Protective Effects of Jujubosides on 6-OHDA-Induced Neurotoxicity in SH-SY5Y and SK-N-SH Cells
Abstract
:1. Introduction
2. Results
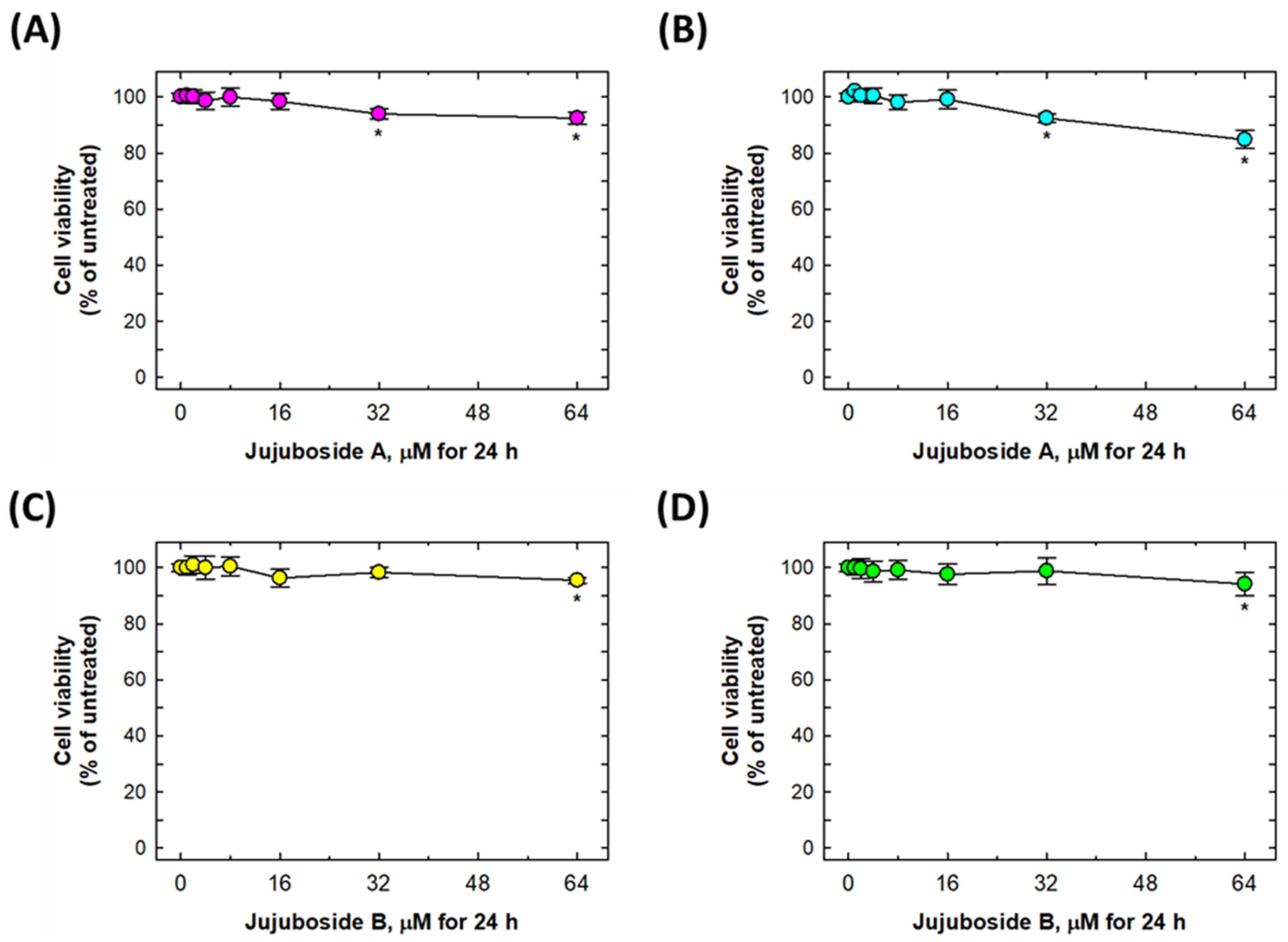
2.1. 6-OHDA Induced Suppression in Cell Viability
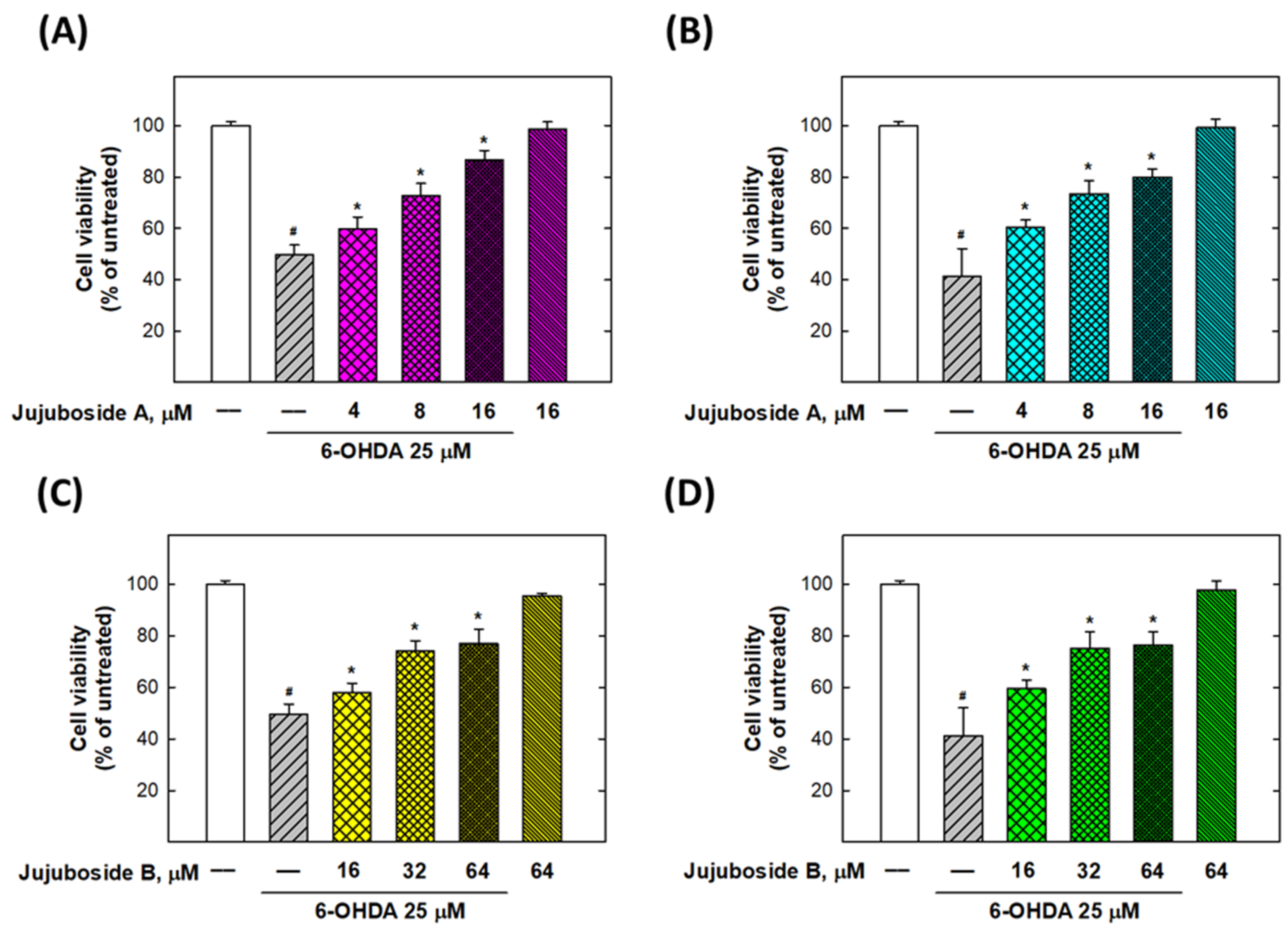
2.2. Alleviation Effects of Jujubosides on 6-OHDA-Induced Decease in Cell Viability
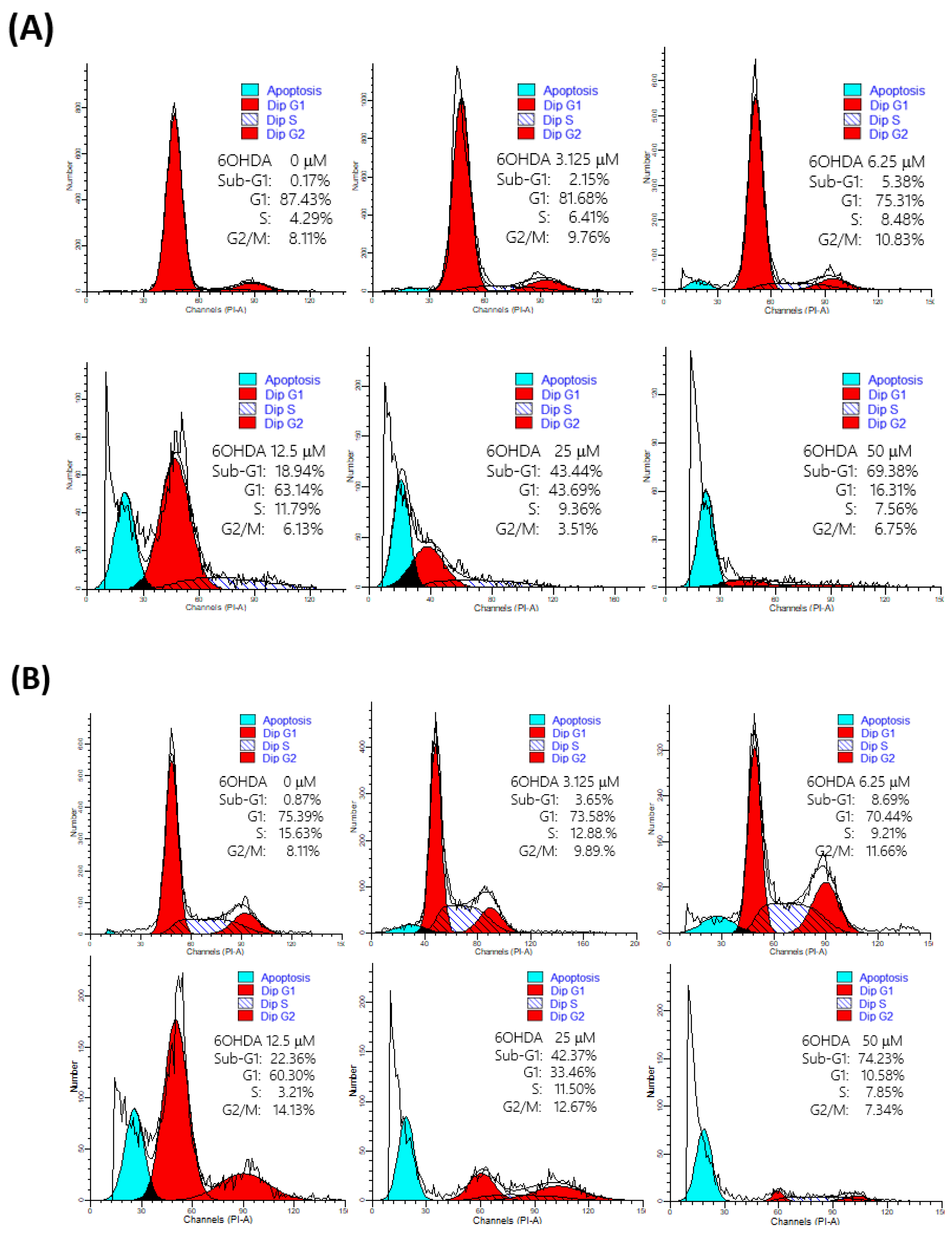
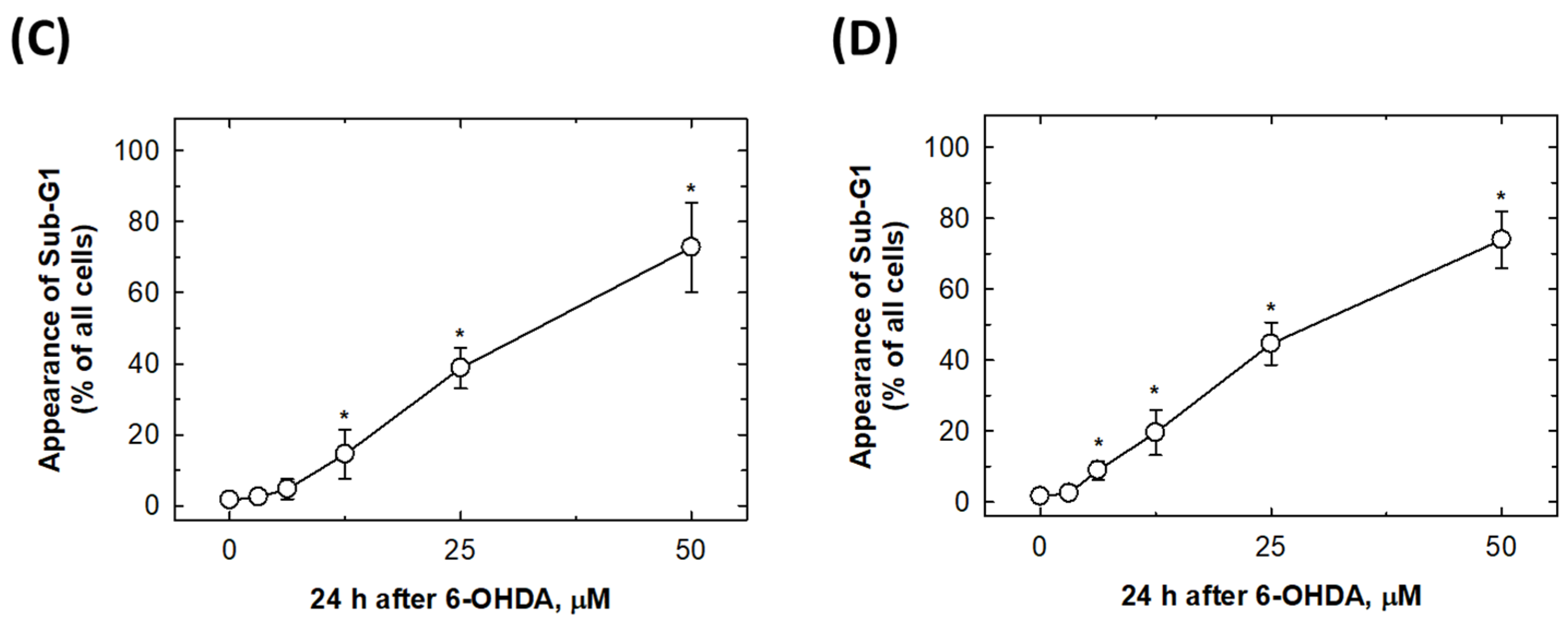
2.3. 6-OHDA Would Induce Cell Apoptosis
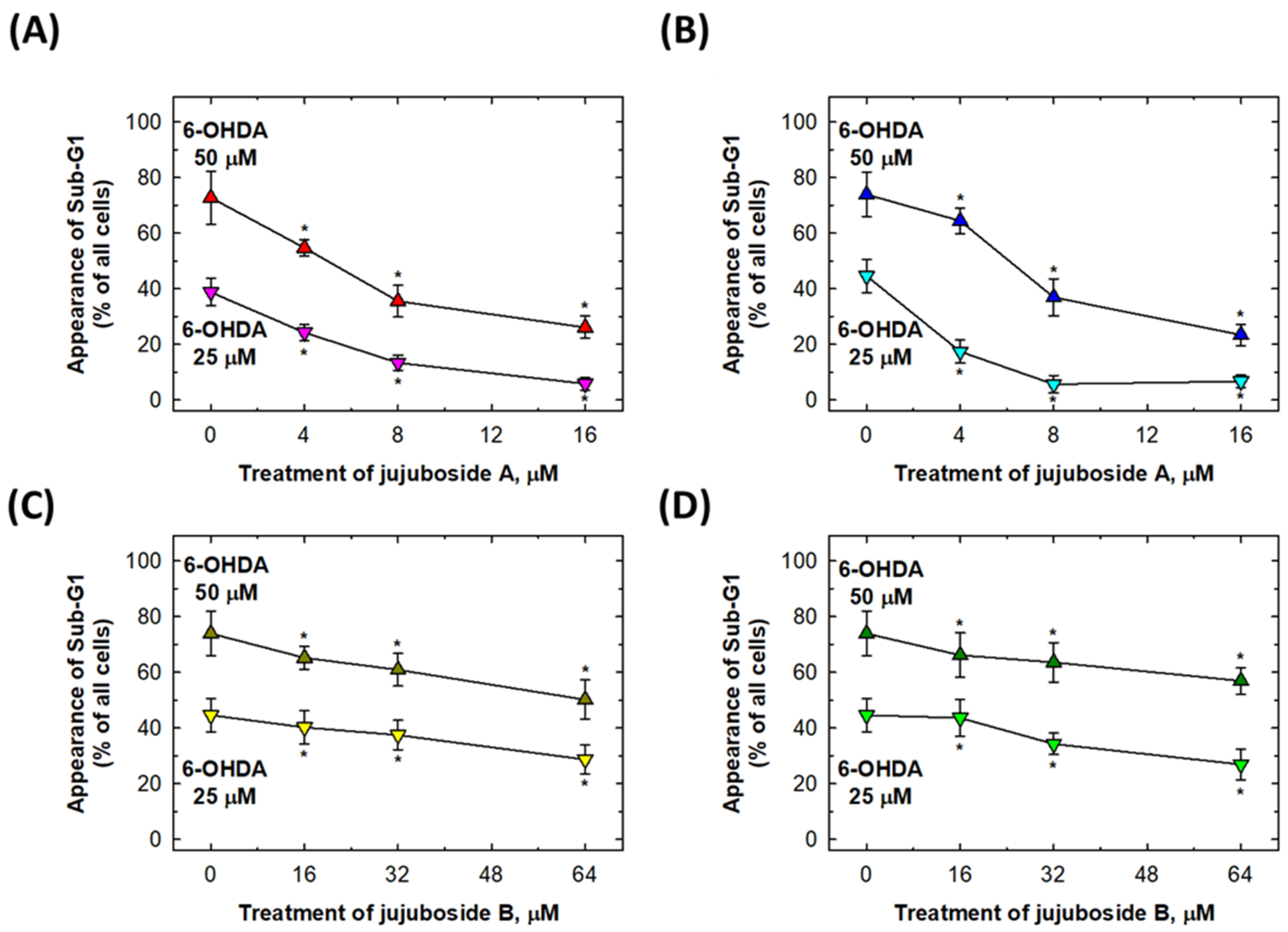
2.4. Rescuing Effects of Jujubosides on 6-OHDA-Induced Cell Apoptosis
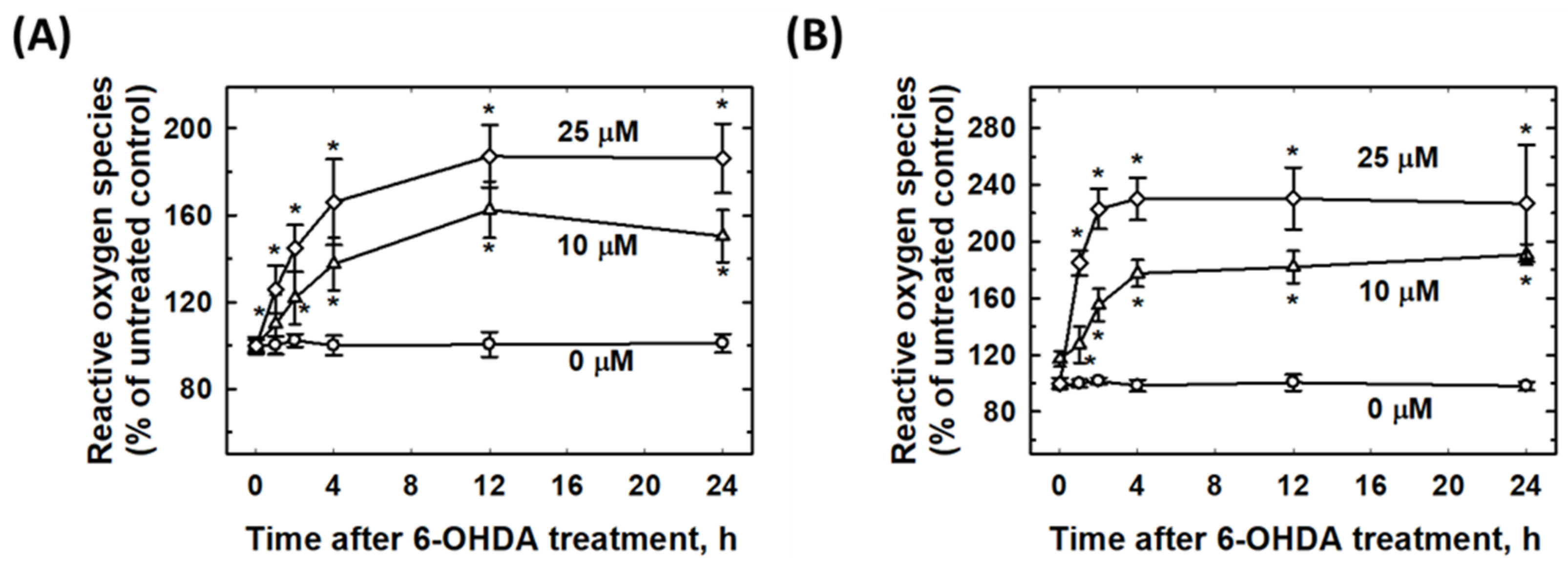
2.5. 6-OHDA Induced Intracellular ROS Elevation
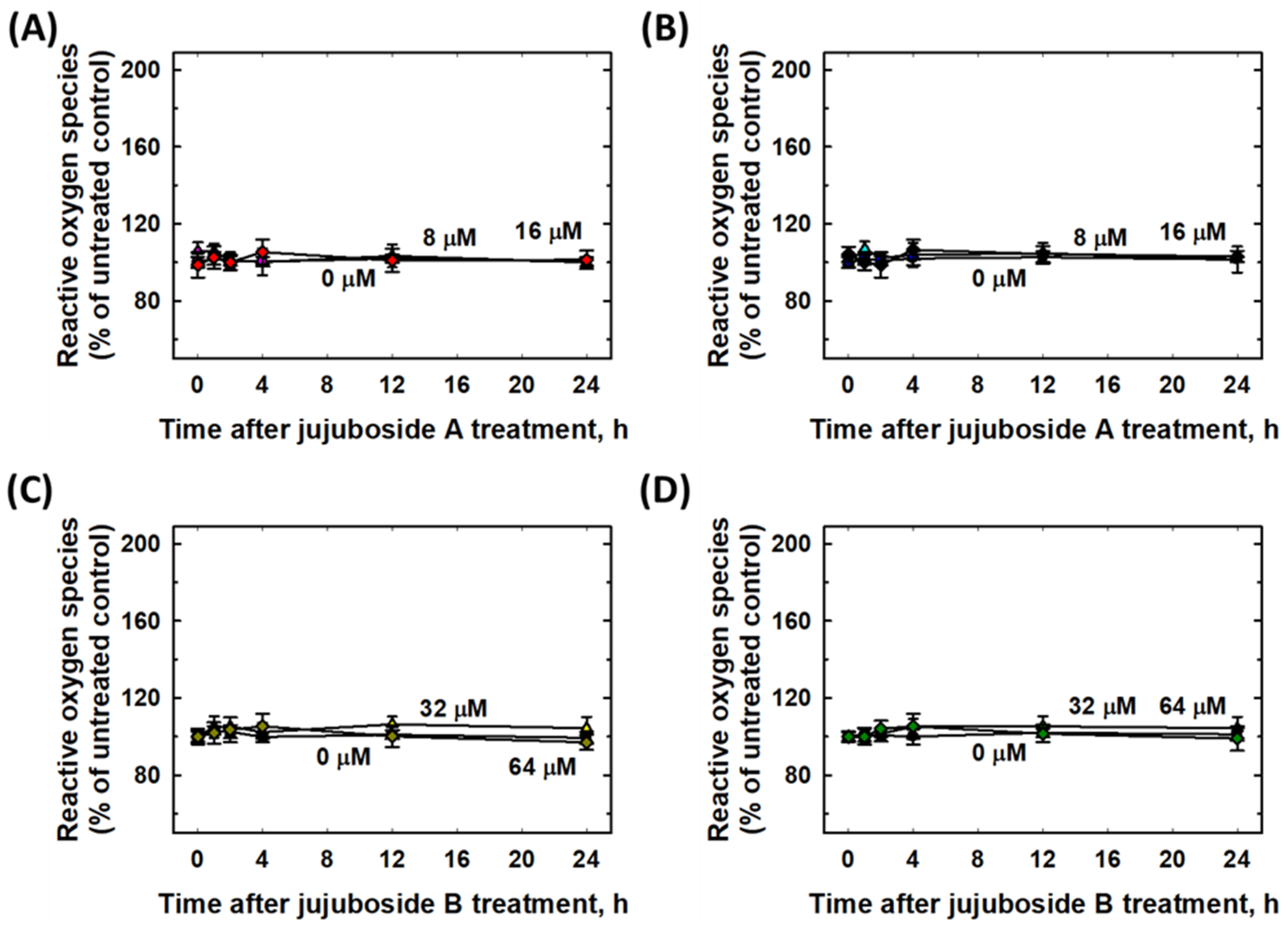
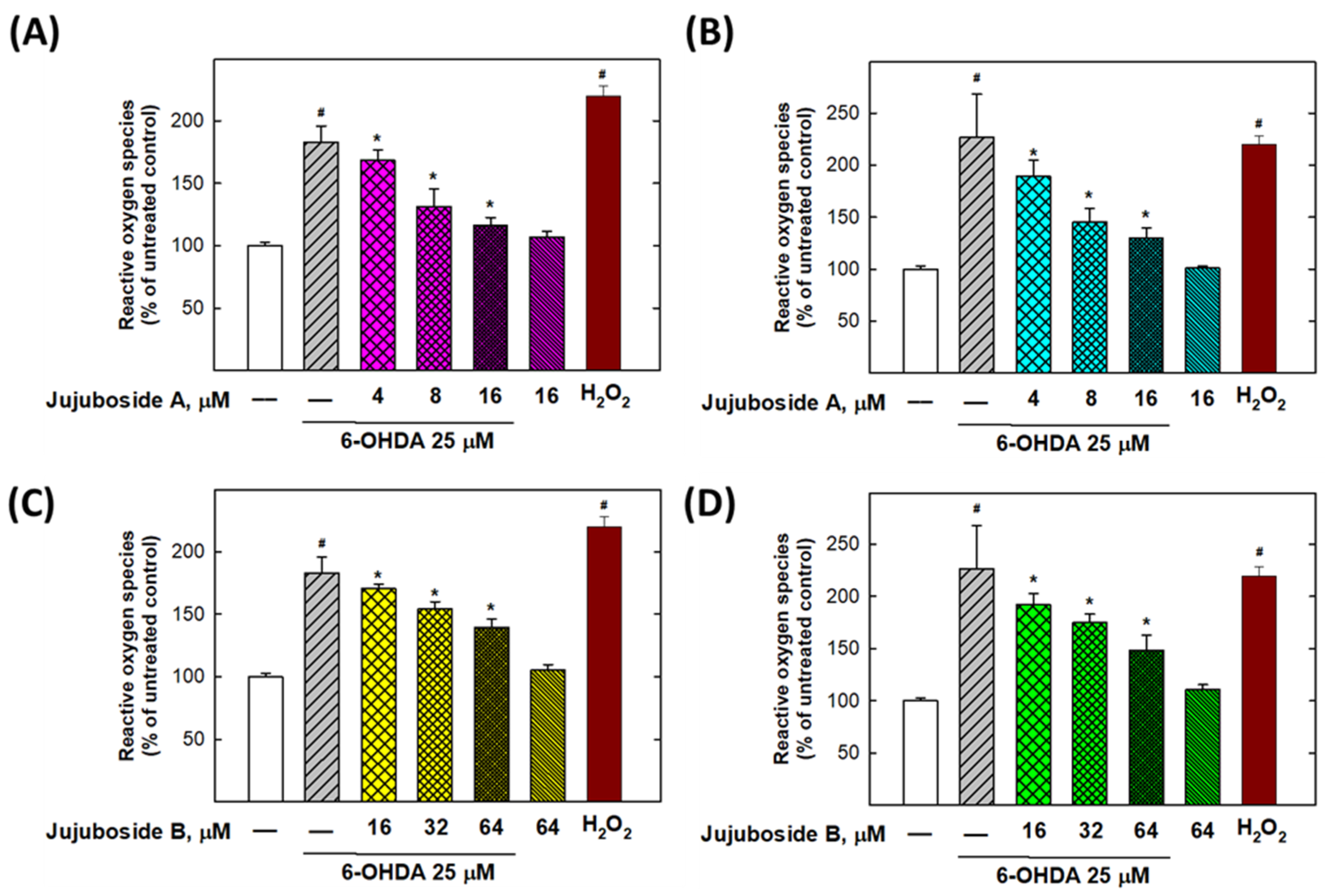
2.6. Eliminative Effects of Jujubosides on 6-OHDA-Induced ROS
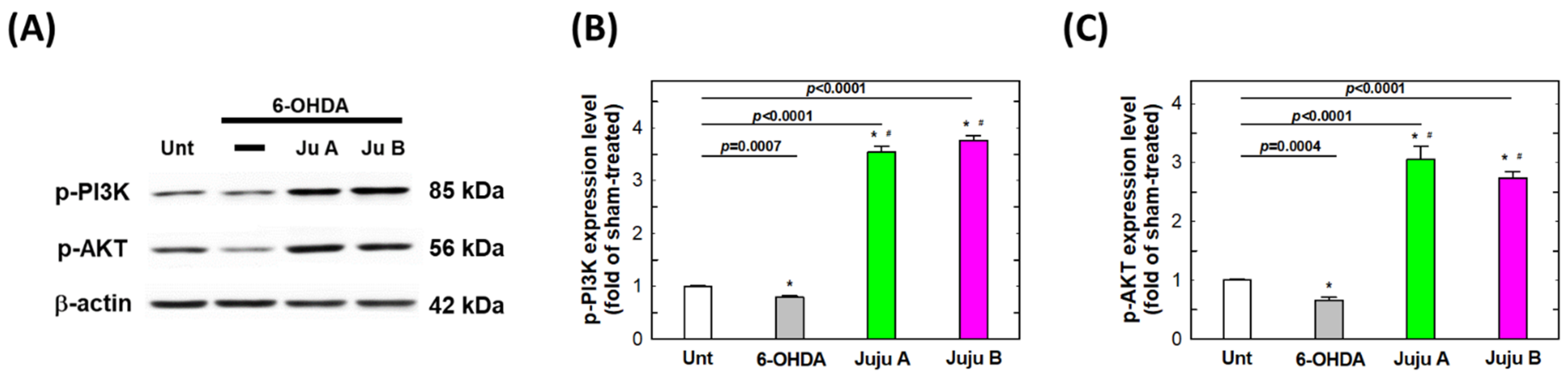
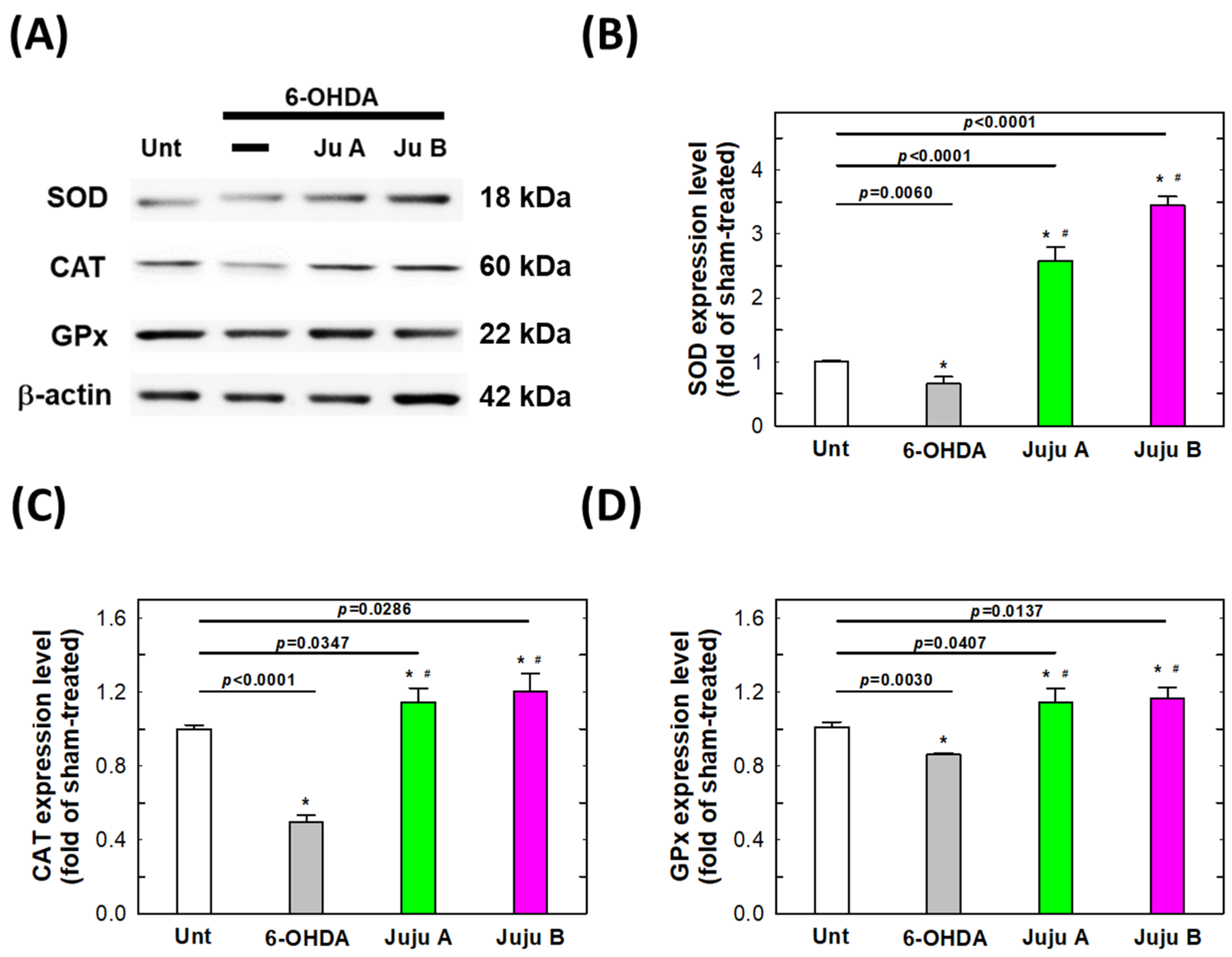
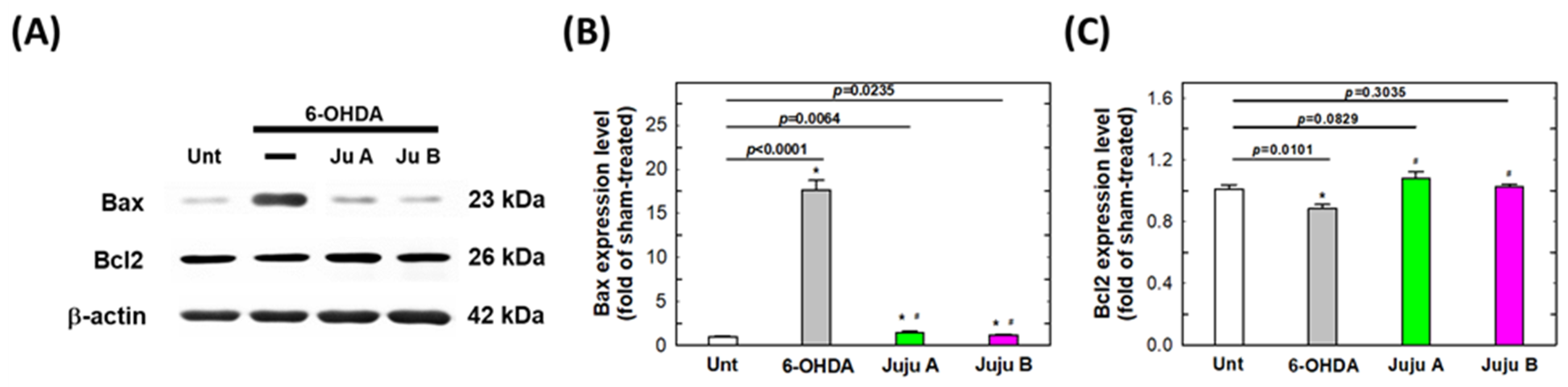
2.7. Alterations of Apoptotic-Related and Redox-Related Proteins
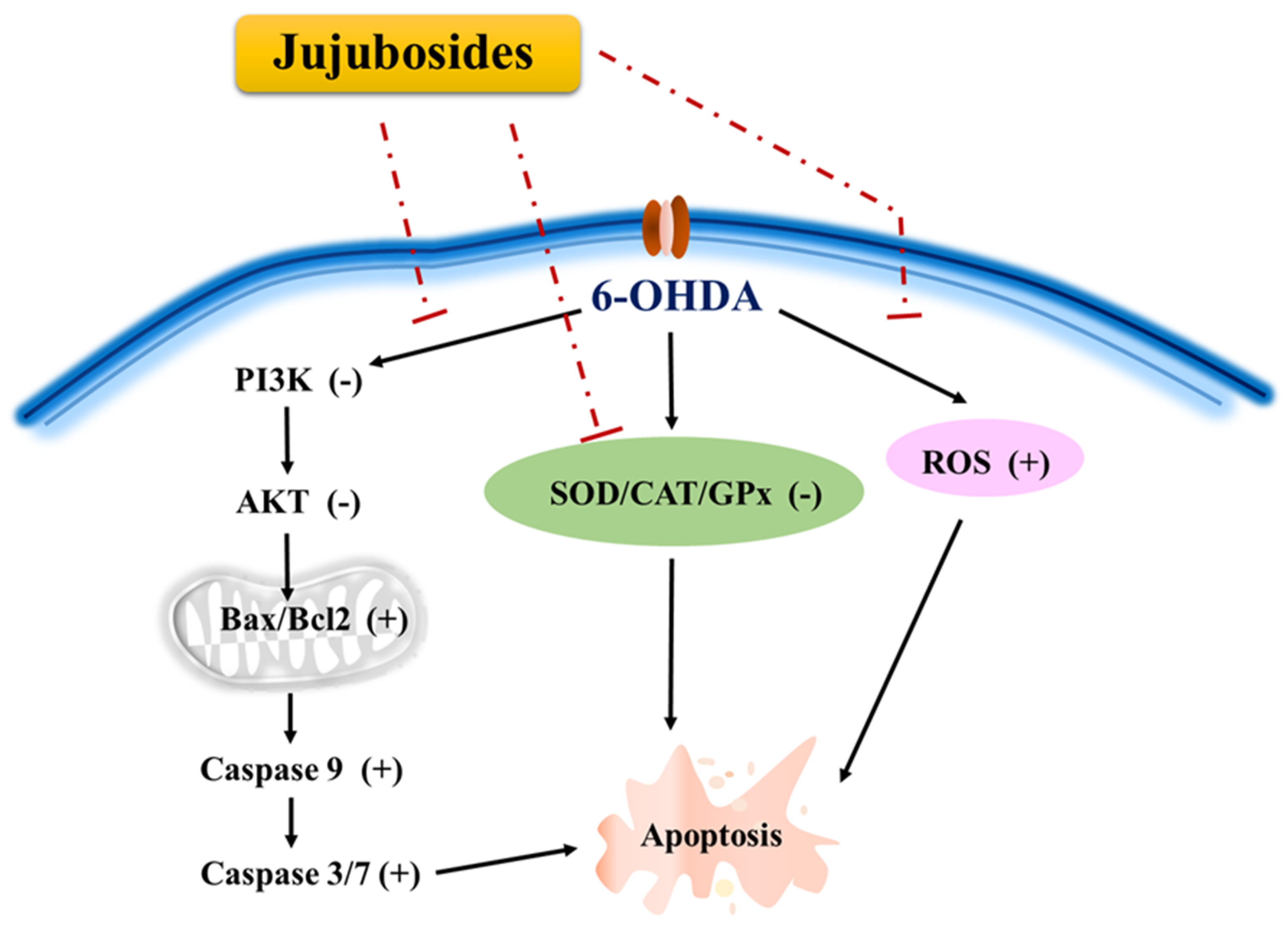
3. Discussion
4. Materials and Methods
4.1. Cell Culturing Conditions
4.2. Chemicals and Common Treatment Protocols
4.3. Determination of Cell Viability by MTT Assay
4.4. Measurement of ROS Production
4.5. Determination of Apoptosis
4.6. Western Blotting Procedure
4.7. Statistical Methodology
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Parkinsons Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Wang, S.; Tian, J.; Chen, L.; Zhang, W.; Zhao, J.; Tang, H.; Zhang, X.; Chen, J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside in the MPTP-induced mouse model of Parkinson’s disease: Involvement of reactive oxygen species-mediated JNK, P38 and mitochondrial pathways. Eur. J. Pharmacol. 