Recent Progress in Iron-Based Microwave Absorbing Composites: A Review and Prospective
Abstract
:1. Introduction
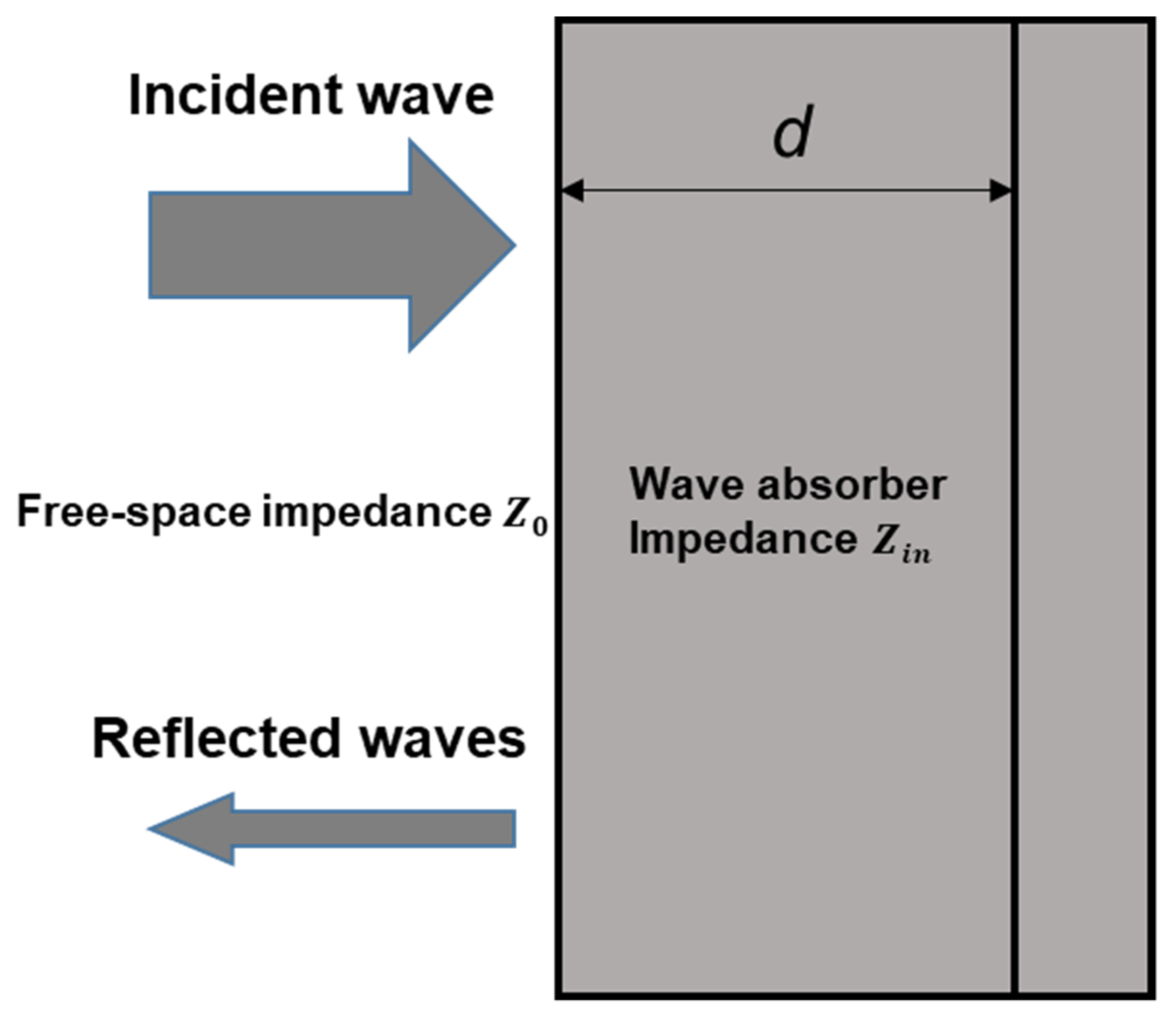
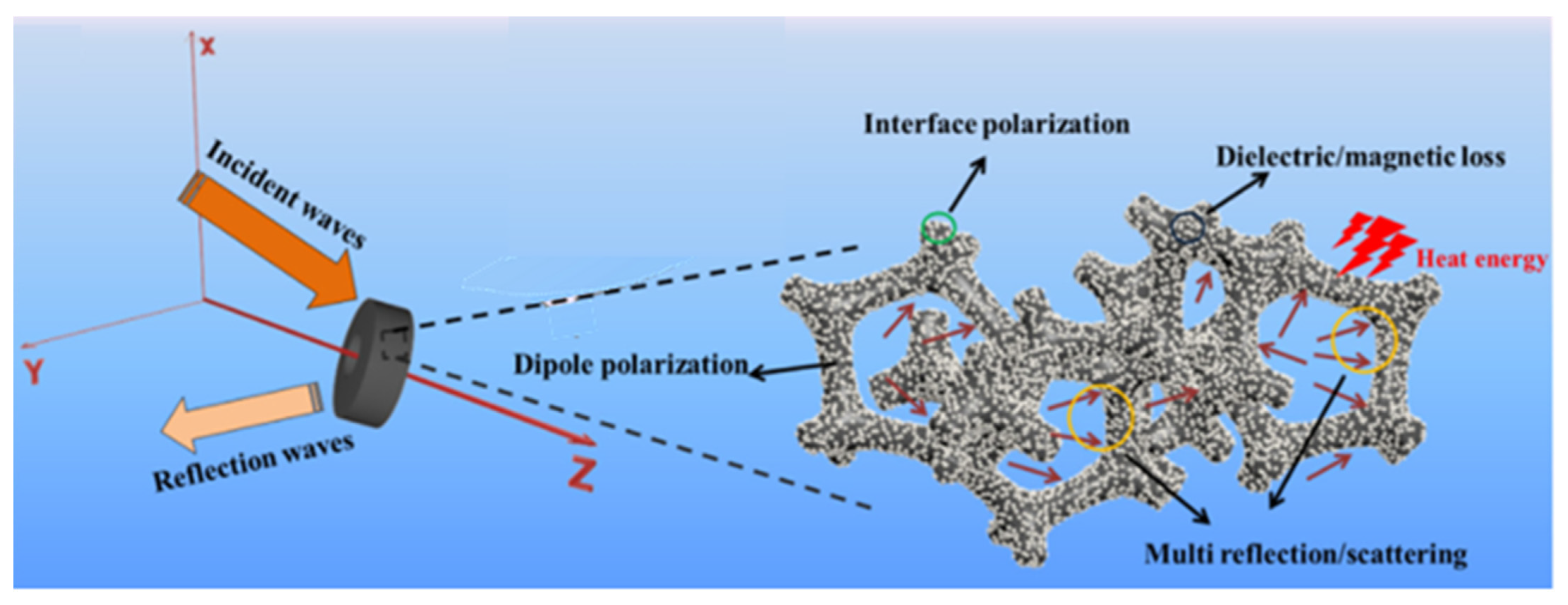
2. Wave Absorption Mechanism of EMWAMs
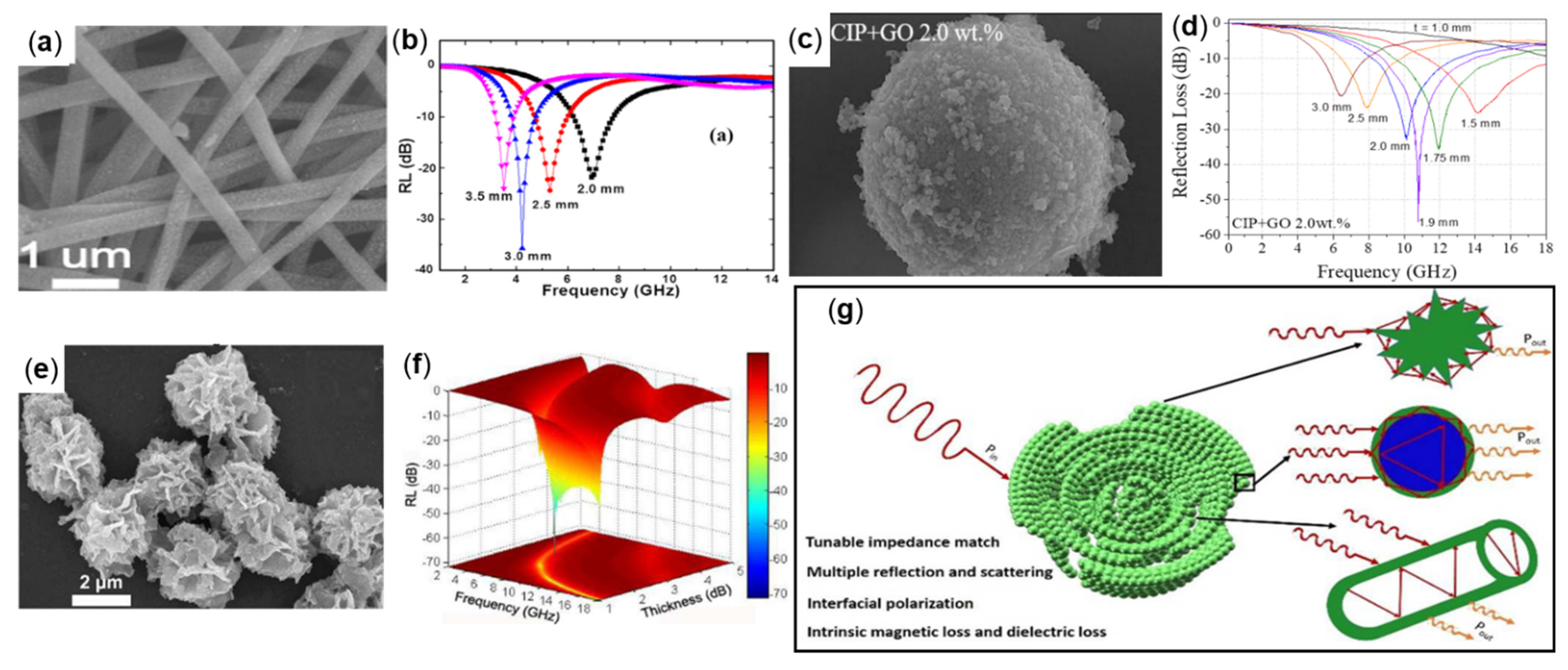
3. Preparation and Absorption Properties of Fe with Different Morphologies
3.1. Sphere-Like Fe
3.2. Flaky-Like Fe
3.3. Wire-Like Fe
| Samples | Methods | ƒE (GHz) | Thickness (mm) | Filling Ratio | RLmin (dB) | Reference |
|---|---|---|---|---|---|---|
| Fe3O4 nanofibers | Electrospinning | 2−18 | - | - | - | [53] |
| Fe nanofibers | Pyrolysis | 9.9 | 2 | 50 wt% | −17.8 | [54] |
| Fe nanowires | Situ reduction | 1.3 | 3.5 | 50 vol% | −32 | [55] |
| Fe NWs | Situ reduction | 2.72 | 1.42 | 20 wt% | −44.67 | [56] |
| Chain-like Fe NWs | Hydrothermal | 3.68 | 3 | 20 wt% | −27.28 | [57] |
| Dendrite-like α-Fe | Electric field-induced, Electrochemical reduction | 10 | 1.9 | 70 wt% | −32.3 | [58] |
| Dendrite-like α-Fe2O3 | Hydrothermal | 2.5 | 3 | 70 wt% | −25 | [59] |
| Cube shape-like Fe | Low-temperature solution reduction | 9.1 | 2 | 26 vol% | −56 | [60] |
3.4. Dendrite-Like Fe and Cube Shape-Like Fe
4. Preparation and Wave Absorption Properties of Fe Matrix Composites
4.1. Blending
4.2. Surface Coating
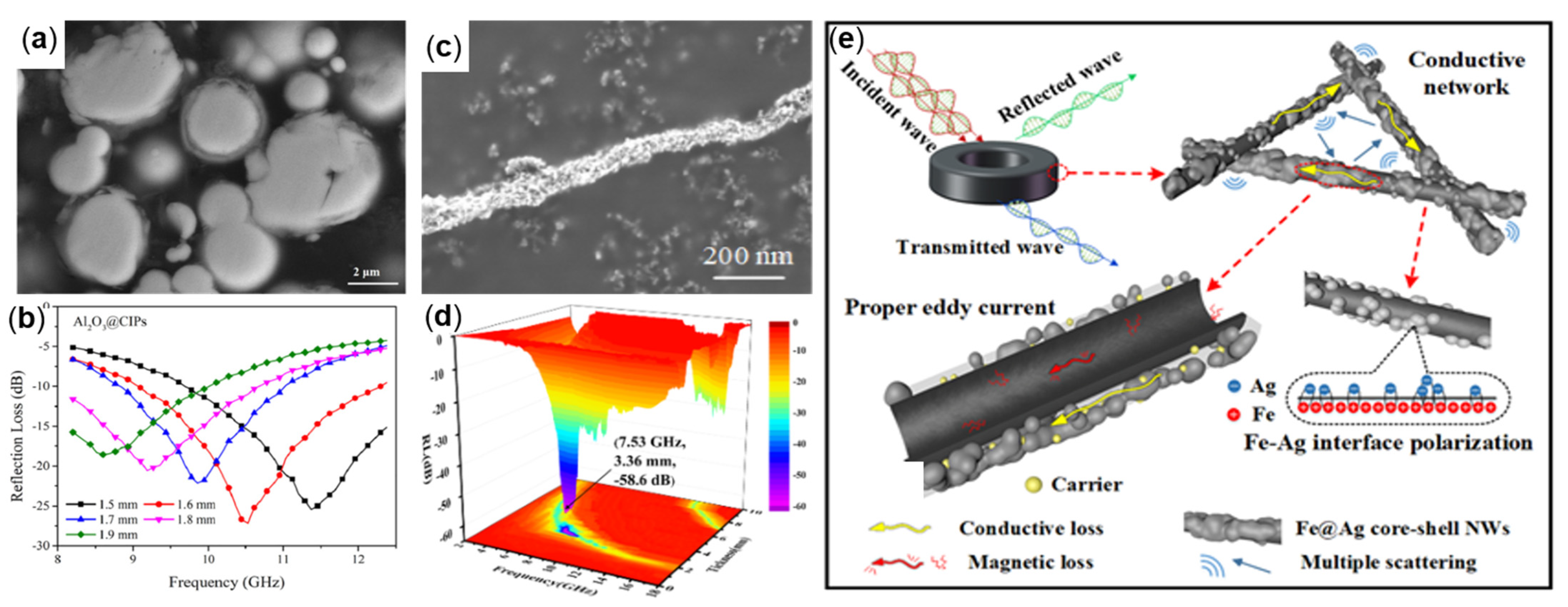
4.2.1. Carbon Material-Coated Fe
4.2.2. Metal-Coated Fe
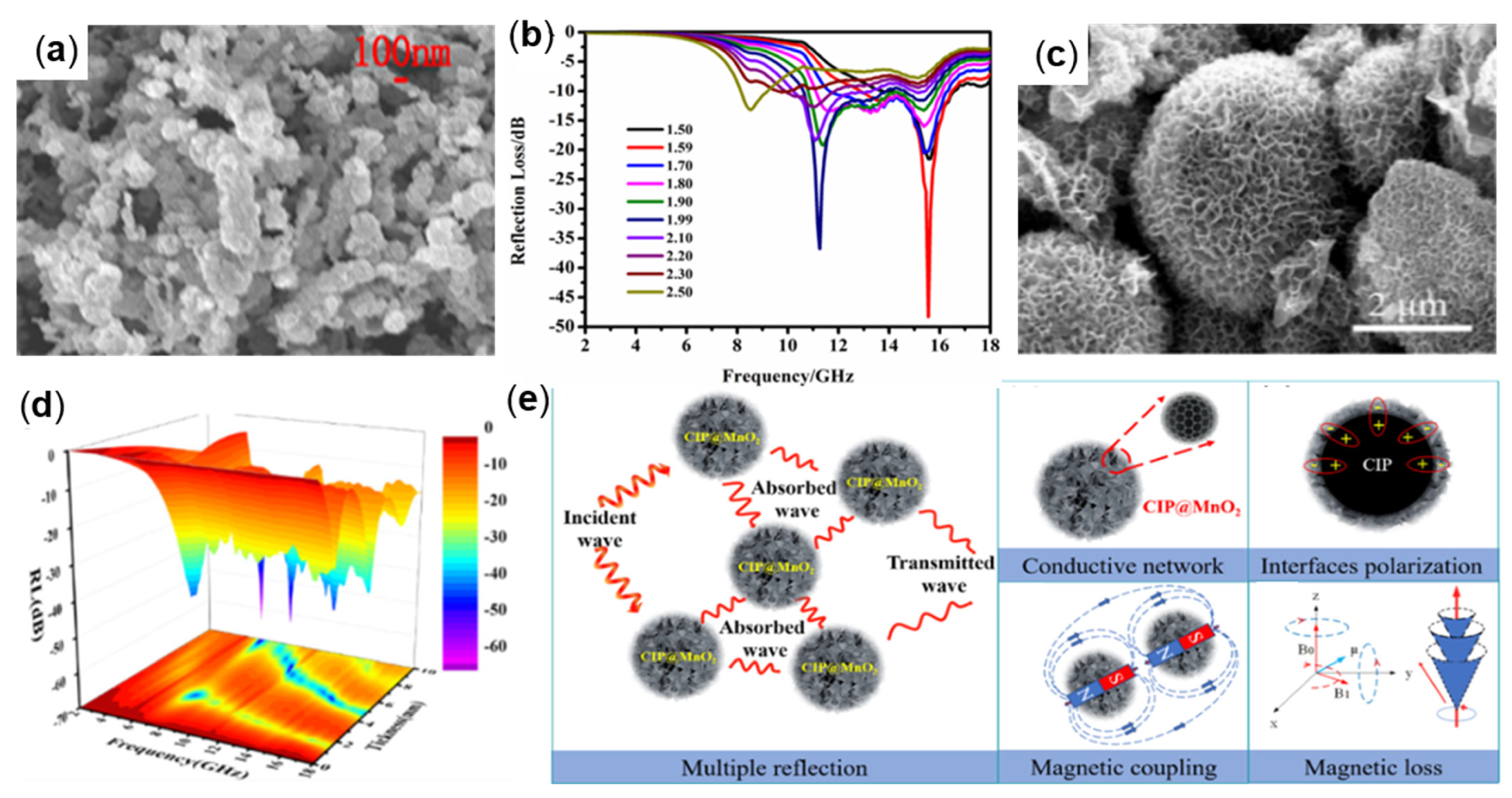
4.2.3. Semiconductor-Coated Fe
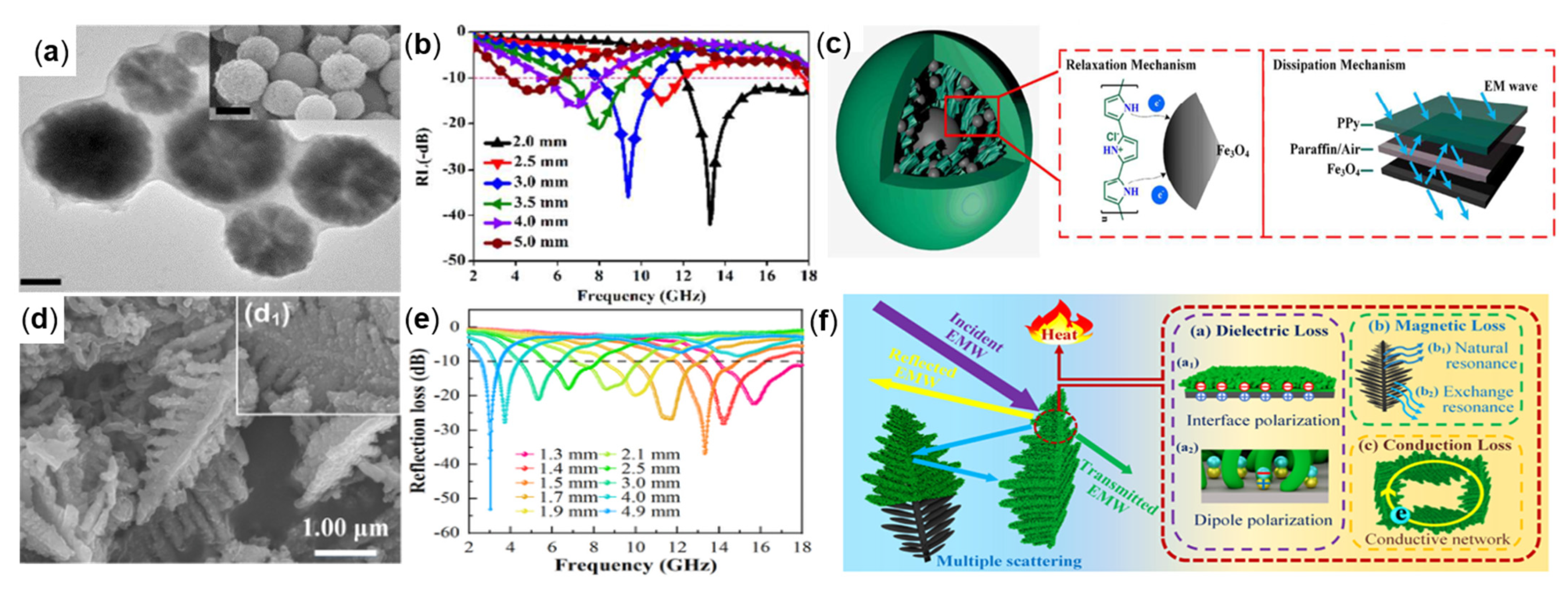
4.2.4. Conductive Polymer-Coated Fe
| Samples | Methods | ƒE (GHz) | Thickness (mm) | Filling Ratio (wt%) | RLmin (dB) | Reference |
|---|---|---|---|---|---|---|
| Fe-C nanofibers | Electrospinning | 4.2 | 3 | - | −44 | [71] |
| GO@CIPs | Wet stirring | 5.1 | 1.9 | 72 | −56.4 | [78] |
| Fe@C | Situ reduction | 11.6 | 1.48 | 50 | −71.47 | [79] |
| Al @CIPs | Ball milling | 10.5 | 1.6 | 70 | −27.2 | [81] |
| Fe@Ag | Liquid-phase reduction etc. | 7.53 | 3.36 | 25 | −58.69 | [83] |
| Fe/ZnO | Low-temperature wet chemical | 15.55 | 1.59 | 50 | −48.28 | [92] |
| CIP@MnO2 | Redox reaction | 6.32 | 10 | 40 | −63.87 | [96] |
| Fe3O4@PPy | Corrosion, etc. | 13.3 | 2.0 | 50 | −41.9 | [101] |
| Fe3O4@PANI | Hydrothermal | 3.04 | 1.3 | 60 | −53.08 | [102] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature
| Fe | Iron |
| EM | Electromagnetic |
| EMWAMs | Electromagnetic wave absorbing materials |
| Free Space Impedance | |
| Input Impedance | |
| Electromagnetic field strength | |
| Magnetic field strength | |
| Free space permeability | |
| Free space dielectric constant | |
| Reflection coefficient | |
| Attenuation parameter | |
| Permeability real part | |
| Dielectric constant real part | |
| Imaginary part of permeability | |
| Imaginary part of dielectric constant | |
| RLmin | The minimum reflection loss |
| RL | The reflection loss |
| SEM | Scanning electron microscope |
| FCI | Flake-shaped carbonyl iron |
| CIP | Carbonyl-iron particles |
| GO | Graphene oxide |
| PPy | Polypyrrole |
| PANI | Polyaniline |
References
- Zhou, C.; Geng, S.; Xu, X.; Wang, T.; Zhang, L.; Tian, X.; Yang, F.; Yang, H.; Li, Y. Lightweight hollow carbon nanospheres with tunable sizes towards enhancement in microwave absorption. Carbon 2016, 108, 234–241. [Google Scholar] [CrossRef]
- Jia, Z.; Lan, D.; Lin, K.; Qin, M.; Kou, K.; Wu, G.; Wu, H. Progress in low-frequency microwave absorbing materials. J. Mater. Sci. Mater. Electron. 2018, 29, 17122–17136. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, B.; Liu, Z.; Li, R.; Hou, L.; Li, Z.; Duan, Y.; Yan, X.; Yang, F.; Li, Y. Magnetic coupling engineered porous dielectric carbon within ultralow filler loading toward tunable and high-performance microwave absorption. J. Mater. Sci. Technol. 2021, 70, 214–223. [Google Scholar] [CrossRef]
