Biopolymer from Water Kefir as a Potential Clean-Label Ingredient for Health Applications: Evaluation of New Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of the Growth Rate of Water Kefir Grains (GGR)
2.2. Production and Purification of EPSwk
2.3. Cryoprotection of Water Kefir Grains
2.4. Characterization of EPSwk
2.4.1. Physico-Chemical Parameters and Zeta Potential (ζ)
2.4.2. Determination of Molecular Weight (Mw)
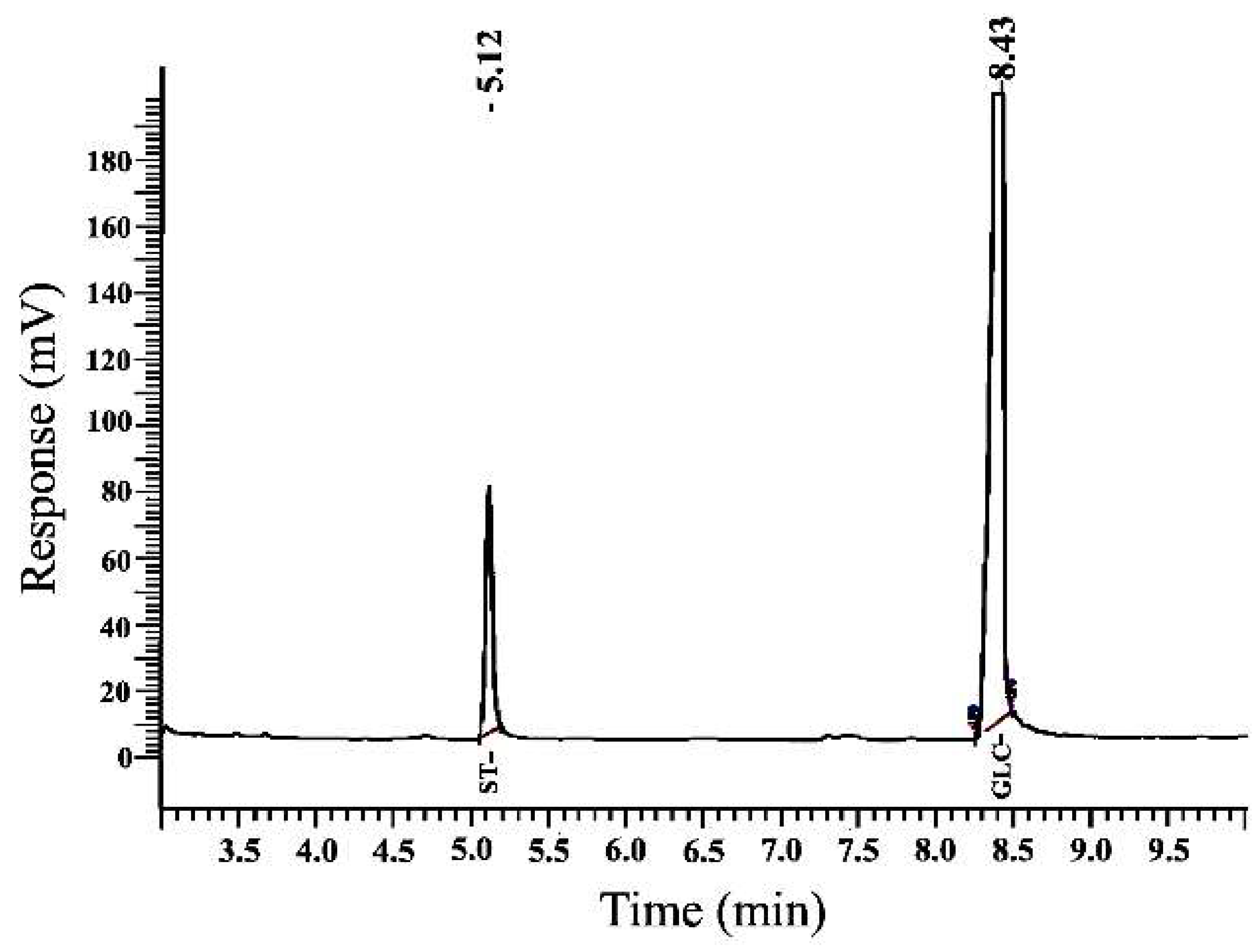
2.4.3. Monosaccharide Content
2.4.4. Protein Content
2.4.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.6. X-ray Diffraction (XRD)
2.4.7. Thermal Properties
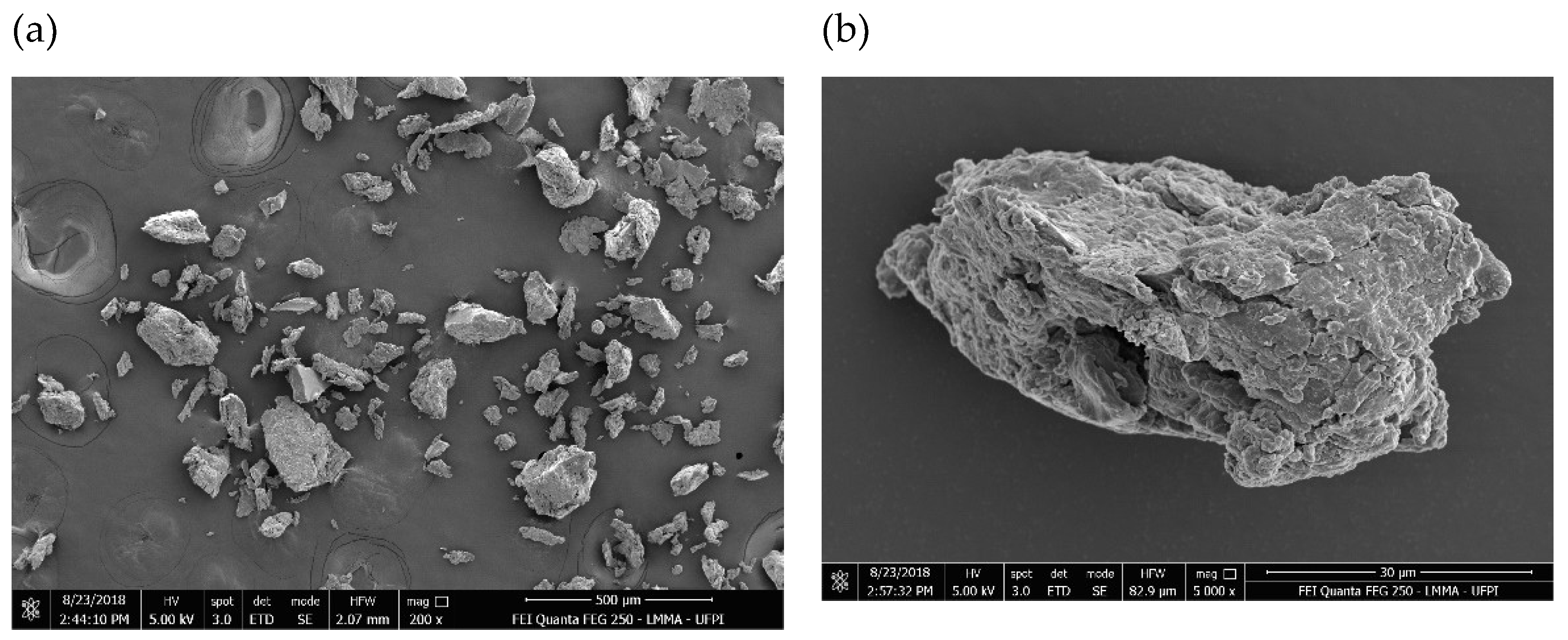
2.4.8. Scanning Electron Microscopy (SEM)
2.5. Functional Properties
2.6. Photostability Study
2.7. Biological Assays
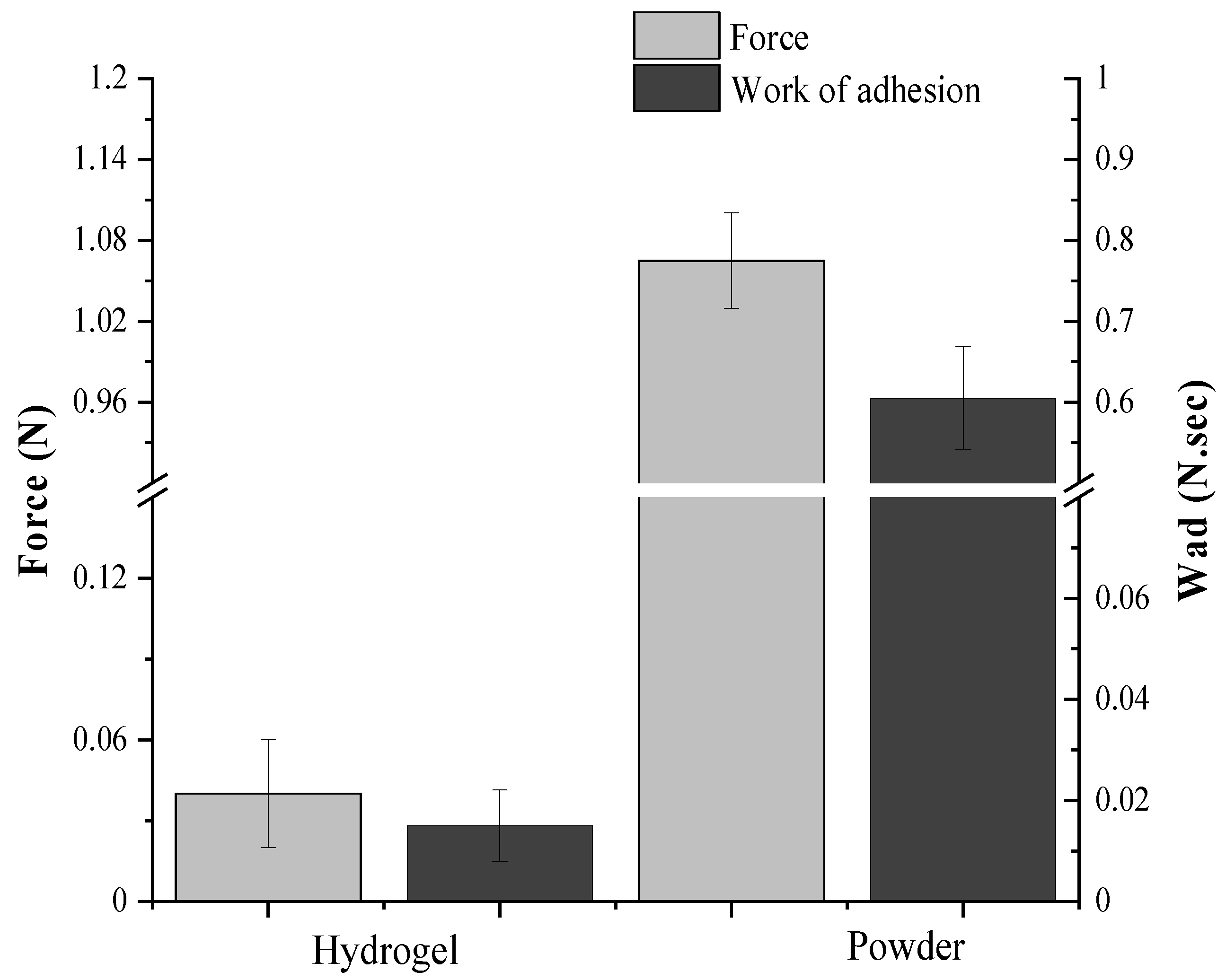
2.7.1. Ex Vivo Mucoadhesiveness
2.7.2. Antimicrobial Activity
2.7.3. In Vitro Cytotoxicity Assay
2.7.4. Hemocompatibility Assay
2.7.5. Microbiological Control and Water Activity (Aw)
3. Materials and Methods
3.1. Materials
3.2. Evaluation of the Growth Rate (GGR) of Water Kefir Grains
3.3. Extraction of Exopolysaccharide from Water Kefir Grains (EPSwk)
3.4. Cryoprotection of Water Kefir Grains
3.5. Characterization of EPSwk
3.5.1. Physico-Chemical Parameters and Zeta Potential (ζ)
3.5.2. Molecular Weight
3.5.3. Protein Content
3.5.4. Analysis of the Monosaccharide Composition
3.5.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.5.6. X-ray Diffraction (XRD)
3.5.7. Thermogravimetry (TGA) and Differential Scanning Calorimetry (DSC)
3.5.8. Scanning Electron Microscopy (SEM)
3.6. Functional Properties
3.6.1. Emulsifying Ability (EA) and Emulsifying Stability (ES)
3.6.2. Water Holding Capacity (WHC)
3.6.3. Water Solubility (Sol)
3.6.4. Foaming Ability (FA) and Foam Stability (FS)
3.6.5. Swelling Index
3.6.6. Film-Forming Ability
3.7. Photostability Study
3.8. Biological Assays
3.8.1. Ex Vivo Mucoadhesiveness
3.8.2. Antimicrobial Activity
3.8.3. In Vitro Cytotoxicity Assay
3.8.4. Hemocompatibility Assay
3.9. Microbiological Control and Water Activity (Aw)
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Norberto, A.P.; Marmentini, R.P.; Carvalho, P.H.; Campagnollo, F.B.; Takeda, H.H.; Alberte, T.M.; Rocha, R.S.; Cruz, A.G.; Alvarenga, V.O.; Sant’Ana, A.S. Impact of partial and total replacement of milk by water-soluble soybean extract on fermentation and growth parameters of kefir microorganisms. LWT 2018, 93, 491–498. [Google Scholar] [CrossRef]
- Luang-In, V.; Deeseenthum, S. Exopolysaccharide-producing isolates from Thai milk kefir and their antioxidant activities. LWT 2016, 73, 592–601. [Google Scholar] [CrossRef]
- Laureys, D.; Van Jean, A.; Dumont, J.; De Vuyst, L. Investigation of the instability and low water kefir grain growth during an industrial water kefir fermentation process. Appl. Microbiol. Biotechnol. 2017, 101, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.T.; de MPereira, G.V.; Dias, D.R.; Schwan, R.F. Microbial communities and chemical changes during fermentation of sugary Brazilian kefir. World J. Microbiol. Biotechnol. 2010, 26, 1241–1250. [Google Scholar] [CrossRef]
- Zanirati, D.F.; Abatemarco, M.; Sandes, S.H.C.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation-A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef]
- Corona, O.; Randazzo, W.; Miceli, A.; Guarcello, R.; Francesca, N.; Erten, H.; Moschetti, G.; Settanni, L. Characterization of kefir-like beverages produced from vegetable juices. LWT 2016, 66, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: A Review and New Perspectives on Engineering Strategies and Applications. Carbohydr. Polym. 2018, 205, 8–26. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Sellamuthu, P.S.; Perumal, A.B.; Sadiku, E.R.; Phiri, G.; Jayaramudu, J. Characterization of an exopolysaccharide produced by Lactobacillus plantarum HM47 isolated from human breast milk. Process Biochem. 