2015, 767, 175–182. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.K. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drechsel, D.A.; Patel, M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic. Biol. Med. 2008, 44, 1873–1886. [Google Scholar] [CrossRef] [Green Version]
- Hermida-Ameijeiras, A.; Mendez-Alvarez, E.; Sanchez-Iglesias, S.; Sanmartin-Suarez, C.; Soto-Otero, R. Autoxidation and MAO-mediated metabolism of dopamine as a potential cause of oxidative stress: Role of ferrous and ferric ions. Neurochem. Int. 2004, 45, 103–116. [Google Scholar] [CrossRef]
- Perfeito, R.; Cunha-Oliveira, T.; Rego, A.C. Reprint of: Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic. Biol. Med. 2013, 62, 186–201. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Mendez-Alvarez, E.; Hermida-Ameijeiras, A.; Munoz-Patino, A.M.; Labandeira-Garcia, J.L. Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: Potential implication in relation to the pathogenesis of Parkinson’s disease. J. Neurochem. 2000, 74, 1605–1612. [Google Scholar] [CrossRef]
- Saito, Y.; Nishio, K.; Ogawa, Y.; Kinumi, T.; Yoshida, Y.; Masuo, Y.; Niki, E. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: Involvement of hydrogen peroxide-dependent and -independent action. Free Radic. Biol. Med. 2007, 42, 675–685. [Google Scholar] [CrossRef]
- Izumi, Y.; Sawada, H.; Sakka, N.; Yamamoto, N.; Kume, T.; Katsuki, H.; Shimohama, S.; Akaike, A. P-Quinone mediates 6-hydroxydopamine-induced dopaminergic neuronal death and ferrous iron accelerates the conversion of p-quinone into melanin extracellularly. J. Neurosci. Res. 2005, 79, 849–860. [Google Scholar] [CrossRef]