- Zhou, W.; Long, L.; Li, Y. Mechanical and electromagnetic wave absorption properties of Cf-Si3N4 ceramics with PyC/SiC interphases. J. Mater. Sci. Technol. 2019, 35, 2809–2813. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Zhao, W.; Shi, Y.; Yu, L.; Dong, Y.; Zhu, Y.; Fu, Y.; Liu, X.; Fu, F. Prism-shaped hollow carbon decorated with polyaniline for microwave absorption. Chem. Eng. J. 2020, 379, 122393. [Google Scholar] [CrossRef]
- Singh, S.K.; Prakash, H.; Akhtar, M.J.; Kar, K.K. Lightweight and High-Performance microwave absorbing Heteroatom-Doped carbon derived from chicken feather fibers. ACS Sustain. Chem. Eng. 2018, 6, 5381–5393. [Google Scholar] [CrossRef]
- Quan, B.; Shi, W.; Ong, S.J.H.; Lu, X.; Wang, P.L.; Ji, G.; Guo, Y.; Zheng, L.; Xu, Z.J. Defect engineering in two common types of dielectric materials for electromagnetic absorption applications. Adv. Funct. Mater. 2019, 29, 1901236. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric loss mechanism in electromagnetic wave absorbing materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Zhong, B.; Wu, T.; Wang, H.; Zhang, T.; Bai, N.; Zhou, G.; Pan, H.; Wen, G.; et al. Balancing interface polarization strategy for enhancing electromagnetic wave absorption of carbon materials. Chem. Eng. J. 2020, 391, 123538. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Chen, Y.; Liu, Y.; Xia, L.; Zhang, T.; Lin, H.; Jia, D.; Zhong, B.; Wen, G.; et al. A theoretical strategy of pure carbon materials for lightweight and excellent absorption performance. Carbon 2021, 174, 662–672. [Google Scholar] [CrossRef]
- He, P.; Hou, Z.; Zhang, K.; Li, J.; Yin, K.; Feng, S.; Bi, S. Lightweight ferroferric oxide nanotubes with natural resonance property and design for broadband microwave absorption. J. Mater. Sci. 2017, 52, 8258–8267. [Google Scholar] [CrossRef]
- Shanenkov, I.; Sivkov, A.; Ivashutenko, A.; Zhuravlev, V.; Guo, Q.; Li, L.; Li, G.; Wei, G.; Han, W. Magnetite hollow microspheres with a broad absorption bandwidth of 11.9 GHz: Toward promising lightweight electromagnetic microwave absorption. Phys. Chem. Chem. Phys. 2017, 19, 19975–19983. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, P.; Li, Y.; Wen, D.; Luo, J.; Wang, S.; Wu, F.; Fang, L.; Pang, Y. A Facile Synthesis of NiFe-Layered Double Hydroxide and Mixed Metal Oxide with Excellent Microwave Absorption Properties. Molecules 2021, 26, 5046. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Wang, M.; Shang, C.; Zhang, H.; Du, Y. FeCo/ZnO composites with enhancing microwave absorbing properties: Effect of hydrothermal temperature and time. RSC Adv. 2014, 4, 57529–57533. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Y.; Ma, Y.; Zheng, H.; Wang, L.; Zhang, Q.; Chen, Y.; Peng, D. Facile preparation and microwave absorption properties of porous Co/CoO microrods. J. Alloy. Compd. 2017, 721, 411–418. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, J.; Zhang, X.; Yu, L.; Zhang, P.; Wu, Z.; Zhang, Z. Strategy for ultrafine ni fibers and investigation of the electromagnetic characteristics. J. Phys. Chem. C 2010, 114, 10101–10107. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Zhang, H.; Yang, Z.; Che, R. Dimensional design and core–shell engineering of nanomaterials for electromagnetic wave absorption. Adv. Mater. 2022, 34, 2107538. [Google Scholar] [CrossRef]