2018, 73, 15–22. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Xu, D.; Fels, L.; Wefers, D.; Behr, J.; Jakob, F.; Vogel, R.F. Lactobacillus hordei dextrans induce Saccharomyces cerevisiae aggregation and network formation on hydrophilic surfaces. Int. J. Biol. Macromol. 2018, 115, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Le Balc’h, R.; Brodu, N.; Gilbert, M.; Le Gouic, B.; Le Gall, S.; Sinquin, C.; Massé, A. Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem. 2018, 74, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Wang, X.; Pan, W.; Shen, X.; He, Y.; Yin, H.; Zhou, K.; Zou, L.; Chen, S.; Liu, S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2018, 121, 342–349. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012, 87, 581–588. [Google Scholar] [CrossRef]
- Paiva, I.M.; da Silva Steinberg, R.; Lula, I.S.; de Souza-Fagundes, E.M.; de Oliveira Mendes, T.; Bell, M.J.V.; Nicoli, J.R.; Nunes, A.C.; Neumann, E. Lactobacillus kefiranofaciens and Lactobacillus satsumensis isolated from Brazilian kefir grains produce alpha-glucans that are potentially suitable for food applications. LWT 2016, 72, 390–398. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.G.; Lin, Y.H.; Zhao, D.X.; Wu, Y.K.; Yan, R.R.; Zhao, H.B.; Tan, Z.L.; Jia, S.R.; Han, P.P. Comparisons of Functional Properties of Polysaccharides from Nostoc flagelliforme under Three Culture Conditions. Polymers 2019, 11, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, I.F.S.; Magalhães, L.M.; Pessoa, C.O.; Ferreira, P.M.P.; Rizzo, M.S.; Osajima, J.A.; Silva-Filho, E.C.; Nunes, C.; Raposo, F.; Coimbra, M.A.; et al. New properties of chia seed mucilage (Salvia hispanica L.) and potential application in cosmetic and pharmaceutical products. Ind. Crops Prod. 2021, 171, 113981. [Google Scholar] [CrossRef]
- Nascimento, R.Q.; Deamici, K.M.; Tavares, P.P.L.G.; de Andrade, R.B.; Guimarães, L.C.; Costa, J.A.V.; Magalhães-Guedes, K.T.; Druzian, J.I.; de Souza, C.O. Improving water kefir nutritional quality via addition of viable Spirulina biomass. Bioresour. Technol. Rep. 2022, 17, 100914. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Cao, C.; Zhu, X.; Wang, C.; Wu, R.; Wu, J. Extraction and biological activity of exopolysaccharide produced by Leuconostoc mesenteroides SN-8. Inter J Biol Macromol 2020, 157, 36–44. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, J.; Liu, L.; Wang, S.; Ping, W.; Ge, J. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int. J. Biol. Macromol. 2021, 178, 306–315. [Google Scholar] [CrossRef]
- Rao, T.J.M.; Goyal, A. A novel high dextran yielding Weissella cibaria JAG8 for cereal food application. Int. J. Food Sci. Nutr. 2013, 64, 346–354. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the exemplary knowledge of microbial exopolysaccharides in a nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Fonseca, F.; Meneghel, J.; Cenard, S.; Passot, S.; Morris, G.J. Determination of Intracellular Vitrification Temperatures for Unicellular Micro Organisms under Conditions Relevant for Cryopreservation. PLoS ONE 2016, 11, e0152939. [Google Scholar] [CrossRef] [Green Version]
- Meneghel, J.; Passot, S.; Dupont, S.; Fonseca, F. Biophysical Characterization of the Lactobacillus Delbrueckii Subsp. Bulgaricus Membrane during Cold and Osmotic Stress and Its Relevance for Cryopreservation. Appl. Microbiol. Biotechnol. 2017, 101, 1427–1441. [Google Scholar] [CrossRef]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef]
- Ghimici, L.; Suflet, D.M. Phosphorylated polysaccharide derivatives as efficient separation agents for zinc and ferric oxides particles from water. Sep. Purif. Technol. 2015, 144, 31–36. [Google Scholar] [CrossRef]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, S.; Wu, D.; Liu, H. Electric conductivity and electric convertibility of potassium acetate in water, ethanol, 2, 2, 2-trifluoroethanol, 2–propanol and their binary blends. Chin. J. Chem. Eng. 2018, 26, 2581–2591. [Google Scholar] [CrossRef]
