Changes in Isoflavone Profile from Soybean Seeds during Cheonggukjang Fermentation Based on High-Resolution UPLC-DAD-QToF/MS: New Succinylated and Phosphorylated Conjugates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of 38 Isoflavone Derivatives in Raw, Steamed and Fermented Soybean Seeds
2.1.1. Glucosides, Malonyl-Glucosides and Acetyl-Glucosides in Raw and Steamed Seeds
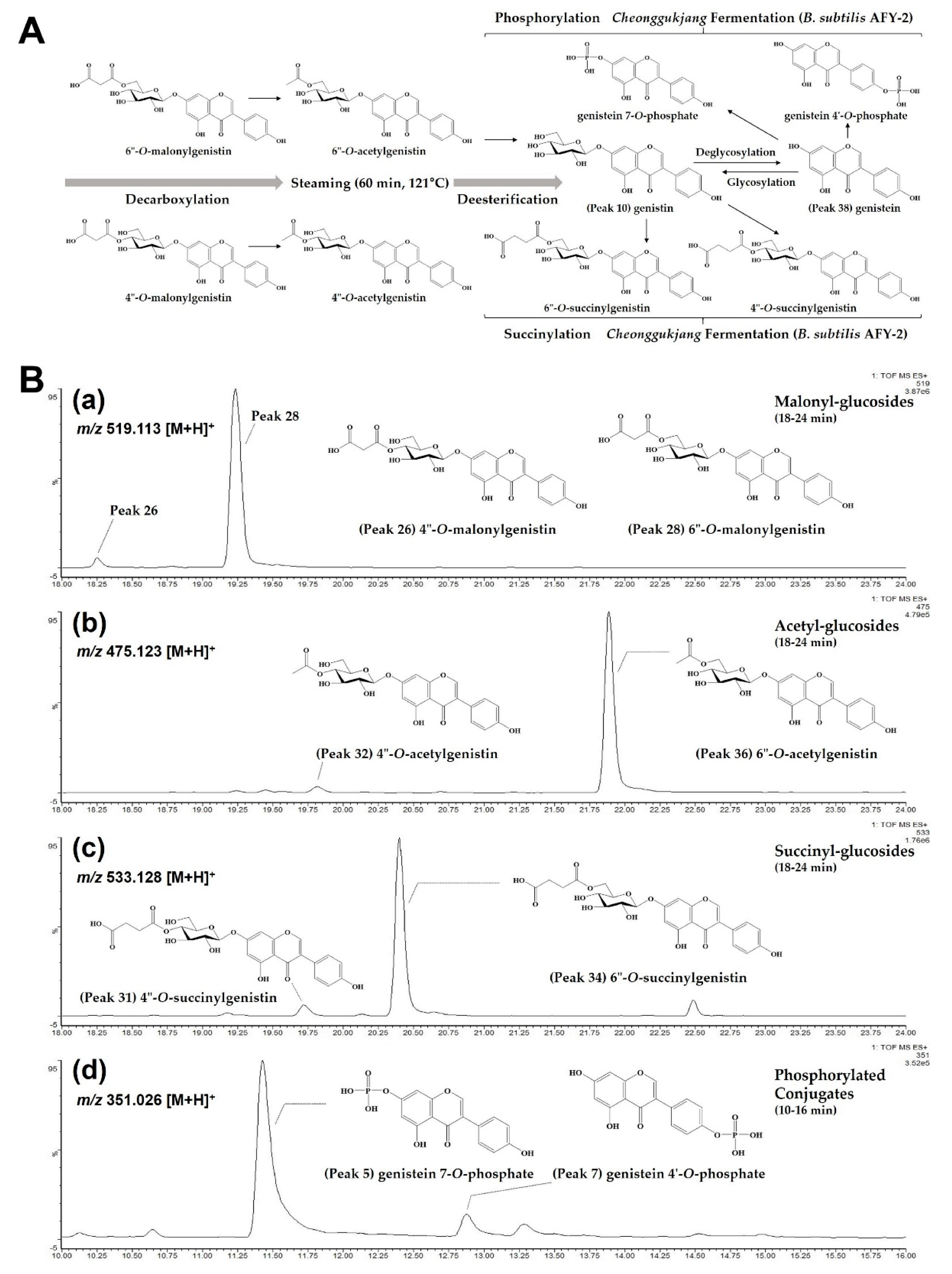
2.1.2. New Succinyl-Glucosides and Phosphorylated Conjugates in Fermented Seeds
2.2. Changes of 38 Isoflavone Derivatives during Steaming and Fermentation in Soybean Seeds
2.2.1. Glucosides, Malonyl-Glucosides and Acetyl-Glucosides during Steaming
2.2.2. Succinyl-Glucosides and Phosphorylated Conjugates during Fermentation
2.2.3. Proposed Roadmap on Biotransformation of Soy Isoflavones during Steaming and Cheonggukjang Fermentation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Preparation of Cheonggukjang with B. Subtilis AFY-2
3.4. Extraction and UPLC-DAD-QToF/MS Analysis of Isoflavone Derivatives
3.5. Identification and Quantification of Isoflavone Derivatives
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones Derived from Plant Raw Materials: Bioavailability, Anti-Cancer, Anti-Aging Potentials, and Microbiome Modulation. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef]
- Kim, J.-B.; Kim, H.-W.; Lee, M.-K.; Lee, S.-H.; Kim, Y.-J.; Choi, B.-K.; Cho, S.-Y.; Kim, H.-J.; Lee, S.-H.; Jang, H.-H.; et al. Flavonoid Library. RDA DB 1.0—Flavonoids; Rural Development Administration (RDA): Wanju, Korea, 2016; pp. 59–522. [Google Scholar]