- Shu, X.; Zhou, J.; Lian, W.; Jiang, Y.; Wang, Y.; Shu, R.; Liu, Y.; Han, J.; Zhuang, Y. Size-morphology control, surface reaction mechanism and excellent electromagnetic wave absorption characteristics of Fe3O4 hollow spheres. J. Alloy. Compd. 2021, 854, 157087. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Shi, X.; Huang, M.; Li, X.; Zeng, Q.; Che, R. Recent progress of microwave absorption microspheres by magnetic–dielectric synergy. Nanoscale 2021, 13, 2136–2156. [Google Scholar] [CrossRef]
- Jin, L.; Wang, J.; Wu, F.; Yin, Y.; Zhang, B. MXene@Fe3O4 microspheres/fibers composite microwave absorbing materials: Optimum composition and performance evaluation. Carbon 2021, 182, 770–780. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Fu, Y.; Liu, T. A review on carbon/magnetic metal composites for microwave absorption. J. Mater. Sci. Technol. 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Deng, L.; Han, M. Microwave absorbing performances of multiwalled carbon nanotube composites with negative permeability. Appl. Phys. Lett. 2007, 91, 23119. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Guo, S.; Wang, Y.; Shen, Z. Electromagnetic and microwave-absorbing properties of highly ordered mesoporous carbon supported by gold nanoparticles. Mater. Chem. Phys. 2012, 133, 965–970. [Google Scholar] [CrossRef]
- Li, W.; Lv, B.; Wang, L.; Li, G.; Xu, Y. Fabrication of Fe3O4@C core–shell nanotubes and their application as a lightweight microwave absorbent. RSC Adv. 2014, 4, 55738–55744. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Mater. 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Shukla, V. Review of electromagnetic interference shielding materials fabricated by iron ingredients. Nanoscale Adv. 2019, 1, 1640–1671. [Google Scholar] [CrossRef]
- Qiao, Z.; Pan, S.; Xiong, J.; Cheng, L.; Yao, Q.; Lin, P. Magnetic and microwave absorption properties of La-Nd-Fe alloys. J. Magn. Magn. Mater. 2017, 423, 197–202. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, C.; Fan, Y.; Zhan, J. Preparation of Fe20Ni80 submicron fibers by an oxalate precipitation-thermal decomposition process and their microwave absorbing properties. J. Mater. Sci. Mater. Electron. 2017, 28, 13548–13555. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Effects of Physical and Chemical States of Iron-Based Catalysts on Formation of Carbon-Encapsulated Iron Nanoparticles from Kraft Lignin. Materials 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akay, G. Plasma generating-chemical looping catalyst synthesis by microwave plasma shock for nitrogen fixation from air and hydrogen production from water for agriculture and energy technologies in global warming prevention. Catalysts 2020, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Bulychev, O.A.; Shleenkov, S.A.; Lisienko, V.G.; Shleenkov, A.S. A study of the possibility of detecting surface defects in ferromagnetic products using a pyroelectromagnetic method. Russ. J. Nondestruct. Test. 2010, 46, 274–280. [Google Scholar] [CrossRef]
- Alphandéry, E. Light-interacting iron-based nanomaterials for localized cancer detection and treatment. Acta Biomater. 2021, 124, 50–71. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, K.; Wu, Y.; Li, S.; Guo, J.; Zhou, B.; Zhao, J.; Guo, L.; Chen, C. Rapid magnetic enrichment and sensitive detection of sudan pollutants with nanoscale zero valent iron-based nanomaterials in combination with liquid chromatography-ultraviolet detector. Chemosphere 2021, 281, 130900. [Google Scholar] [CrossRef]
- Sui, M.; Sun, X.; Lou, H.; Li, X.; Lv, X.; Li, L.; Gu, G. Synthesis of hollow Fe3O4 particles via one-step solvothermal approach for microwave absorption materials: Effect of reactant concentration, reaction temperature and reaction time. J. Mater. Sci. Mater. Electron. 2018, 29, 7539–7550. [Google Scholar] [CrossRef]