- Ponrasu, T.; Chen, B.H.; Chou, T.H.; Wu, J.J.; Cheng, Y.S. Fast Dissolving Electrospun Nanofibers Fabricated from Jelly Fig Polysaccharide/Pullulan for Drug Delivery Applications. Polymers 2021, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutiérrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Bratuša, A.; Elschner, T.; Heinze, T.; Fröhlich, E.; Hribernik, S.; Božič, M.; Žagar, E.; Kleinschek, K.S.; Thonhofer, M.; Kargl, R. Functional dextran amino acid ester particles derived from N-protected S-trityl-L-cysteine. Colloids. Surf. B Biointerfaces 2019, 181, 561–566. [Google Scholar] [CrossRef]
- Xiao, L.; Li, Y.; Tian, J.; Zhou, J.; Xu, Q.; Feng, L.; Rui, X.; Fan, X.; Zhang, Q.; Chen, X.; et al. Influences of drying methods on the structural, physicochemical and antioxidant properties of exopolysaccharide from Lactobacillus helveticus MB2-1. Int. J. Biol. Macromol. 2020, 157, 220–231. [Google Scholar] [CrossRef]
- Montero, X.A.; Alves, A.; Ribeiro, M.P.; Lazzari, M.; Coutinho, P.; Otero, A. Biochemical characterization of Nostoc sp. exopolysaccharides and evaluation of potential use in wound healing. Carbohydr. Polym. 2021, 254, 117303. [Google Scholar] [CrossRef]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Interaction of colloidal silver nanoparticles (SNPs) with exopolysaccharides (EPS) and its adsorption isotherms and kinetics. Colloids. Surf. A Physicochem. Eng. Asp. 2011, 381, 99–105. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.S.; Chen, Y.; Juang, R.S.; Chang, T.W.; Mdlovu, N.B. Formulation and characterization of multifunctional polymer modified-iron oxide magnetic nanocarrier for doxorubicin delivery. J. Taiwan Inst. Chem. Eng. 2019, 104, 260–272. [Google Scholar] [CrossRef]
- Sibaja-Hernández, R.; Román-Guerrero, A.; Sepúlveda-Jiménez, G.; Rodríguez-Monroy, M. Physicochemical, shear flow behaviour and emulsifying properties of Acacia cochliacantha and Acacia farnesiana gums. Ind. Crops Prod. 2015, 67, 161–168. [Google Scholar] [CrossRef]
- Kang, X.; Xia, Z.; Chen, R.; Liu, P.; Yang, W. Effects of inorganic cations and organic polymers on the physicochemical properties and microfabrics of kaolinite suspensions. Appl. Clay Sci. 2019, 176, 38–48. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Shobsngob, S.; Oonkhanond, B.; Varavinit, S. Zeta potential (ζ) and pasting properties of phosphorylated or crosslinked rice starches. Starche. Weinh. 2005, 57, 32–37. [Google Scholar] [CrossRef]
- Bogataj, M.; Vovk, T.; Kerec, M.; Dimnik, A.; Grabnar, I.; Mrhar, A. The correlation between zeta potential and mucoadhesion strength on pig vesical mucosa. Biol. Pharm. Bull. 2003, 26, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Uetani, K.; Yano, H. Zeta Potential Time Dependence Reveals the Swelling Dynamics of Wood Cellulose Nanofibrils. Langmuir 2012, 28, 818–827. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Enríquez-Ramírez, K.E.; García-Márquez, E.; Ramírez-Santiago, C.; Lobato-Calleros, C.; Vernon-Carter, J. Interrelationship between the zeta potential and viscoelastic properties in coacervates complexes. Carbohydr. Polym. 2013, 95, 161–166. [Google Scholar] [CrossRef]
- Singh, S.; Bothara, S.B. Physico-chemical and structural characterization of mucilage isolated from seeds of Diospyros melonoxylon Roxb. Braz. J. Pharm. Sci. 2014, 50, 713–725. [Google Scholar] [CrossRef]
