2,5-Furandicarboxylic Acid: An Intriguing Precursor for Monomer and Polymer Synthesis
Abstract
:1. Introduction
2. FDCA Synthesis
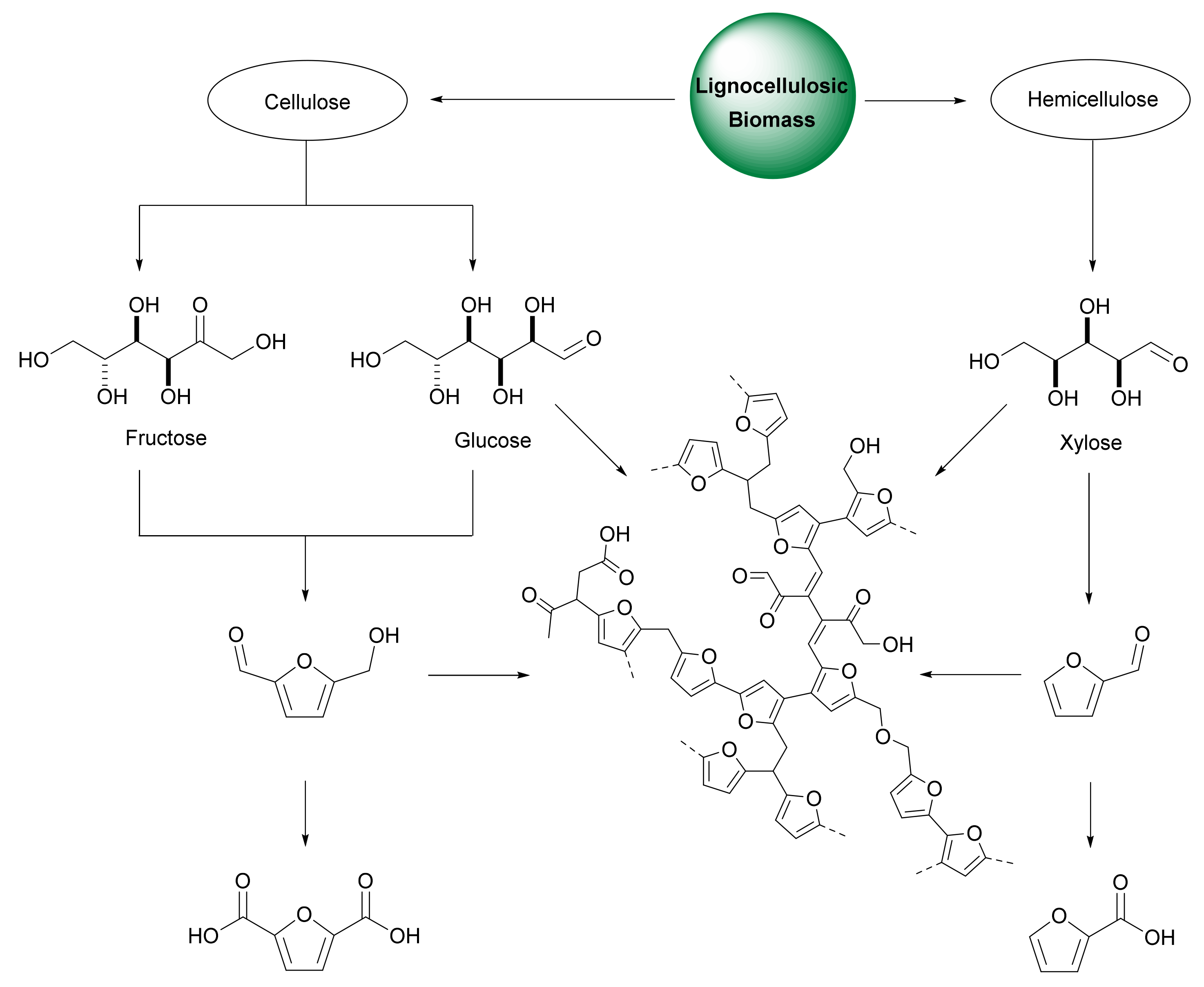
2.1. FDCA from Cellulose
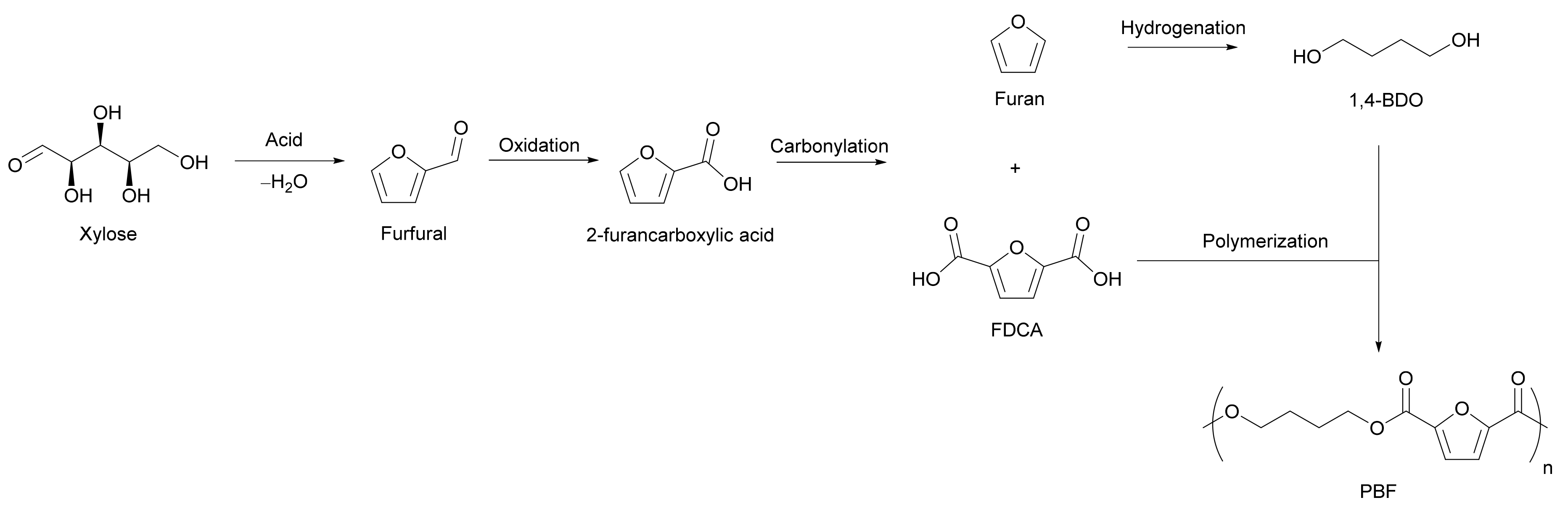
2.2. FDCA from Hemicellulose
3. FDCA as a Monomer
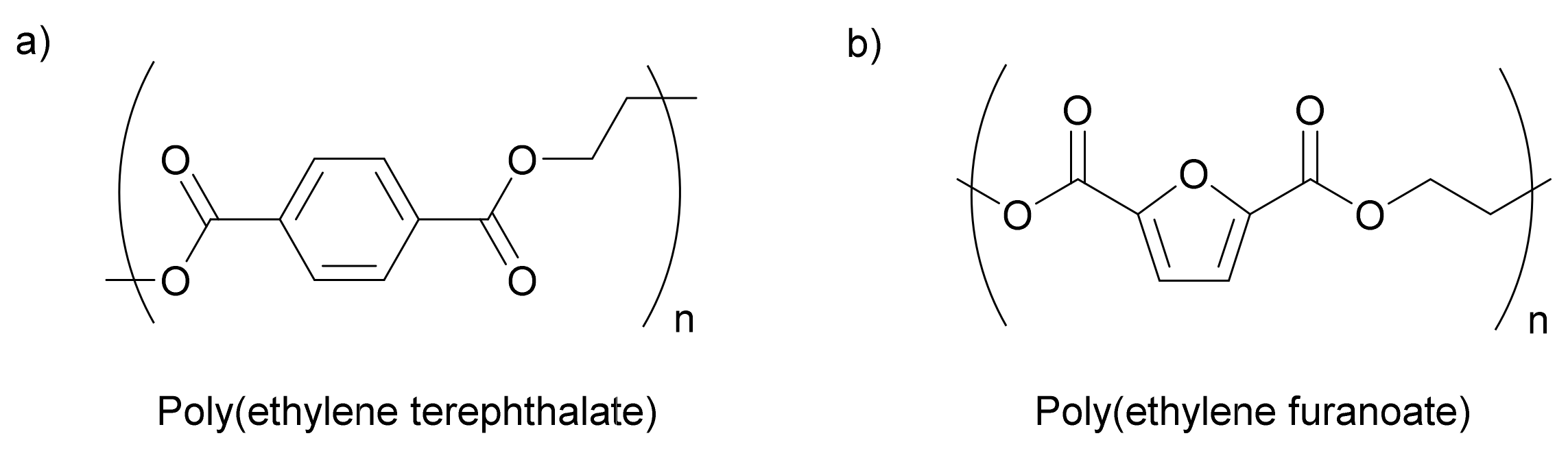
3.1. PET vs. PEF
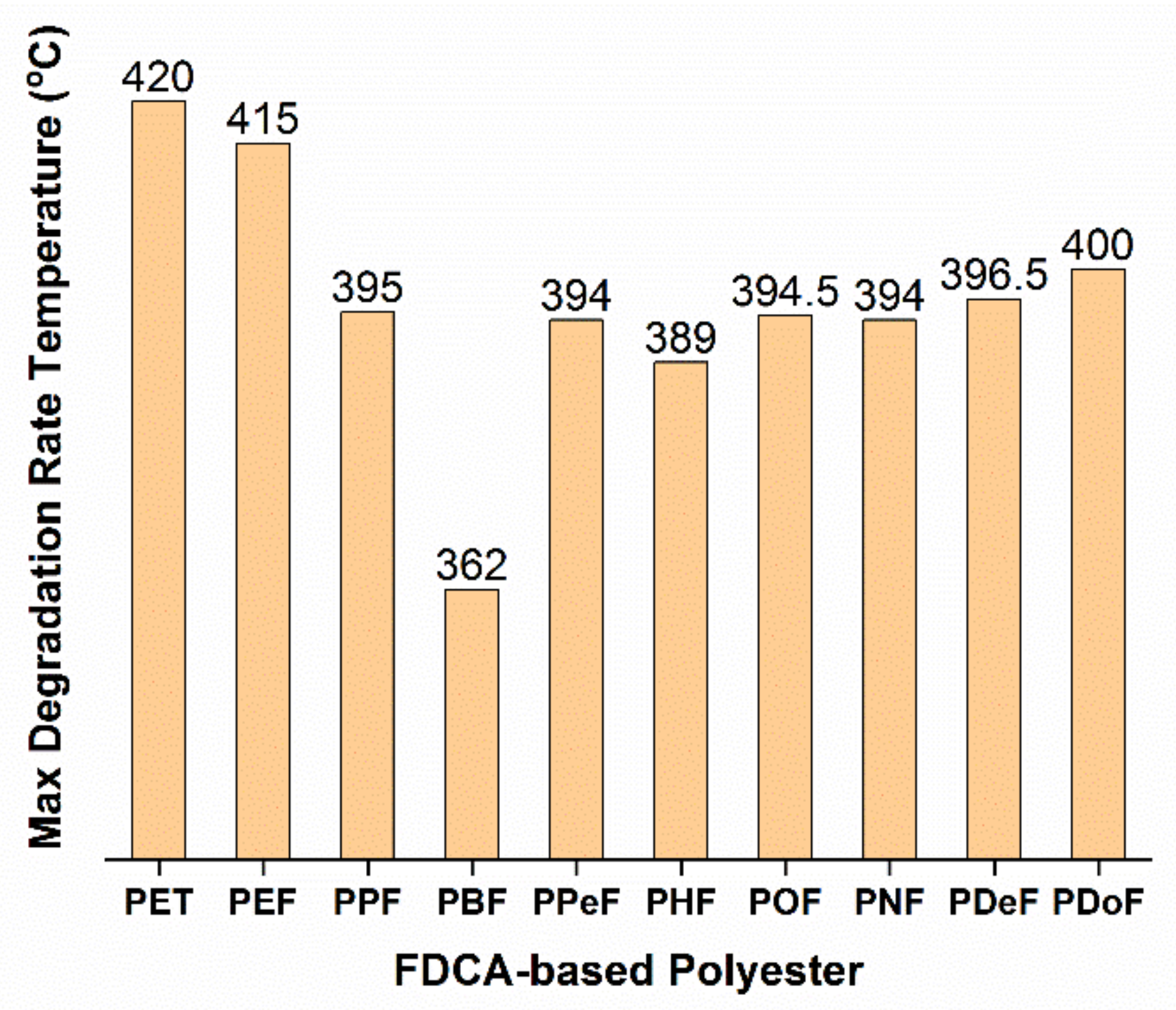
3.2. Polymer Production Challenges: Thermal Degradation and Stability
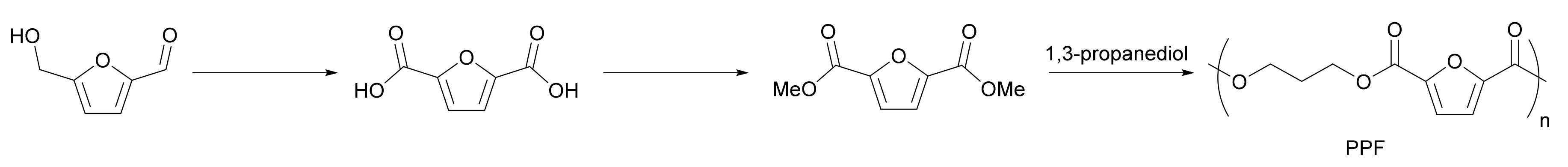
3.3. FDCA-Based Polymer Production
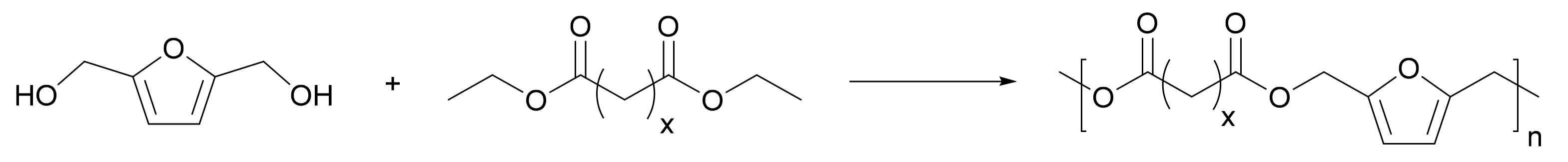
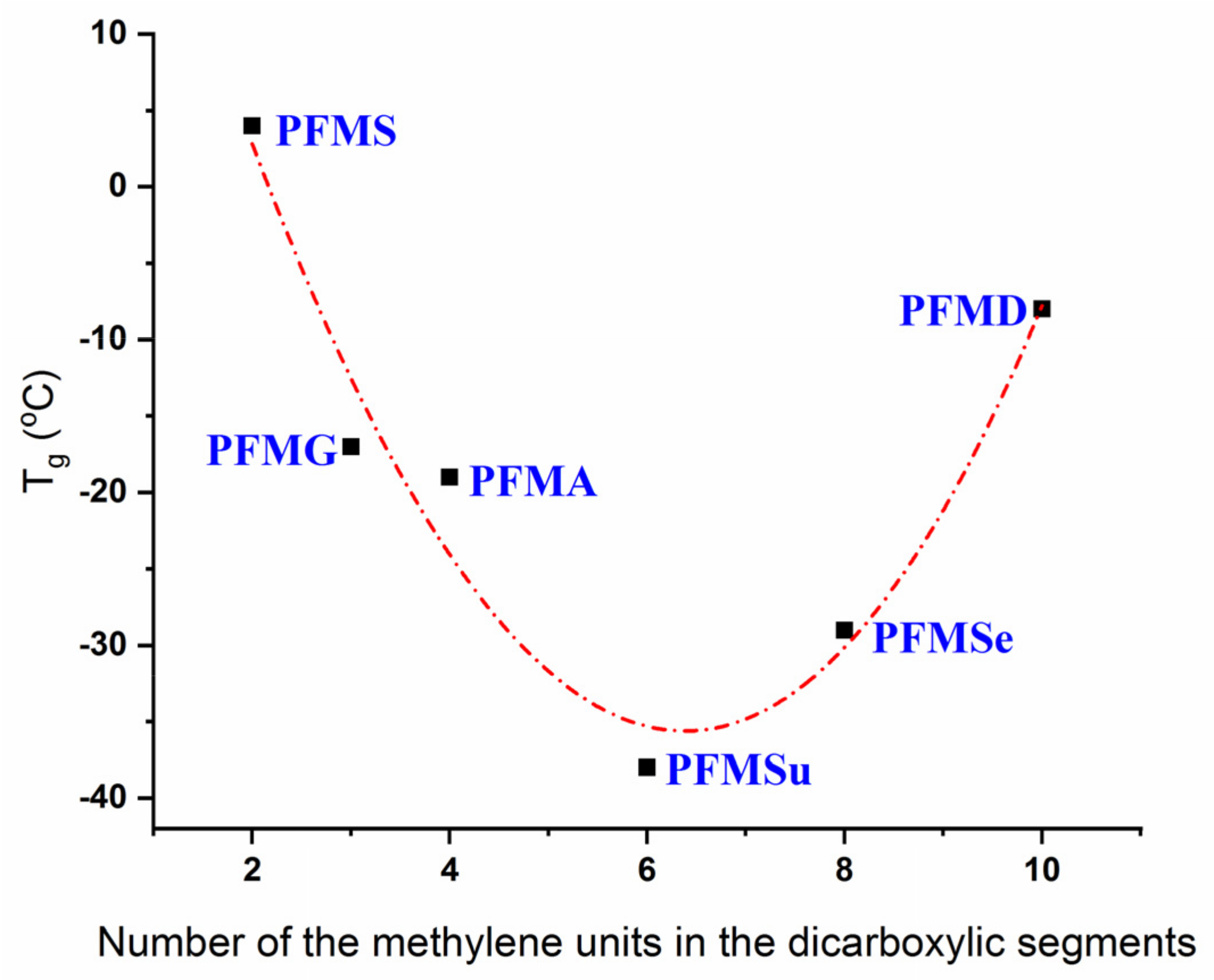
3.4. FDCA Copolymers beyond PEF and PPF
4. BHMF as a Monomer
4.1. BHMF-Based Polymer Production
4.2. BHMF Ethers
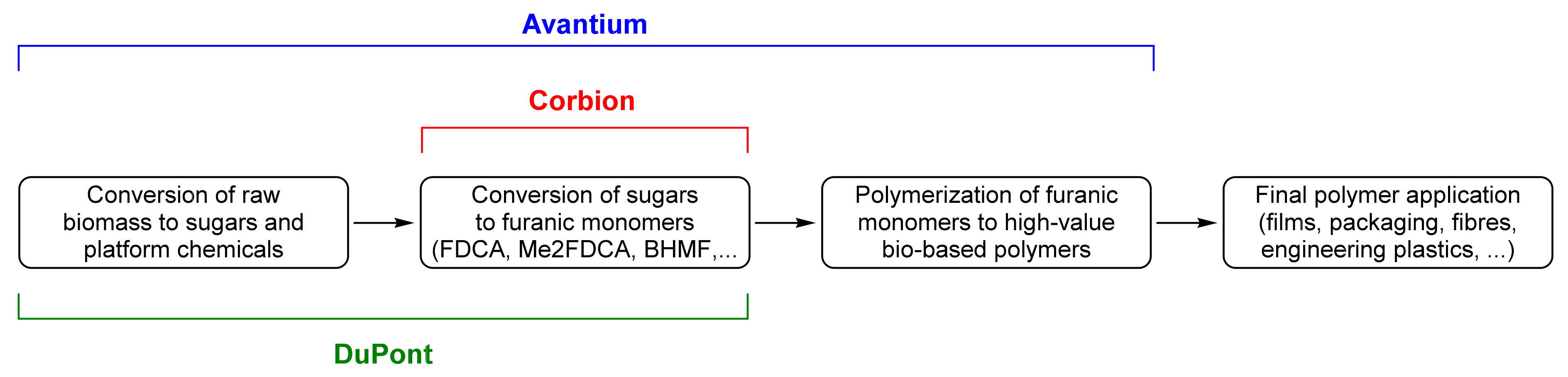
5. Industrial Production and Economics
5.1. Advances in the Industrial Production of FDCA and Esters
5.2. Improving FDCA Process Economics: Humin Valorization
5.3. FDCA Market Economics
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fouilloux, H.; Thomas, C.M. Production and polymerization of biobased acrylates and analogs. Macromol. Rapid Commun. 2021, 42, 2000530. [Google Scholar] [CrossRef] [PubMed]
- Oil Consumption. Available online: https://www.bpf.co.uk/press/Oil_Consumption (accessed on 11 November 2021).
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass, Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Laboratory, US Department of Energy: Richland, WA, USA, 2004.
- Fittig, R. Mittheilungen aus dem chemischen Institut der Universitat Strassburg. Ber. Dtsch. Chem. Ges. 1876, 9, 1189–1199. [Google Scholar] [CrossRef] [Green Version]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Arkivoc 2001, 1, 17–54. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.; Lee, S.; Choi, E.; Kim, J.W. Efficient synthetic routes of biomass-derived platform chemicals. Appl. Chem. Eng. 2019, 30, 280–289. [Google Scholar]