- Zhao, B.; Fan, B.; Xie, Y.; Zhang, R. Effect of particle sizes on the microwave absorption properties of monodispersed Ni submicrospheres. Optik 2015, 126, 4597–4600. [Google Scholar] [CrossRef]
- Ge, C.; Wang, L.; Liu, G.; Wang, T.; Chen, H. Effects of particle size on electromagnetic properties of spherical carbonyl iron. J. Mater. Sci. Mater. Electron. 2019, 30, 8390–8398. [Google Scholar] [CrossRef]
- Tong, G.; Wu, W.; Hu, Q.; Yuan, J.; Qiao, R.; Qian, H. Enhanced electromagnetic characteristics of porous iron particles made by a facile corrosion technique. Mater. Chem. Phys. 2012, 132, 563–569. [Google Scholar] [CrossRef]
- Li, D.; Choi, C.J.; Han, Z.; Liu, X.G.; Hu, W.J.; Li, J.; Zhang, Z.D. Magnetic and electromagnetic wave absorption properties of α-Fe(N) nanoparticles. J. Magn. Magn. Mater. 2009, 321, 4081–4085. [Google Scholar] [CrossRef]
- Yu, M.; Xu, Y.; Mao, Q.; Li, F.; Wang, C. Electromagnetic and absorption properties of nano-sized and micro-sized Fe4N particles. J. Alloy. Compd. 2016, 656, 362–367. [Google Scholar] [CrossRef]
- Qu, B.; Zhu, C.; Li, C.; Zhang, X.; Chen, Y. Coupling hollow Fe3O4–Fe nanoparticles with graphene sheets for High-Performance electromagnetic wave absorbing material. ACS Appl. Mater. Interfaces 2016, 8, 3730–3735. [Google Scholar] [CrossRef]
- Guo, C.; Yang, Z.; Shen, S.; Liang, J.; Xu, G. High microwave attenuation performance of planar carbonyl iron particles with orientation of shape anisotropy field. J. Magn. Magn. Mater. 2018, 454, 32–38. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Long, C.; Li, W.; Guan, J. Flaky carbonyl iron particles with both small grain size and low internal strain for broadband microwave absorption. J. Alloy. Compd. 2015, 637, 106–111. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Kumar, D.; Sinha, G.R.; Moon, A.P. Carbonyl iron powders as absorption material for microwave interference shielding: A review. J. Alloy. Compd. 2021, 853, 157251. [Google Scholar] [CrossRef]
- Yin, C.; Fan, J.; Bai, L.; Ding, F.; Yuan, F. Microwave absorption and antioxidation properties of flaky carbonyl iron passivated with carbon dioxide. J. Magn. Magn. Mater. 2013, 340, 65–69. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Z.; Zhou, L.; Heidarshenas, B.; Zhang, C.; Xia, J.; Zhi, L.; Shen, G.; Wu, H. Influence of heat treatment on the microwave absorption properties of flaky carbonyl iron powder. Int. J. Lightweight Mater. Manuf. 2020, 3, 258–264. [Google Scholar] [CrossRef]
- Ji, P.; Xie, G.; Xie, N.; Li, J.; Chen, J.; Chen, J. Microwave absorbing properties of flaky carbonyl iron powder prepared by rod milling method. J. Electron. Mater. 2019, 48, 2495–2500. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, L.; Wang, X.; Zhang, D. Two-step milling on the carbonyl iron particles and optimizing on the composite absorption. J. Alloy. Compd. 2016, 676, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liu, T.; Zhou, L.; Xu, Y. Electromagnetic absorbing property of the flaky carbonyl iron particles by chemical corrosion process. J. Magn. Magn. Mater. 2016, 419, 119–124. [Google Scholar] [CrossRef]
- Dang, S.; Lin, Y.; Wei, X.; Ye, H. Design and preparation of an ultrawideband gradient triple-layered planar microwave absorber using flaky carbonyl iron as absorbent. J. Mater. Sci. Mater. Electron. 2018, 29, 17651–17660. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Yang, P.; Ruan, H. High-aspect-ratio iron nanowires: Magnetic field-assisted in situ reduction synthesis and extensive parametric study. Nanotechnology 2020, 31, 145601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jia, Z.; Wu, H.; Gao, Z.; Zhang, Y.; Kou, K.; Huang, Z.; Feng, A.; Wu, G. Morphology-Dependent Electromagnetic Wave Absorbing Properties of Iron-Based Absorbers: One-Dimensional, Two-Dimensional, and Three-Dimensional Classification. Eur. Phys. J. Appl. Phys. 2019, 87, 20901. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Chen, Y.; Yu, J.; Zhang, J.; Sang, T.; Tao, G.; Zhu, H. Fabrication and electromagnetic loss properties of Fe3O4 nanofibers. J. Mater. Sci. Mater. Electron. 2015, 26, 3474–3478. [Google Scholar] [CrossRef]