- Choudhuri, I.; Khanra, K.; Maity, P.; Patra, A.; Maity, G.N.; Pati, B.R.; Nag, A.; Mondal, S.; Bhattacharyya, N. Structure and biological properties of exopolysaccharide isolated from Citrobacter freundii. Int. J. Biol. Macromol. 2021, 168, 537–549. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. High molecular weight bioemulsifiers, main properties and potential environmental and biomedical applications. World J. Microbiol. Biotechnol. 2015, 31, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hang, F.; Guo, B.; Liu, Z.; You, C.; Wu, Z. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr. Polym. 2014, 112, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Du, C.; Xu, Z.Y.; Qian, H.; Zhang, W.G. Rheological properties of phosphorylated exopolysaccharide produced by Sporidiobolus pararoseus JD-2. Int. J. Biol. Macromol. 2016, 88, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lü, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Besrour-Aouam, N.; Fhoula, I.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Najjari, A.; Prieto, A.; Ruas-Madiedo, P.; López, P.; Ouzari, H.I. The role of dextran production in the metabolic context of Leuconostoc and Weissella Tunisian strains. Carbohydr. Polym. 2021, 253, 117254. [Google Scholar] [CrossRef]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Q.; Guo, Y.; Han, Y.; Xiao, H.; Zhou, Z. Isolation and characterization of dextran produced by Leuconostoc citreum NM105 from manchurian sauerkraut. Carbohydr. Polym. 2015, 133, 365–372. [Google Scholar] [CrossRef]
- Baruah, R.; Maina, N.H.; Katina, K.; Juvonen, R.; Goyal, A. Functional food applications of dextran from Weissella cibaria RBA12 from pummelo (Citrus maxima). Int. J. Food Microbiol. 2017, 242, 124–131. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, Q.; Yang, Y.; Zhao, F.; Du, R.; Han, Y.; Xiao, H.; Zhou, Z. Characterization of highly branched dextran produced by Leuconostoc citreum B-2 from pineapple fermented product. Int. J. Biol. Macromol. 2018, 113, 45–50. [Google Scholar] [CrossRef]
- Banerjee, A.; Rudra, S.G.; Mazumder, K.; Nigam, V.; Bandopadhyay, R. Structural and Functional Properties of Exopolysaccharide Excreted by a Novel Bacillus anthracis (Strain PFAB2) of Hot Spring Origin. Indian J. Microbiol. 2018, 58, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Feng, F.; Yang, Y.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Characterization of a dextran produced by Leuconostoc pseudomesenteroides XG5 from homemade wine. Int. J. Biol. Macromol. 2018, 107, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.M.; Gamal, A.A.; Mansour, N.M.; Salama, B.M.; Hassanein, N.M.; Awad, G.E.; Esawy, M.A. Optimization of Enterococcus faecalis Esawy KR758759 dextransucrase and evaluation of some dextran bioactivities. Biocatal. Agric. Biotechnol. 2018, 15, 348–358. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Chen, H.; Chen, W.; Zhang, H.; Zhang, M.; Zhong, Q.; Chen, W. Physicochemical Characterization of an Exopolysaccharide Produced by Lipomyces sp. and Investigation of Rheological and Interfacial. Behavior. Gels 2021, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Bajpai, V.K.; Rather, I.A.; Park, Y.H. Partially purified exopolysaccharide from Lactobacillus plantarum YML009 with total phenolic content, antioxidant and free radical scavenging efficacy. Indian J. Pharm. Educ. Res. 2015, 49, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Tinzl-Malang, S.K.; Grattepanche, F.; Rast, P.; Fischer, P.; Sych, J.; Lacroix, C. Purified exopolysaccharides from Weissella confusa, 11GU-1 and Propionibacterium freudenreichii JS15 act synergistically on bread structure to prevent staling. LWT 2020, 127, 109375. [Google Scholar] [CrossRef]
- Shi, K.; An, W.; Meng, Q.; Gu, Y.; Liu, S. Partial characterization and lyoprotective activity of exopolysaccharide from Oenococcus oeni 28A-1. Process Biochem. 2021, 101, 128–136. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Iqbal, D.N.; Shafiq, S.; Khan, S.M.; Ibrahim, S.M.; Abubshait, S.A.; Nazir, A.; Abbas, M.; Iqbal, M. Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. Int. J. Biol. Macromol. 2020, 164, 499–509. [Google Scholar] [CrossRef]