- He, Y.; Lee, T.; Leong, S.K. 6-Hydroxydopamine induced apoptosis of dopaminergic cells in the rat substantia nigra. Brain Res. 2000, 858, 163–166. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Lombardo, G.E.; Russo, C.; Musumeci, L.; Gangemi, S.; Calapai, G.; Barreca, D.; Navarra, M. A Flavonoid-Rich Extract of Mandarin Juice Counteracts 6-OHDA-Induced Oxidative Stress in SH-SY5Y Cells and Modulates Parkinson-Related Genes. Antioxidant 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.W.; Hsu, P.C.; Chang, W.S.; Yu, C.C.; Wang, Y.C.; Yang, J.S.; Tsai, F.J.; Chen, K.Y.; Tsai, C.W.; Bau, D.T. Protective effects of valproic acid on 6-hydroxydopamine-induced neuroinjury. Environ. Toxicol. 2020, 35, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Wu, Y.; Nissou, M.F.; Arnaud, S.; Alim Louis, B.; Verna, J.M. p53 and Bax activation in 6-hydroxydopamine-induced apoptosis in PC12 cells. Brain Res. 1997, 751, 139–142. [Google Scholar] [CrossRef]
- Rehfeldt, S.C.H.; Silva, J.; Alves, C.; Pinteus, S.; Pedrosa, R.; Laufer, S.; Goettert, M.I. Neuroprotective Effect of Luteolin-7-O-Glucoside against 6-OHDA-Induced Damage in Undifferentiated and RA-Differentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 2914. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of Jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Li, J.; Shan, L.; Liu, Y.; Fan, L.; Ai, L. Screening of a functional polysaccharide from Zizyphus Jujuba cv. Jinsixiaozao and its property. Int. J. Biol. Macromol. 2011, 49, 255–259. [Google Scholar] [CrossRef]
- Chen, J.; Tsim, K.W.K. A Review of Edible Jujube, the Ziziphus jujuba Fruit: A Heath Food Supplement for Anemia Prevalence. Front. Pharmacol. 2020, 11, 593655. [Google Scholar] [CrossRef]