- Liu, Q.; Zi, Z.; Wu, D.; Sun, Y.; Dai, J. Controllable synthesis and morphology-dependent microwave absorption properties of iron nanocrystals. J. Mater. Sci. 2012, 47, 1033–1037. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Liu, T.; Zheng, X.; Bai, J. Shape-controlled synthesis of Fe nanostructures and their enhanced microwave absorption properties at L-band. Mater. Res. Bull. 2014, 59, 137–141. [Google Scholar] [CrossRef]
- Yang, P.; Ruan, H.; Sun, Y.; Li, R.; Lu, Y.; Xiang, C. Excellent microwave absorption performances of high length-diameter ratio iron nanowires with low filling ratio. Nanotechnology 2020, 31, 395708. [Google Scholar] [CrossRef]
- Shen, J.; Yao, Y.; Liu, Y.; Leng, J. Tunable hierarchical Fe nanowires with a facile template-free approach for enhanced microwave absorption performance. J. Mater. Chem. C 2016, 4, 7614–7621. [Google Scholar] [CrossRef]
- Yu, Z.; Yao, Z.; Zhang, N.; Wang, Z.; Li, C.; Han, X.; Wu, X.; Jiang, Z. Electric field-induced synthesis of dendritic nanostructured α-Fe for electromagnetic absorption application. J. Mater. Chem. A 2013, 1, 4571–4576. [Google Scholar] [CrossRef]
- Sun, G.; Dong, B.; Cao, M.; Wei, B.; Hu, C. Hierarchical Dendrite-Like magnetic materials of Fe3O4, γ-Fe2O3, and fe with high performance of microwave absorption. Chem. Mater. 2011, 23, 1587–1593. [Google Scholar] [CrossRef]
- Fan, X.; Guan, J.; Li, Z.; Mou, F.; Tong, G.; Wang, W. One-pot low temperature solution synthesis, magnetic and microwave electromagnetic properties of single-crystal iron submicron cubes. J. Mater. Chem. 2010, 20, 1676–1682. [Google Scholar] [CrossRef]
- Gargama, H.; Thakur, A.K.; Chaturvedi, S.K. Polyvinylidene fluoride/nanocrystalline iron composite materials for EMI shielding and absorption applications. J. Alloy. Compd. 2016, 654, 209–215. [Google Scholar] [CrossRef]
- Wongyu, J.; Shanigaram, M.; Ki, H.K. Microwave absorption properties of carbonyl iron particles filled in polymer composites. Orig. New Phys. Sae Mulli 2020, 70, 311–314. [Google Scholar] [CrossRef]
- Zhu, B.; Tian, Y.; Wang, Y.; Mao, L.; Gao, F.; Wen, G.; Liang, L.; Zhang, K.; Li, G. Synthesis and microwave absorption properties of Fe-Loaded fly Ash-Based ceramic composites. ACS Appl. Electron. Mater. 2020, 2, 3307–3319. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.; Gao, X.; Chen, L.; Ma, S.; Xie, C.; Zhang, X.; Lu, W. Preparation and microwave absorption properties of carbon nanotubes/iron oxide/polypyrrole/carbon composites. Synth. Met. 2020, 260, 116282. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhang, M.; Feng, A.; Qu, S.; Zhang, Y.; Liu, X.; Jia, Z.; Wu, G. Synthesis of Fe3O4/carbon foams composites with broadened bandwidth and excellent electromagnetic wave absorption performance. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105627. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Wang, Z.; Li, Y.; He, X.; Yuan, Y. Microwave absorption enhancement of porous C@CoFe2O4 nanocomposites derived from eggshell membrane. Carbon 2019, 143, 507–516. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Song, C.; Han, M.; Xu, H.; Duan, W.; Cheng, L.; Zhang, L. Self-Assembly core-shell Graphene-Bridged hollow MXenes spheres 3D foam with ultrahigh specific EM absorption performance. Adv. Funct. Mater. 2018, 28, 1803938. [Google Scholar] [CrossRef]