- Mobika, J.; Rajkumar, M.; Sibi, S.L.; Priya, V.N. Investigation on hydrogen bonds and conformational changes in protein/polysaccharide/ceramic based tri-component system. Spectrochim Acta A Mol. Biomol. Spectrosc. 2021, 244, 118836. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohydr. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, P.X.; Huang, S.X.; Hou, D.Z.; Xu, X.D.; Ci, L.Y.; Chen, S. Quantitative analysis of straight-chain/branched-chain ratio during enzymatic synthesis of dextran based on periodate oxidation. Biochem. Biophys. Res. Commun. 2020, 523, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Savi, A.; Calegari, G.C.; Santos, V.A.Q.; Pereira, E.A.; Teixeira, S.D. Chemical characterization and antioxidant of polysaccharide extracted from Dioscorea bulbifera. J. King Saud. Univ. Sci. 2018, 32, 636–642. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Tiwari, S.; Soni, S. Swelling behavior of cross-linked dextran hydrogels and preliminary Gliclazide release behavior. Int. J. Biol. Macromol. 2016, 93, 978–987. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Puertas, A.I.; Prieto, A.; López, P.; Cobos, M.; Miranda, J.I.; Marieta, C.; Ruas-Madiedo, P.; Dueñas, M.T. Characterization of dextrans produced by Lactobacillus mali CUPV271 and Leuconostoc carnosum CUPV411. Food Hydrocoll. 2019, 89, 613–622. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Faucard, P.; Grimaud, F.; Lourdin, D.; Maigret, J.E.; Moulis, C.; Remaud-Siméon, M.; Putaux, J.; Potocki-Véronèse, G.; Rolland-Sabaté, A. Macromolecular structure and film properties of enzymatically-engineered high molar mass dextrans. Carbohydr. Polym. 2018, 181, 337–344. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Yao, D.; Zhao, J.; Wu, Y.; Lu, B.; Zheng, X. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int. J. Biol. Macromol. 2019, 139, 252–261. [Google Scholar] [CrossRef]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Jayamurthy, H.; Dhar, K.S.; Pandey, A. Studies on structural and physical characteristics of a novel exopolysaccharide from Pseudozyma sp. NII 08165. Int. J. Biol. Macromol. 2013, 59, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Zeng, Y.J.; Xu, F.Z.; Li, F.Z.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Joulak, I.; Azabou, S.; Finore, I.; Poli, A.; Nicolaus, B.; Donato, P.D.; Bkhairia, I.; Dumas, E.; Gharsallaoui, A.; Immirzi, B.; et al. Structural characterization and functional properties of novel exopolysaccharide from the extremely halotolerant Halomonas elongata S6. Int. J. Biol. Macromol. 2020, 164, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Andrade, F.; Martinez-Trucharte, F.; Basas, J.; Seras-Franzoso, J.; Palau, M.; Gomis, X.; Pérez-Burgos, M.; Blanco, A.; López-Fernández, A.; et al. Sterilization Procedure for Temperature-Sensitive Hydrogels Loaded with Silver Nanoparticles for Clinical Applications. Nanomaterials 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid. Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Li, Z.; Wu, F.; Du, R.; Feng, Y. A two-step approach for fluidized bed granulation in pharmaceutical processing: Assessing different models for design and control. PLoS ONE 2017, 2, e0180209. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.P.; Kim, Y.; Hu, Y.; Jung, S. Bacterial Succinoglycans: Structure, Physical Properties, and Applications. Polymers 2022, 14, 276. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, C.C. Thermal and mechanical properties of biodegradable hydrophilic-hydrophobic hydrogels based on dextran and poly (lactic acid). J. Mater Sci. Mater. Med. 2002, 13, 773–781. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef]
- Rosca, I.; Petrovici, A.R.; Peptanariu, D.; Nicolescu, A.; Dodi, G.; Avadanei, M.; Ivanov, I.C.; Bostanaru, A.C.; Mares, M.; Ciolacu, D. Biosynthesis of dextran by Weissella confusa and its. In vitro functional characteristics. Int. J. Biol. Macromol. 2018, 107, 1765–1772. [Google Scholar] [CrossRef]