- Shen, G.; Shi, J.; Lei, Y.; Fu, C.; Chen, Z.; Andrioletti, B.; Yin, G. Aqueous carbonylation of furfural-derived 5-bromofuroic acid to 2,5-furandicarboxylic acid with supported palladium catalyst. Ind. Eng. Chem. Res. 2019, 58, 22951–22957. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc. Chem. Res. 2002, 35, 728–737. [Google Scholar] [CrossRef]
- Pandey, S.; Dumont, M.-J.; Orsat, V.; Rodrigue, D. Biobased 2,5-furandicarboxylic acid (FDCA) and its emerging copolyesters’ properties for packaging applications. Eur. Polym. J. 2021, 160, 110778. [Google Scholar] [CrossRef]
- Pan, T.; Deng, J.; Xu, Q.; Zuo, Y.; Guo, Q.; Fu, Y. Catalytic conversion of furfural into a 2,5-furandicarboxylic acid-based polyester with total carbon utilization. ChemSusChem 2013, 6, 47–50. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Lukasik, E.; Bogel-Lukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural—a promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Thomás, R.A.F.; Bordado, J.C.M.; Gomes, J.F.P. p-Xylene oxidation to terephthalic acid: A literature review oriented toward process optimization and development. Chem. Rev. 2013, 133, 7421–7469. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Waal, J.C.V.D.; Varini, M.; Gruter, G.J.M. Process for the Preparation of an Aromatic Dicarboxylic Acid. WO Patent EP3297995B1, 10 July 2019. [Google Scholar]
- Kang, E.; Hong, Y.; Chae, D.W.; Kim, B.; Kim, B.; Kim, Y.J.; Cho, J.K.; Kim, Y.G. From lignocellulosic biomass to furans via 5-acetoxymethylfurfural as an alternative to 5-hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188. [Google Scholar] [CrossRef]