- Wang, L.; Huang, M.; Qian, X.; Liu, L.; You, W.; Zhang, J.; Wang, M.; Che, R. Confined Magnetic-Dielectric balance boosted electromagnetic wave absorption. Small 2021, 17, 2100970. [Google Scholar] [CrossRef]
- Sheng, A.; Yang, Y.; Yan, D.; Dai, K.; Duan, H.; Zhao, G.; Liu, Y.; Li, Z. Self-assembled reduced graphene oxide/nickel nanofibers with hierarchical core-shell structure for enhanced electromagnetic wave absorption. Carbon 2020, 167, 530–540. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Liu, H.; Jin, S.; Wu, G. Interconnected magnetic carbon@NixCo1-xFe2O4 nanospheres with core–shell structure: An efficient and thin electromagnetic wave absorber. J. Colloid Interface Sci. 2022, 606, 526–536. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Chi, X.; Li, R.; Wang, J. Synthesis and microwave absorption properties of Fe-C nanofibers by electrospinning with disperse Fe nanoparticles parceled by carbon. Carbon 2014, 74, 312–318. [Google Scholar] [CrossRef]
- Liu, T.; Pang, Y.; Kikuchi, H.; Kamada, Y.; Takahashi, S. Superparamagnetic property and high microwave absorption performance of FeAl@(Al, Fe)2O3 nanoparticles induced by surface oxidation. J. Mater. Chem. C 2015, 3, 6232–6239. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, Y.; Wang, S.; Jiang, W. Controlled synthesis and electromagnetic wave absorption properties of core-shell Fe3O4@SiO2 nanospheres decorated graphene. Ceram. Int. 2017, 43, 1887–1894. [Google Scholar] [CrossRef]
- Lin, T.; Yu, H.; Wang, L.; Fahad, S.; Khan, A.; Naveed, K.; Haq, F.; Nazir, A.; Amin, B.U. A review of recent advances in the preparation of polyaniline-based composites and their electromagnetic absorption properties. J. Mater. Sci. 2021, 56, 5449–5478. [Google Scholar] [CrossRef]
- Pan, K.; Leng, T.; Song, J.; Ji, C.; Zhang, J.; Li, J.; Novoselov, K.S.; Hu, Z. Controlled reduction of graphene oxide laminate and its applications for ultra-wideband microwave absorption. Carbon 2020, 160, 307–316. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yu, H.Y.; Yang, Z.T.; Zhang, A.B.; Zhang, Q.Y.; Zhang, B.L. Tubular carbon nanofibers: Synthesis, characterization and applications in microwave absorption. Carbon 2019, 152, 255–266. [Google Scholar] [CrossRef]
- Jinxiao, W.; Jianfeng, Y.; Jun, Y.; Hui, Z. An Ni-Co bimetallic MOF-derived hierarchical CNT/CoO/Ni2O3 composite for electromagnetic wave absorption. J. Alloy. Compd. 2021, 876, 160126. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, J.; Kim, K.H. Microwave absorption properties of graphene oxide capsulated carbonyl iron particles. Appl. Surf. Sci. 2019, 475, 1065–1069. [Google Scholar] [CrossRef]
- Li, X.; Du, D.; Wang, C.; Wang, H.; Xu, Z. In situ synthesis of hierarchical rose-like porous Fe@C with enhanced electromagnetic wave absorption. J. Mater. Chem. C 2018, 6, 558–567. [Google Scholar] [CrossRef]
- Zhorin, V.A.; Kiselev, M.R.; Roldugin, V.I. Thermogravimetric analysis of the aluminum-polypropylene mixtures after plastic deformation under high pressure. Russ. J. Appl. Chem. 2013, 86, 15–19. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, L.; Li, R.; Chen, D.; Lu, Y.; Cheng, Y.; Luo, X.; Xie, H.; Zhou, W. Enhanced heat-resistance property of aluminum-coated carbonyl iron particles as microwave absorption materials. J. Magn. Magn. Mater. 2021, 524, 167681. [Google Scholar] [CrossRef]
- Wei, B.; Zhou, J.; Yao, Z.; Haidry, A.A.; Guo, X.; Lin, H.; Qian, K.; Chen, W. The effect of Ag nanoparticles content on dielectric and microwave absorption properties of β-SiC. Ceram. Int. 2020, 46, 5788–5798. [Google Scholar] [CrossRef]