- Jiang, G.; Gan, L.; Li, X.; He, J.; Zhang, S.; Chen, J.; Zhang, R.; Xu, Z.; Tian, Y. Characterization of Structural and Physicochemical Properties of an Exopolysaccharide Produced by Enterococcus sp. F2 From Fermented Soya Beans. Front. Microbiol. 2021, 12, 744007. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.M.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kureel, A.K.; Dutta, P.K.; Mehrotra, G.K. Phenolic compounds based conjugates from dextran aldehyde and BSA: Preparation, characterization and evaluation of their anti-cancer efficacy for therapeutic applications. Int. J. Biol. Macromol. 2018, 110, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Jamir, K.; Badithi, N.; Venumadhav, K.; Seshagirirao, K. Characterization and comparative studies of galactomannans from Bauhinia vahlii, Delonix elata, and Peltophorum pterocarpum. Int. J. Biol. Macromol. 2019, 134, 498–506. [Google Scholar] [CrossRef]
- Kumar, P.; Kulkarni, G.T. Characterization of mucilage from Artocarpus heterophyllus as pharmaceutical excipient. J. Chronother. Drug Deliv. 2013, 4, 31–43. [Google Scholar]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Alpizar-Reyes, E.; Carrillo-Navas, H.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Alvarez-Ramirez, J.; Pérez-Alonso, C. Functional properties and physicochemical characteristics of tamarind (Tamarindus indica L.) seed mucilage powder as a novel hydrocolloid. J. Food Eng. 2017, 209, 68–75. [Google Scholar] [CrossRef]
- Ahmadi, S.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Alihosseini, F.; Yadav, H. Effects of different drying methods on the physicochemical properties and antioxidant activities of isolated acorn polysaccharides. LWT 2019, 100, 1–9. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Trigui, I.; Yaich, H.; Sila, A.; Cheikh-Rouhou, S.; Bougatef, A.; Blecker, C.; Attia, H.; Ayadi, M.A. Physicochemical properties of water-soluble polysaccharides from black cumin seeds. Int. J. Biol. Macromol. 2018, 117, 937–946. [Google Scholar] [CrossRef]
- Kavitake, D.; Delattre, C.; Devi, P.B.; Pierre, G.; Michaud, P.; Shetty, P.H.; Andhare, P. Physical and functional characterization of succinoglycan exopolysaccharide produced by Rhizobium radiobacter CAS from curd sample. Int. J. Biol. Macromol. 2019, 134, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Bu, F.; Chen, X.; Li, C.; Wang, S.; Kan, J. Ultrasonic extraction, structural characterization, physicochemical properties and antioxidant activities of polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2018, 112, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zeng, R.; Hu, L.; Maffucci, K.G.; Qu, Y. Polysaccharides from tubers of Bletilla striata: Physicochemical characterization, formulation of buccoadhesive wafers and preliminary study on treating oral ulcer. Int. J. Biol. Macromol. 2019, 122, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Chadni, M.; Grimi, N.; Bals, O.; Ziegler-Devin, I.; Desobry, S.; Brosse, N. Elaboration of hemicellulose-based films: Impact of the extraction process from spruce wood on the film properties. Carbohydr. Res. 2020, 497, 108111. [Google Scholar] [CrossRef]
- Kurdziel, M.; Łabanowska, M.; Pietrzyk, S.; Sobolewska-Zielińska, J.; Michalec, M. Changes in the physicochemical properties of barley and oat starches upon the use of environmentally friendly oxidation methods. Carbohydr. Polym. 2019, 210, 339–349. [Google Scholar] [CrossRef]
- Wondraczek, H.; Kotiaho, A.; Fardim, P.; Heinze, T. Photoactive polysaccharides. Carbohydr. Polym. 2011, 83, 1048–1061. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wis’niewska, J.; Kozłowska, J.; Płanecka, A.; Kurzawa, M. Photochemical behaviour of hydrolysed keratin. Int. J. Cosmet. Sci. 2011, 33, 503–508. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.G.; Detoni, C.B.; da Silva, T.L.; Colomé, L.M.; Pohlmann, A.R.; Guterres, S.S. α-Tocopherol acetate-loaded chitosan microparticles: Stability during spray drying process, photostability and swelling evaluation. J. Drug Deliv. Sci. Technol. 2015, 30, 220–224. [Google Scholar] [CrossRef]