- Lam, C.T.W.; Chan, P.H.; Lee, P.S.C.; Lau, K.M.; Kong, A.Y.Y.; Gong, A.G.W.; Xu, M.L.; Lam, K.Y.C.; Dong, T.T.X.; Lin, H.; et al. Chemical and biological assessment of Jujube (Ziziphus jujuba)-containing herbal decoctions: Induction of erythropoietin expression in cultures. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2016, 1026, 254–262. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, B.P.; Luo, D.; Shen, X.C.; Guo, S.; Duan, J.A.; Tang, Y.P. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine 2012, 19, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Abedini, M.R.; Mitra, M.; Fard, M.H.; Beydokhti, H. “Ziziphus jujuba”: A red fruit with promising anticancer activities. Pharmacogn. Rev. 2015, 9, 99–106. [Google Scholar] [PubMed] [Green Version]
- Steinkamp-Fenske, K.; Bollinger, L.; Xu, H.; Yao, Y.; Horke, S.; Forstermann, U.; Li, H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharmacol. Exp. Ther. 2007, 322, 836–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, E.J.; Lee, S.Y.; Kang, S.S.; Jung, Y.S. Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytother. Res. 2013, 27, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.X.; Zhang, Q.Y.; Cui, S.Y.; Cui, X.Y.; Zhang, J.; Zhang, Y.H.; Bai, Y.J.; Zhao, Y.Y. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. J. Ethnopharmacol. 2010, 130, 163–166. [Google Scholar] [CrossRef]
- Wang, X.X.; Ma, G.I.; Xie, J.B.; Pang, G.C. Influence of JuA in evoking communication changes between the small intestines and brain tissues of rats and the GABAA and GABAB receptor transcription levels of hippocampal neurons. J. Ethnopharmacol. 2015, 159, 215–223. [Google Scholar] [CrossRef]
