. | Longer average lifetime. Decreased intensity. An anomaly. |
| Bisphenol A (Bis A) | Longer average lifetime. Intensity increase. Consistent observations | Shorter average lifetime. Decreased intensity. Consistent observations. | No change in average lifetime. Decreased intensity. Anomaly? |
| Humic acid (HA) | Longer average lifetime. Decreased intensity. An anomaly. | Shorter average lifetime. Decreased intensity. Consistent observations. | Longer average lifetime. Decreased intensity. An anomaly. |
| Human serum Albumin (HSA) | Longer average lifetime. Intensity increase. Consistent observations | No change in average lifetime. Decreased intensity. Anomaly? | No change in average lifetime. Decreased intensity. Anomaly? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szablewski, M.; Thompson, R.L.; Pålsson, L.-O. Modulated Fluorescence in LB Films Based on DADQs—A Potential Sensing Surface? Molecules 2022, 27, 3893. https://doi.org/10.3390/molecules27123893
Szablewski M, Thompson RL, Pålsson L-O. Modulated Fluorescence in LB Films Based on DADQs—A Potential Sensing Surface? Molecules. 2022; 27(12):3893. https://doi.org/10.3390/molecules27123893
Chicago/Turabian StyleSzablewski, Marek, Richard L. Thompson, and Lars-Olof Pålsson. 2022. "Modulated Fluorescence in LB Films Based on DADQs—A Potential Sensing Surface?" Molecules 27, no. 12: 3893. https://doi.org/10.3390/molecules27123893
APA StyleSzablewski, M., Thompson, R. L., & Pålsson, L.-O. (2022). Modulated Fluorescence in LB Films Based on DADQs—A Potential Sensing Surface? Molecules, 27(12), 3893. https://doi.org/10.3390/molecules27123893