- Jones, D.S.; Bruschi, M.L.; de Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, D.J.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Developing synthetic mucosa-mimetic hydrogels to replace animal experimentation in characterisation of mucoadhesive drug delivery systems. Soft Matter 2011, 7, 9620–9623. [Google Scholar] [CrossRef]
- Sobczyński, J.; Chudzik-Rząd, B. Organic Nanocarriers for the Delivery of Antiinfective Agents; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Carreras, J.J.; Canales, P.; Zaera, A.M. Mucoadhesion of Polymeric Drug Delivery Systems: Polymeric Nanoparticles and its Interactions with the Intestinal Barrier. JSM Nanotechnol. Nanomed. 2016, 4, 1041. [Google Scholar]
- Lam, H.T.; Zupančič, O.; Laffleur, F.; Bernkop-Schnürch, A. Mucoadhesive properties of polyacrylates: Structure–function relationship. Int. J. Adhes. Adhes. 2021, 107, 102857. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Hasheminya, S.M.; Dehghannya, J. Novel ultrasound-assisted extraction of kefiran biomaterial, a prebiotic exopolysaccharide, and investigation of its physicochemical, antioxidant and antimicrobial properties. Mater. Chem. Phys. 2020, 243, 122645. [Google Scholar] [CrossRef]
- Marques, V.D.; Franzolin, M.R.; Sanabani, S.S.; Vigerelli, H.; Piazza, R.M.F.; Pimenta, D.C.; Venâncio, T.; Neves, I.V.; de Sousa Silva, H.G.; Dos Santos Courrol, D.; et al. A new class of antimicrobial molecules derived from kefir, effective against Pseudomonas aeruginosa and methicillin resistant Staphylococcus aureus (MRSA) strains. Sci. Rep. 2020, 10, 17434. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, H.; Jiang, Q.; Xia, W. The characterization and biological activities of synthetic N, O-selenized chitosan derivatives. Int. J. Biol. Macromol. 2021, 173, 504–512. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Sousa, A.S.; Reis, C.A.; Pintado, M. Chitosan-olive oil microparticles for phenylethyl isothiocyanate delivery: Optimal formulation. PLoS ONE 2021, 16, e0248257. [Google Scholar] [CrossRef]
- Garriga, R.; Herrero-Continente, T.; Palos, M.; Cebolla, V.L.; Osada, J.; Muñoz, E.; Rodríguez-Yoldi, M.J. Toxicity of Carbon Nanomaterials and Their Potential Application as Drug Delivery Systems: In Vitro Studies in Caco-2 and MCF-7 Cell Lines. Nanomaterials 2020, 10, 1617. [Google Scholar] [CrossRef]
- Shahnaz, G.; Perera, G.; Sakloetsakun, D.; Rahmat, D.; Bernkop-Schnürch, A. Synthesis, characterization, mucoadhesion and biocompatibility of thiolated carboxymethyl dextran–cysteine conjugate. J. Control. Release 2010, 144, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jesus, L.I.; Smiderle, F.R.; Ruthes, A.C.; Vilaplana, F.; Dal’Lin, F.T.; Maria-Ferreira, D.; Werner, M.F.; Van Griensven, L.J.L.D.; Iacomini, M. Chemical characterization and wound healing property of a β-D-glucan from edible mushroom Piptoporus betulinus. Int. J. Biol. Macromol. 2018, 117, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Srichamroen, A. Effect of extracted malva nut gum on reducing high glucose levels by Caco-2 cells. Food Biosci. 2018, 21, 107–116. [Google Scholar] [CrossRef]
- Naeye, B.; Deschout, H.; Röding, M.; Rudemo, M.; Delanghe, J.; Devreese, K.; Demeester, J.; Braeckmans, K.; de Smedt, S.C.; Raemdonck, K. Hemocompatibility of siRNA loaded dextran nanogels. Biomaterials 2011, 32, 9120–9127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Montes, E.; Yáñez-Fernández, J.; Castro-Muñoz, R. Microfiltration-mediated extraction of dextran produced by Leuconostoc mesenteroides SF3. Food Bioprod. Process 2020, 119, 317–328. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Jeddou, K.B.; Kallel, F.; Helbert, C.B.; Khelfa, A.R.; Ghorbel, R.E.; Chaabouni, S.E. Water-soluble polysaccharides and hemicelluloses from almond gum: Functional and prebiotic properties. Int. J. Biol. Macromol. 2016, 93, 359–368. [Google Scholar] [CrossRef]
- Alves, V.; Scapini, T.; Camargo, A.F.; Bonatto, C.; Stefanski, F.S.; de Jesus, E.P.; Diniz, L.G.T.; Bertan, L.C.; Maldonado, R.R.; Treichel, H. Development of fermented beverage with water kefir in water-soluble coconut extract (Cocos nucifera L.) with inulin addition. LWT 2021, 145, 111364. [Google Scholar] [CrossRef]