- Yang, P.; Huang, Y.; Li, R.; Huang, X.; Ruan, H.; Shou, M.; Li, W.; Zhang, Y.; Li, N.; Dong, L. Optimization of Fe@Ag core-shell nanowires with improved impedance matching and microwave absorption properties. Chem. Eng. J. 2022, 430, 132878. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Q.; Yan, F.; Wang, W.; Wu, K.; Liu, Y.; Lv, Q.; Zhang, H.; Zhang, Q.; Li, J.; et al. Enhanced microwave absorption in ZnO/carbonyl iron nano-composites by coating dielectric material. J. Magn. Magn. Mater. 2012, 324, 1720–1725. [Google Scholar] [CrossRef]
- Zhang, F.; Du, H.; Shang, S.; Qaim, M.; Bao, S.; Su, T.; Zhang, R.; Lu, H.; Wang, H.; Xu, H.; et al. Constructing γ-MnO2 hollow spheres with tunable microwave absorption properties. Adv. Powder Technol. 2020, 31, 4642–4647. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, H.; Zhou, W.; Ren, Z. Enhanced antioxidation and microwave absorbing properties of SiO2-coated flaky carbonyl iron particles. J. Magn. Magn. Mater. 2018, 446, 143–149. [Google Scholar] [CrossRef]
- Pan, J.; Sun, X.; Wang, T.; Zhu, Z.; He, Y.; Xia, W.; He, J. Porous coin-like Fe@MoS2 composite with optimized impedance matching for efficient microwave absorption. Appl. Surf. Sci. 2018, 457, 271–279. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Wu, H. Hierarchical flower-like Fe3O4/MoS2 composites for selective broadband electromagnetic wave absorption performance. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105760. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Li, L. Synthesis of the SiO2@C composites with high-performance electromagnetic wave absorption. Powder Technol. 2019, 343, 129–136. [Google Scholar] [CrossRef]
- Javid, M.; Zhou, Y.; Zhou, T.; Wang, D.; Zhou, L.; Shah, A.; Duan, Y.; Dong, X.; Zhang, Z. In-situ fabrication of Fe@ZrO2 nanochains for the heat-resistant electromagnetic wave absorber. Mater. Lett. 2019, 242, 199–202. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Chen, J.; Wang, L.; Guo, L.; Ouyang, J.; Jia, D.; Zhou, Y. Reduced graphene oxide decorated with in-situ growing ZnO nanocrystals: Facile synthesis and enhanced microwave absorption properties. Carbon 2016, 108, 52–60. [Google Scholar] [CrossRef]
- Liu, Q.; Dai, J.; Hu, F.; Zhang, Z.; Xiong, K.; Xu, G. Core-shell structured Fe/ZnO composite with superior electromagnetic wave absorption performance. Ceram. Int. 2021, 47, 14506–14514. [Google Scholar] [CrossRef]
- Liu, R.; Sun, S.; Yang, Y.; Chi, J. Wave-absorbing properties of a cement-based coating with MnO2/Activated carbon composite. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2018, 33, 394–402. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wu, X.; Zhang, W.; Wang, Q.; Li, J. Synthesis of hierarchical core-shell NiFe2O4@MnO2 composite microspheres decorated graphene nanosheet for enhanced microwave absorption performance. Ceram. Int. 2017, 43, 11367–11375. [Google Scholar] [CrossRef]
- Guan, H.; Xie, J.; Chen, G.; Wang, Y. Facile synthesis of α-MnO2 nanorods at low temperature and their microwave absorption properties. Mater. Chem. Phys. 2014, 143, 1061–1068. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, Y.; Yang, P.; Zheng, W.; Li, N.; Bai, J.; Zhang, Y.; Li, K.; Wang, D.; Liu, Z.; et al. Enhanced Electromagnetic Wave Absorption Properties of Ultrathin MnO2 Nanosheet-Decorated Spherical Flower-Shaped Carbonyl Iron Powder. Molecules 2022, 27, 135. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Chen, X.; Wei, C. Conducting polymers-NiFe2O4 coated on reduced graphene oxide sheets as electromagnetic (EM) wave absorption materials. Synth. Met. 2016, 221, 291–298. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Ganguly, S.; Das, P.; Ravindren, R.; Sit, S.; Chakraborty, G.; Das, N.C. Highly conductive and flexible nano-structured carbon-based polymer nanocomposites with improved electromagnetic-interference-shielding performance. Mater. Res. Express 2017, 4, 105039. [Google Scholar] [CrossRef]