- Han, D.; Wan, C.; Liu, F.; Xu, X.; Jiang, L.; Xu, J. Jujuboside A Protects H9C2 Cells from Isoproterenol-Induced Injury via Activating PI3K/Akt/mTOR Signaling Pathway. Evid.-Based Complement. Altern. Med. 2016, 2016, 9593716. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.G.; Huang, X.J.; Chen, J.; Lin, Q.S. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat. Prod. Res. 2007, 21, 310–320. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Xie, J. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J. Pharm. Biomed. Anal. 2008, 48, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Zhu, H.; Xie, L.; Ma, J.; Xu, Y.; Zhou, Q.; Wu, Z.; Cai, B. Simultaneous Quantification of Six Bioactive Components in Decoction of Ziziphi spinosae Semen Using Ultrahigh Performance Liquid Chromatography Coupled with Triple-Quadrupole Mass Spectrometry. J. Anal. Methods Chem. 2018, 2018, 8397818. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Lee, S.Y.; Kang, S.S.; Kim, Y.S. Antitumor activity of jujuboside B and the underlying mechanism via induction of apoptosis and autophagy. J. Nat. Prod. 2014, 77, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.M.; Li, Y.Q.; Xu, K.Q.; Zhang, Y.Y.; Tan, S.M.; Zhang, Q.; Peng, J.; Luo, X.J. Jujuboside B promotes the death of acute leukemia cell in a RIPK1/RIPK3/MLKL pathway-dependent manner. Eur. J. Pharmacol. 2020, 876, 173041. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liang, Y.; Wang, S.; Li, L.; Cai, L.; Heng, Y.; Yang, J.; Jin, X.; Zhang, J.; Yuan, S.; et al. Jujuboside B Inhibits the proliferation of breast cancer cell lines by inducing apoptosis and autophagy. Front. Pharmacol. 2021, 12, 668887. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Hennequin, C.; Giocanti, N.; Balosso, J.; Favaudon, V. Interaction of ionizing radiation with the topoisomerase I poison camptothecin in growing V-79 and HeLa cells. Cancer Res. 1994, 54, 1720–1728. [Google Scholar]