- Moreira, M.E.C.; Santos, M.D.; Zolini, G.P.P.; Wouters, A.T.B.; Carvalho, J.C.T.; Schneedorf, J.M. Anti-inflammatory and cicatrizing activities of a carbohydrate fraction isolated from sugary kefir. J. Med. Food 2008, 11, 356–361. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Tomić, M.; Gordić, M.; Nešić, A.; Dimitrijević, S. Response surface methodology for optimisation of edible coatings based on dextran from Leuconostoc mesenteroides T3. Carbohydr. Polym. 2018, 184, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Geronço, M.S.; Sousa, R.W.R.; Ramos, L.P.S.; Araújo, F.P.; Ribeiro, A.B.; Ferreira, P.M.P.; Osajima, J.A.; Costa, M.P. Biopolymer from Adenanthera pavonina L. seeds: Characterization, photostability, antioxidant activity, and biotoxicity evaluation. Int. J. Polym. Sci. 2018, 2018, 1385830. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, H. Mechanism of photoinitiated degradation of poly (ethylene oxide) by copper complexes in acetonitrile. J. Photochem. Photobiol. A 1996, 95, 61–65. [Google Scholar] [CrossRef]
- NCCLS Document M7-A6 [ISBN (2003), 1-56238-486-4]; NCCLS (National Committee for Clinical Laboratory Standards) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard. 6th ed. NCCLS: Wayne, PA, USA, 2003; pp. 1898–19087.
- Silva, L.R.; Teixeira, R. Phenolic profile and biological potential of Endopleura uchi extracts. Asian Pac. J. Trop. Med. 2015, 8, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalegari, M.; Miguel, M.D.; Dias, J.D.F.G.; Lordello, A.L.L.; Lima, C.P.D.; Miyazaki, C.M.S.; Zanin, S.M.W.; Verdam, M.C.S.; Miguel, O.G. Phytochemical constituents and preliminary toxicity evaluation of leaves from Rourea induta Planch. (Connaraceae). Braz. J. Pharm. Sci. 2011, 47, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Gallina, D.A.; Silva, A.T.; de Souza Trento, F.K.H.; Carusi, J. Characterization of Fermented Milk, and Probiotics and Prebiotics Free Milk, and Viability Evaluation of Lactic Acid and Probiotic Bacteria Luring the Shelf Life. J. Health Sci. 2011, 13, 239–244. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena, M.d.A.; Ramos, I.F.d.S.; Geronço, M.S.; de Araújo, R.; da Silva Filho, F.L.; da Silva, L.M.L.R.; de Sousa, R.W.R.; Ferreira, P.M.P.; Osajima, J.A.; Silva-Filho, E.C.; et al. Biopolymer from Water Kefir as a Potential Clean-Label Ingredient for Health Applications: Evaluation of New Properties. Molecules 2022, 27, 3895. https://doi.org/10.3390/molecules27123895
Lucena MdA, Ramos IFdS, Geronço MS, de Araújo R, da Silva Filho FL, da Silva LMLR, de Sousa RWR, Ferreira PMP, Osajima JA, Silva-Filho EC, et al. Biopolymer from Water Kefir as a Potential Clean-Label Ingredient for Health Applications: Evaluation of New Properties. Molecules. 2022; 27(12):3895. https://doi.org/10.3390/molecules27123895
Chicago/Turabian StyleLucena, Monalisa de Alencar, Igor Frederico da Silveira Ramos, Maurycyo Silva Geronço, Ricardo de Araújo, Francisco Lopes da Silva Filho, Luís Manuel Lopes Rodrigues da Silva, Rayran Walter Ramos de Sousa, Paulo Michel Pinheiro Ferreira, Josy Anteveli Osajima, Edson Cavalcanti Silva-Filho, and et al. 2022. "Biopolymer from Water Kefir as a Potential Clean-Label Ingredient for Health Applications: Evaluation of New Properties" Molecules 27, no. 12: 3895. https://doi.org/10.3390/molecules27123895
APA StyleLucena, M. d. A., Ramos, I. F. d. S., Geronço, M. S., de Araújo, R., da Silva Filho, F. L., da Silva, L. M. L. R., de Sousa, R. W. R., Ferreira, P. M. P., Osajima, J. A., Silva-Filho, E. C., Rizzo, M. d. S., Ribeiro, A. B., & da Costa, M. P. (2022). Biopolymer from Water Kefir as a Potential Clean-Label Ingredient for Health Applications: Evaluation of New Properties. Molecules, 27(12), 3895. https://doi.org/10.3390/molecules27123895