- Janzowski, C.; Glaab, V.; Samimi, E.; Schlatter, J.; Eisenbrand, G. 5-Hydroxymethylfurfural: Assessment of mutagenicity, DNA-damaging potential and reactivity towards cellular glutathione. Food Chem. Toxicol. 2000, 38, 801–809. [Google Scholar] [CrossRef]
- Harrisson, R.J.; Moyle, M. 2-Furoic Acid. Org. Synth. 1956, 36, 36. [Google Scholar]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- Nocito, F.; Ditaranto, N.; Dibenedetto, A. Valorization of C5 polyols by direct carboxylation to FDCA: Synthesis and characterization of a key intermediate and role of carbon dioxide. J. CO2 Util. 2019, 32, 170–177. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Pukin, A.; van Haveren, J.; Lutz, M.; van Es, D.S. Concurrent formation of furan-2,5- and furan-2,4-dicarboxylic acid: Unexpected aspects of the Henkel reaction. RSC Adv. 2013, 3, 15678–15686. [Google Scholar] [CrossRef] [Green Version]
- Rosenboom, J.G.; Hohl, D.K.; Fleckenstein, P.; Storti, G.; Morbidelli, M. Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers. Nat. Commun. 2018, 9, 2701. [Google Scholar] [CrossRef] [Green Version]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Manair, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A perspective on PEF synthesis, properties, and end-life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Wahbi, M.; Kasmi, N.; Papergeorgiou, G.Z.; Bikiaris, D.N. Effect of additives on the thermal and thermo-oxidative stability of poly(ethylene furanoate) biobased polyester. Thermochim. Acta 2020, 686, 178549. [Google Scholar] [CrossRef]