- Guo, S.; Bezard, E.; Zhao, B. Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic. Biol. Med. 2005, 39, 682–695. [Google Scholar] [CrossRef]
- Szabo, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Ma, C.; Cao, M.; Yan, J.; Liang, J.; Ke, K.; Cao, M.; Gu, X. Skin-derived precursor Schwann cells protect SH-SY5Y cells against 6-OHDA-induced neurotoxicity by PI3K/AKT/Bcl-2 pathway. Brain Res. Bull 2020, 161, 84–93. [Google Scholar] [CrossRef]
- Wan, C.R.; Han, D.D.; Xu, J.Q.; Yin, P.; Xu, X.L.; Mei, C.; Liu, F.H.; Xia, Z.F. Jujuboside A attenuates norepinephrine-induced apoptosis of H9c2 cardiomyocytes by modulating MAPK and AKT signaling pathways. Mol. Med. Rep. 2018, 17, 1132–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shou, C.; Feng, Z.; Wang, J.; Zheng, X. The inhibitory effects of jujuboside A on rat hippocampus in vivo and in vitro. Planta Med. 2002, 68, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Misrani, A.; Tang, B.L.; Chen, J.; Yang, L.; Long, C. Jujuboside A prevents sleep loss-induced disturbance of hippocampal neuronal excitability and memory impairment in young APP/PS1 mice. Sci. Rep. 2019, 9, 4512. [Google Scholar] [CrossRef]
- Zhong, Y.; Luo, R.; Liu, Q.; Zhu, J.; Lei, M.; Liang, X.; Wang, X.; Peng, X. Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem. Toxicol. 2022, 159, 112697. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.M.; Tsai, C.W.; Chang, W.S.; Yang, Y.C.; Hung, Y.W.; Lee, H.T.; Shen, C.C.; Sheu, M.L.; Wang, J.Y.; Gong, C.L.; et al. Novel Combination of Arsenic Trioxide (As2O3) Plus Resveratrol in Inducing Programmed Cell Death of Human Neuroblastoma SK-N-SH Cells. Cancer Genom. Proteom. 2018, 15, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.F.; Yang, J.S.; Chiu, Y.J.; Tsai, C.W.; Bau, D.T.; Chang, W.S. Gadodiamide Induced Autophagy and Apoptosis in Human Keratinocytes. In Vivo 2022, 36, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Tsai, C.W.; Chang, W.S.; Lin, J.C.; Shih, L.C.; He, J.L.; Bau, D.T. Protective Effects of Crocetin on Arsenic Trioxide-induced Oxidative Stress in Human Umbilical Vein Endothelial Cells. In Vivo 2021, 35, 3157–3163. [Google Scholar] [CrossRef]
- Chang, W.S.; Tsai, C.W.; Yang, J.S.; Hsu, Y.M.; Shih, L.C.; Chiu, H.Y.; Bau, D.T.; Tsai, F.J. Resveratrol inhibited the metastatic behaviors of cisplatin-resistant human oral cancer cells via phosphorylation of ERK/p-38 and suppression of MMP-2/9. J. Food Biochem. 2021, 45, e13666. [Google Scholar] [CrossRef]
- Liu, S.P.; Shibu, M.A.; Tsai, F.J.; Hsu, Y.M.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Wang, S.; Li, Q.; et al. Tetramethylpyrazine reverses high-glucose induced hypoxic effects by negatively regulating HIF-1alpha induced BNIP3 expression to ameliorate H9c2 cardiomyoblast apoptosis. Nutr. Metab. 2020, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.P.; Chen, P.C.; Wang, S.W.; Fong, Y.C.; Tsai, C.H.; Tsai, F.J.; Chung, J.G.; Huang, C.Y.; Yang, J.S.; Hsu, Y.M.; et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J. Funct. Foods 2019, 52, 537–544. [Google Scholar] [CrossRef]