- Ali, N.N.; Atassi, Y.; Salloum, A.; Charba, A.; Malki, A.; Jafarian, M. Comparative study of microwave absorption characteristics of (Polyaniline/NiZn ferrite) nanocomposites with different ferrite percentages. Mater. Chem. Phys. 2018, 211, 79–87. [Google Scholar] [CrossRef]
- Lan, D.; Gao, Z.; Zhao, Z.; Kou, K.; Wu, H. Application progress of conductive conjugated polymers in electromagnetic wave absorbing composites. Compos. Commun. 2021, 26, 100767. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, D.; Tian, K.; Hu, W.; Wang, J.; Su, M.; Li, L. Facile preparation of core-shell Fe3O4@polypyrrole composites with superior electromagnetic wave absorption properties. J. Phys. Chem. C 2017, 121, 15784–15792. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Deng, D.; Zheng, L.; Wu, Y.; Luo, W.; Zhang, M.; Gong, R. Preparation and excellent electromagnetic absorption properties of dendritic structured Fe3O4@PANI composites. J. Alloy. Compd. 2022, 891, 161922. [Google Scholar] [CrossRef]

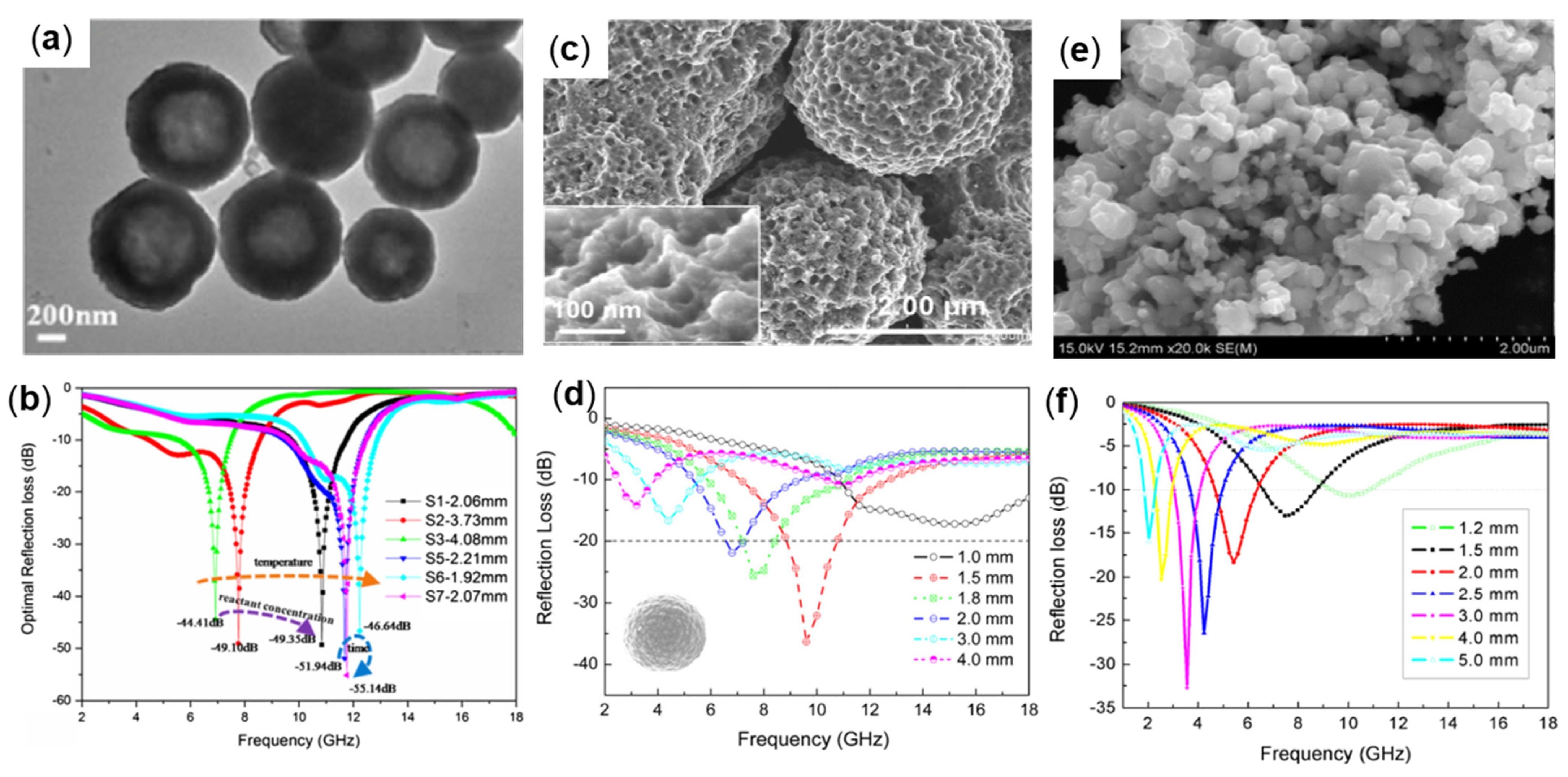


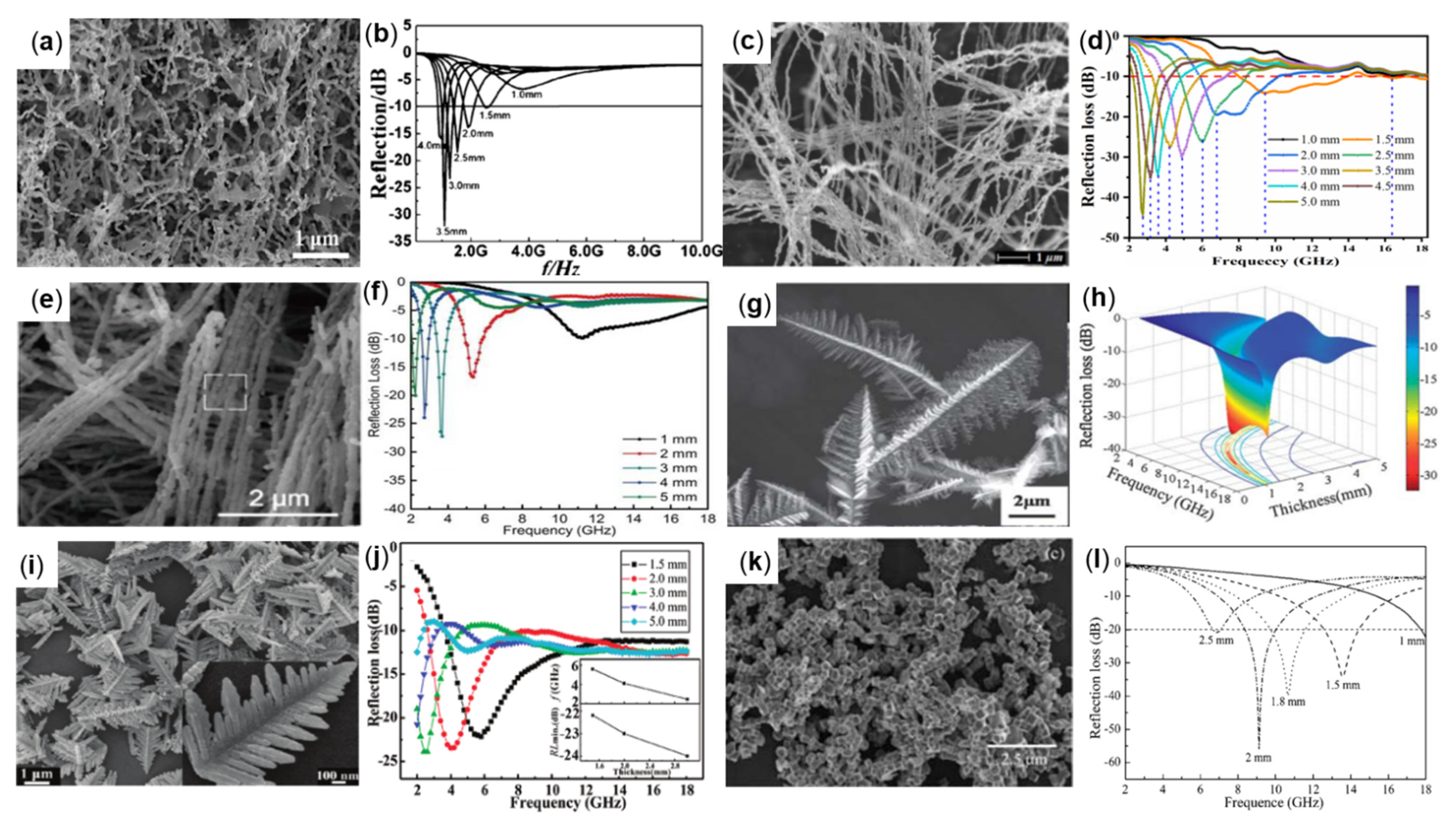
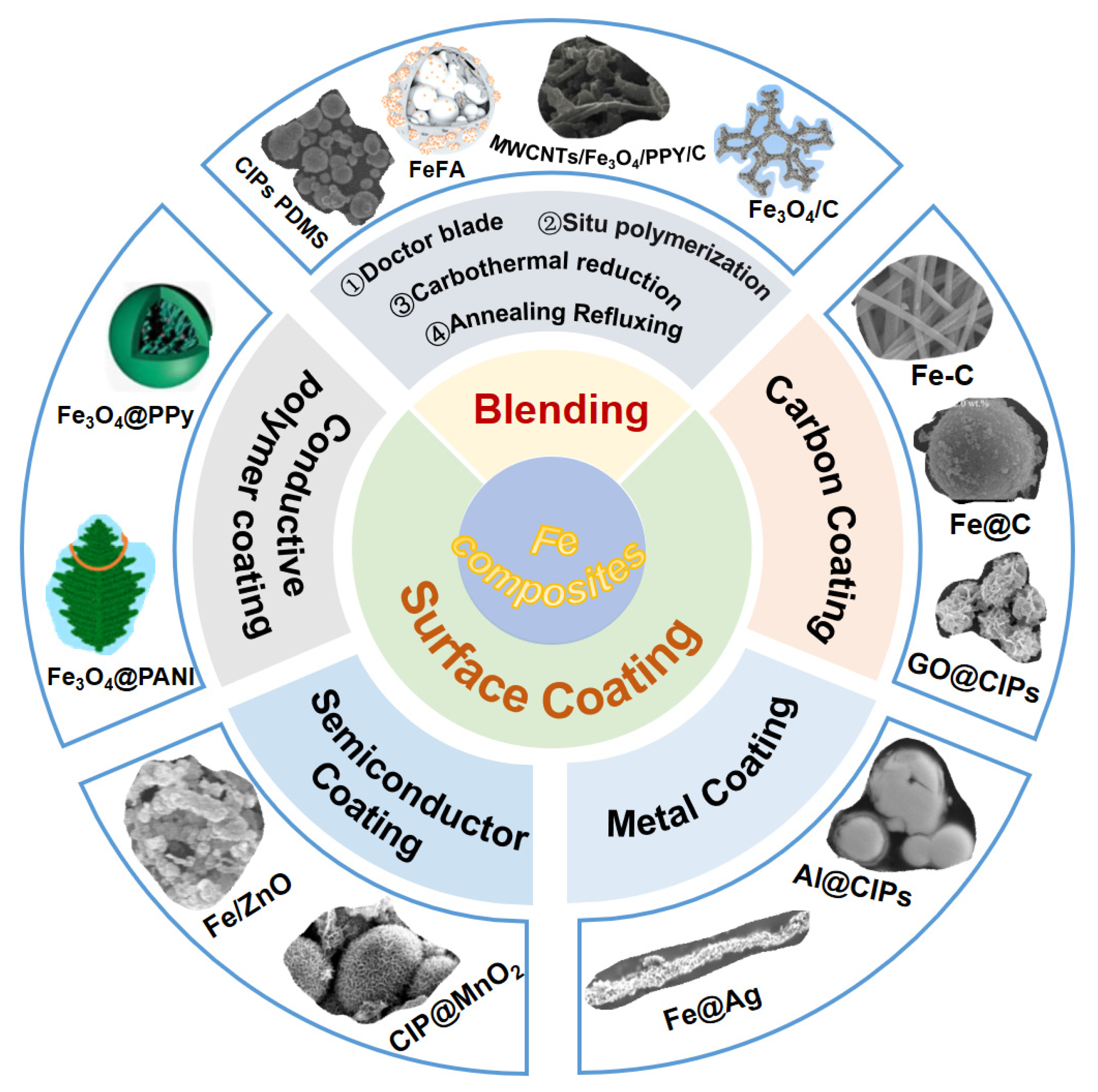

| Samples | Methods | ƒE (GHz) | Thickness (mm) | Filling Ratio | RLmin (dB) | Reference |
|---|---|---|---|---|---|---|
| Fe3O4 | Pyrolysis | 11.76 | 2.07 | 70 wt% | −55.14 | [35] |
| PIPs | Corrosion technique | 13.2 | 1.8 | 20 vol% | −42.2 | [38] |
| Fe4N | Gaseous nitriding | 3.5 | 3.0 | 75 wt% | −33 | [40] |
| Samples | Methods | ƒE (GHz) | Thicknes (mm) | Filling Ratio | RLmin (dB) | Reference |
|---|---|---|---|---|---|---|
| FCI | Ball milling | 2 6.2 | - | 70 vol% | μ′ = 1.61 μ″ = 3.20 | [46] |
| Scale-like FCI | Ball milling | 4.5~8.5 | 1.5 | 85 wt% | <−10 dB | [47] |
| PACI | Ball milling | 2.09 | 3.25 | 70 wt% | −53.1 dB | [42] |
| FCI | Ball milling | 8~18 | 1.47 | 50 wt% | <−10 dB | [48] |
| FCI | Ball milling | 5.92~18 | 0.8 | 40 wt% | <−8 dB | [49] |
| FCIPs | Ball milling | 2~18 26.5~40 | 6 | 75 wt% | <−10 dB | [50] |
| Samples | Methods | ƒE (GHz) | Thickness (mm) | Filling Ratio | RLmin (dB) | Reference |
|---|---|---|---|---|---|---|
| CIPsPDMS | Doctor blade | 14.6 | 1.5 | 72 wt% | −27.5 | [62] |
| FeFA | Carbothermal reduction | 16.1 | 2.5 | 65 wt% | −35.7 | [63] |
| MWCNTs/Fe3O4 /PPY/C | Situ polymerization | 13.92 | 2.2 | 25 wt% | −53.07 | [64] |
| Fe3O4/C | Refluxing, annealing | 12.2−17.8 | 1.9 | 25 wt% | −46.5 | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Ye, W.; Yang, P.; Wang, D.; Xiong, Y.; Liu, Z.; Qi, J.; Zhang, Y. Recent Progress in Iron-Based Microwave Absorbing Composites: A Review and Prospective. Molecules 2022, 27, 4117. https://doi.org/10.3390/molecules27134117
Zheng W, Ye W, Yang P, Wang D, Xiong Y, Liu Z, Qi J, Zhang Y. Recent Progress in Iron-Based Microwave Absorbing Composites: A Review and Prospective. Molecules. 2022; 27(13):4117. https://doi.org/10.3390/molecules27134117
Chicago/Turabian StyleZheng, Wei, Wenxian Ye, Pingan Yang, Dashuang Wang, Yuting Xiong, Zhiyong Liu, Jindong Qi, and Yuxin Zhang. 2022. "Recent Progress in Iron-Based Microwave Absorbing Composites: A Review and Prospective" Molecules 27, no. 13: 4117. https://doi.org/10.3390/molecules27134117
APA StyleZheng, W., Ye, W., Yang, P., Wang, D., Xiong, Y., Liu, Z., Qi, J., & Zhang, Y. (2022). Recent Progress in Iron-Based Microwave Absorbing Composites: A Review and Prospective. Molecules, 27(13), 4117. https://doi.org/10.3390/molecules27134117