- Knoop, R.J.I.; Vogelzang, W.; van Haveren, J.; van Es, D.S. High molecular weight poly (ethylene-2, 5-furanoate); critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part B Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Achilias, D.S.; Vaimakis, T.; Papageorgiou, G.P.; Bikiaris, D.N. Thermal degradation kinetics and decomposition mechanism of polyesters based on 2,5-furandicarboxylic acid and low molecular weight aliphatic diols. J. Anal. Appl. Pyrolysis 2015, 112, 369–378. [Google Scholar]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Decomposition mechanism of polyesters based on 2, 5-furandicarboxylic acid and aliphatic diols with medium and long chain methylene groups. Polym. Degrad. Stab. 2016, 132, 127–136. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Tsanaktsis, V.; Nerantzaki, M.; Achilias, D.S.; Vaimakis, T.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal degradation of biobased polyesters: Kinetics and decomposition mechanism of polyesters from 2,5-furandicarboxylic acid and long-chain aliphatic diols. J. Anal. Appl. Pyrolysis 2016, 117, 162–175. [Google Scholar] [CrossRef]
- Stensrud, K.; Fergusson, S. Organotin Catalysts in Esterification Processes of Furan-2,5-dicarboxylic Acid (fdca). US Patent 20200354329A1, 12 November 2020. [Google Scholar]
- Saltzberg, M.A. Update on DuPont-ADM FDME Program. DuPont Industrial Biosciences, July 2017. Available online: https://www.bio.org/sites/default/files/legacy/bioorg/docs/0830AM-%20Michael%20Saltzberg.pdf (accessed on 21 June 2022).
- Metkar, P.S.; Ozer, R.; Rajagopalan, B. Service Backed Digital Ruled Paper Templates. US Patent US 20190300494 A1, 3 October 2019. [Google Scholar]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papgeorgiou, D.G.; Bikiaris, D.N. Tuning the properties of furandicarboxylic acid-based polyesters with copolymerization: A review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Maniar, D.; Jiang, Y.; Woortman, A.J.J.; Dijken, J.v.; Loos, K. Furan-based copolyesters from renewable resources: Enzymatic synthesis and properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.; Wang, J.; Zhang, X.; Jia, Z.; Jiang, Y.; Liu, X. Recent progress on bio-based polyesters derived from 2,5-furandicarbonxylic acid (FDCA). Polymers 2022, 14, 625. [Google Scholar] [CrossRef]
- Kainulainen, T.P.; Hukka, T.I.; Özeren, H.D.; Sirviö, J.A.; Hedenqvist, M.S.; Heiskanen, J.P. Utilizing furfural-based bifuran diester as monomer and comonomer for high-performance bioplastics: Properties of poly(butylene furanoate), poly(butylene bifuranoate), and their copolyesters. Biomacromolecules 2019, 21, 743–752. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Sousa, A.; Long, Y.; Ying, W.B.; Wang, J.; Zhu, J. Bio-based poly(butylene 2,5-furandicarboxylate)-b-poly(ethylene glycol) copolymers with adjustable degradation rate and mechanical properties: Synthesis and characterization. Eur. Polym. J. 2018, 106, 42–52. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Tang, X.; Peng, L.; Wei, J.; Lin, L. Methods in the synthesis and conversion of 2,5-bis-(hydroxymethyl)furan from bio-derived 5-hydroxymethylfurfural and its great potential in polymerization. BioResources 2018, 13, 7137–7154. [Google Scholar] [CrossRef]
- Kang, E.-S.; Chae, D.W.; Kim, B.; Kim, Y.G. Efficient preparation of DHMF and HMFA from biomass-derived HMF via a Cannizzaro reaction in ionic liquids. J. Ind. Eng. Chem. 2012, 18, 174–177. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Self-healing bio-based furan polymers cross-linked with various bis-maleimides. Polymer 2013, 54, 5351–5357. [Google Scholar] [CrossRef]