- Chang, P.Y.; Tsai, F.J.; Bau, D.T.; Hsu, Y.M.; Yang, J.S.; Tu, M.G.; Chiang, S.L. Potential effects of allyl isothiocyanate on inhibiting cellular proliferation and inducing apoptotic pathway in human cisplatin-resistant oral cancer cells. J. Formos. Med. Assoc. 2021, 120, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Lin, C.; Lu, C.C.; Kuo, S.C.; Tsao, J.W.; Juan, Y.N.; Chiu, H.Y.; Lee, F.Y.; Yang, J.S.; Tsai, F.J. YC-1 induces G0/G1 phase arrest and mitochondria-dependent apoptosis in cisplatin-resistant human oral cancer CAR cells. Biomedicine 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Hsu, P.-C.; Hsu, S.-W.; Hong, K.-T.; Chen, K.-Y.; He, J.-L.; Cho, D.-Y.; Wang, Y.-C.; Chang, W.-S.; Bau, D.-T.; et al. Protective Effects of Jujubosides on 6-OHDA-Induced Neurotoxicity in SH-SY5Y and SK-N-SH Cells. Molecules 2022, 27, 4106. https://doi.org/10.3390/molecules27134106
Chen C-H, Hsu P-C, Hsu S-W, Hong K-T, Chen K-Y, He J-L, Cho D-Y, Wang Y-C, Chang W-S, Bau D-T, et al. Protective Effects of Jujubosides on 6-OHDA-Induced Neurotoxicity in SH-SY5Y and SK-N-SH Cells. Molecules. 2022; 27(13):4106. https://doi.org/10.3390/molecules27134106
Chicago/Turabian StyleChen, Chao-Hsuan, Pei-Chen Hsu, Shih-Wei Hsu, Kun-Ting Hong, Kai-Yuan Chen, Jie-Long He, Der-Yang Cho, Yun-Chi Wang, Wen-Shin Chang, Da-Tian Bau, and et al. 2022. "Protective Effects of Jujubosides on 6-OHDA-Induced Neurotoxicity in SH-SY5Y and SK-N-SH Cells" Molecules 27, no. 13: 4106. https://doi.org/10.3390/molecules27134106
APA StyleChen, C.-H., Hsu, P.-C., Hsu, S.-W., Hong, K.-T., Chen, K.-Y., He, J.-L., Cho, D.-Y., Wang, Y.-C., Chang, W.-S., Bau, D.-T., & Tsai, C.-W. (2022). Protective Effects of Jujubosides on 6-OHDA-Induced Neurotoxicity in SH-SY5Y and SK-N-SH Cells. Molecules, 27(13), 4106. https://doi.org/10.3390/molecules27134106