- Li, H.; Huo, N.; Liu, X.; Cheng, J.; Zhang, J. Effects of the furan ring in epoxy resin on the thermomechanical properties of highly cross-linked epoxy networks: A molecular simulation study. RSC Adv. 2016, 6, 769–777. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Ekenstein, G.O.R.A.v.; Petrović, D.M.; Loos, K. Enzymatic synthesis of biobased polyesters using 2,5-bis(hydroxymethyl)furan as the building block. Biomacromolecules 2014, 15, 2482–2493. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Miletić, N.; Loos, K.; Gross, R.A. Enzymatic Polymerisation of Polyester. In Biocatalysis in Polymer Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 83–129. [Google Scholar]
- Sperling, L.H. Glass-Rubber Transition Behaviour. In Introduction to Physical Polymer Science; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 349–425. [Google Scholar]
- Guillaume, L.; Marshall, A.; Niessen, N.; Ni, P.; Gauvin, R.M.; Thomas, C.M. Multicatalysis from renewable resources: A direct route to furan-based polyesters. Green Chem. 2021, 23, 6931–6935. [Google Scholar] [CrossRef]
- Fouilloux, H.; Rager, M.-N.; Ríos, P.; Conejero, S.; Thomas, C.M. Highly efficient synthesis of poly(silylether)s: Access to degradable polymers from renewable resources. Angew. Chem. Int. Ed. 2022, 61, e202113443. [Google Scholar] [CrossRef]
- Choi, E.H.; Lee, J.; Son, S.U.; Song, C. Biomass-derived furanic polycarbonates: Mild synthesis and control of the glass transition temperature. J. Polym. Sci. A Polym. Chem. 2019, 57, 1796–1800. [Google Scholar] [CrossRef]
- Nguyen, L.-T.T.; Devroede, J.; Plasschaert, K.; Jonckheere, L.; Haucourt, N.; Prez, F.E.D. Providing polyurethane foams with functionality: A kinetic comparison of different “click” and coupling reaction pathway. Polym. Chem. 2013, 4, 1546–1556. [Google Scholar] [CrossRef]
- Oh, C.; Choi, E.H.; Choi, E.J.; Premkumar, T.; Song, C. Facile solid-state mechanochemical synthesis of eco-friendly thermoplastic polyurethanes and copolymers using a biomass-derived furan diol. ACS Sustain. Chem. Eng. 2020, 8, 4440–4446. [Google Scholar] [CrossRef]
- Zhang, L.; Michel, F.C.; Co, A.C. Nonisocyanate route to 2,5-bis(hydroxymethyl)furan-based polyurethanes crosslinked by reversible diels–alder reactions. J. Polym. Sci. 2019, 57, 1495–1499. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Bell, A.T. Biomass conversion to diesel via the etherification of furanyl alcohols catalyzed by Amberlyst-15. J. Catal. 2014, 313, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Liang, W.; Guan, J.; Wang, L.; Qu, Q.; Zhang, X.; Wang, X.; Mu, X. Catalytic synthesis of 2,5-bis-methoxymethylfuran: A promising cetane number improver for diesel. Appl. Catal. A 2014, 481, 49–53. [Google Scholar] [CrossRef]
- De Jong, E.; Vijlbrief, T.; Hijkoop, R.; Gruter, G.-J.M.; Van der Waal, J.C. Promising results with YXY Diesel components in an ESC test cycle using a PACCAR Diesel engine. Biomass Bioenerg. 2012, 36, 151–159. [Google Scholar] [CrossRef]
- Avantium History. Available online: https://www.avantium.com/our-company/history/ (accessed on 14 February 2021).
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Avantium YXY® Technology. Available online: https://www.avantium.com/technologies/yxy/ (accessed on 14 February 2021).
- Avantium Commercialising PEF Production. Available online: https://www.thechemicalengineer.com/news/avantium-commercialising-pef-production/ (accessed on 14 February 2021).
- Krawielitzki, S. AVA Biochem, pioneer in industrial biobased furan chemistry. CHIMIA 2020, 74, 776–778. [Google Scholar] [CrossRef] [PubMed]
- AVA Biochem: Our Products. Available online: https://ava-biochem.com/our-products/ (accessed on 15 February 2021).
- Saltzberg, M.A. Update on DuPont-ADM FDME/Furan Polymer Program, DuPont Industrial Biosciences, July 2018. Available online: https://www.bio.org/sites/default/files/legacy/bioorg/docs/0830%20Saltzberg.pdf (accessed on 21 June 2022).
- Origin Materials: Technology. Available online: https://www.originmaterials.com/technology (accessed on 15 February 2021).
- Eastman Licences FDCA Technology to Origin. Available online: https://www.eastman.com/Company/News_Center/2017/Pages/Eastman-Licenses-2-5-Furandicarboxylic-Acid.aspx (accessed on 15 February 2021).
- Morden, P. Construction Continues on ‘Pioneer’ Biochemical Plant in Sarnia. Sarnia The Observer, November 2020. Available online: https://www.theobserver.ca/news/local-news/construction-continues-on-pioneer-bio-chemical-plant-in-sarnia (accessed on 21 June 2022).
- Mija, A.; Waal, J.C.v.d.; Pin, J.M.; Guigo, N.; de Jong, E. Humins as promising material for producing sustainable carbohydrate-derived building materials. Constr. Build. Mater. 2017, 139, 594–601. [Google Scholar] [CrossRef]
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Thygesen, L.; Marlair, G.; Angelici, C.; Jong, E.d.; Sbirrazzuoli, N. Humin based resin for wood modification and property improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.M.C.; Van Eck, E.R.G.; Bula, W.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Pin, J.-M.; Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N.; Waal, J.C.v.d.; Jong, E.D. Valorization of biorefinery side-stream products: Combination of humins with polyfurfuryl alcohol for composite elaboration. ACS Sustain. Chem. Eng. 2015, 2, 2182–2190. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; Waal, J.C.v.d.; Sbirrazzuoli, N. All ‘green’ composites comprising flax fibres and humins’ resins. Compos. Sci. Technol. 2019, 171, 70–77. [Google Scholar]
- Filiciotto, L.; Balu, A.M.; Romero, A.A.; Castellón, E.R.; van der Waal, J.C.; Luque, R. Benign-by-design preparation of humin-based iron oxide catalytic nanocomposites. Green Chem. 2017, 19, 4423–4434. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Agarwal, S.; Kloekhorst, A.; Heeres, H.J. Catalytic hydrotreatment of humins in mixtures of formic acid/2-propanol with supported ruthenium catalysts. ChemSusChem 2016, 9, 951–961. [Google Scholar] [CrossRef]
- Eerhart, J.E.; Patel, M.K.; Faaij, A.P.C. Fuels and plastics from lignocellulosic biomass via the furan pathway: An economic analysis. Biofuels Bioprod. Bioref. 2015, 9, 307–325. [Google Scholar] [CrossRef]
- Avantium. Annual Report 2019; Avantium NV: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Avantium. Avantium Investor Presentation Q4 2020; Avantium NV: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Corbion Purac. FDCA Bioplastics: Biobased Monomers for PEF; Corbion Purac: Amsterdam, The Netherlands, 2015. [Google Scholar]
- European Commission. “Paired Electrochemical Oxidation Process for Feasible Industrial Production of the Crucial FDCA Building Block for the Bioplastic Industry”, EC, 29 January 2019. Available online: https://cordis.europa.eu/project/id/807221/reporting (accessed on 11 March 2021).










| Property | PET | PEF |
|---|---|---|
| Glass Transition Temperature (°C) | 73 | 85 |
| Melting Point (°C) | 260 | 220 |
| Tensile Strength (MPa) | 50 | 76 |
| Young’s Modulus (GPa) | 1.1 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, A.; Jiang, B.; Gauvin, R.M.; Thomas, C.M. 2,5-Furandicarboxylic Acid: An Intriguing Precursor for Monomer and Polymer Synthesis. Molecules 2022, 27, 4071. https://doi.org/10.3390/molecules27134071
Marshall A, Jiang B, Gauvin RM, Thomas CM. 2,5-Furandicarboxylic Acid: An Intriguing Precursor for Monomer and Polymer Synthesis. Molecules. 2022; 27(13):4071. https://doi.org/10.3390/molecules27134071
Chicago/Turabian StyleMarshall, Adam, Bo Jiang, Régis M. Gauvin, and Christophe M. Thomas. 2022. "2,5-Furandicarboxylic Acid: An Intriguing Precursor for Monomer and Polymer Synthesis" Molecules 27, no. 13: 4071. https://doi.org/10.3390/molecules27134071