- Watanabe, S.; Uesugi, S.; Kikuchi, Y. Isoflavones for Prevention of Cancer, Cardiovascular Diseases, Gynecological Problems and Possible Immune Potentiation. Biomed. Pharmacother. 2002, 56, 302–312. [Google Scholar] [CrossRef]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and Antiangiogenic Activity of Soy Isoflavone Genistein in Mouse Models of Melanoma and Breast Cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, G. The Antioxidant Role of Soy and Soy Foods in Human Health. Antioxidants 2020, 9, 635. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Sakamoto, A.; Takayanagi, T.; Yokotsuka, K. Changes in Isoflavone Compositions of Soybean Foods during Cooking Process. Food Sci. Technol. Res. 2000, 6, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lee, S.; Park, Y.; Lee, S.; Chang, P.-S.; Choi, S.-S.; Lee, J. Succinyl Daidzin and Succinyl Genistin are New Isoflavone Derivatives Found in Cheonggukjang. Food Sci. Biotechnol. 2008, 17, 172–175. [Google Scholar]
- Toda, T.; Uesugi, T.; Hirai, K.; Nukaya, H.; Tsuji, K.; Ishida, H. New 6-O-Acyl Isoflavone Glycosides from Soybeans Fermented with Bacillus subtilis (natto). I. 6-O-Succinylated Isoflavone Glycosides and Their Preventive Effects on Bone Loss in Ovariectomized Rats Fed a Calcium-Deficient Diet. Biol. Pharm. Bull. 1999, 22, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Coward, L.; Smith, M.; Kirk, M.; Barnes, S. Chemical Modification of Isoflavones in Soyfoods during Cooking and Processing. Am. J. Clin. Nutr. 1998, 68, 1486S–1491S. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, B.W.; Kim, B.; Kim, H.T.; Ko, J.M.; Baek, I.-Y.; Seo, W.T.; Kang, Y.M.; Cho, K.M. Changes in Phenolic Compounds (Isoflavones and Phenolic acids) and Antioxidant Properties in High-Protein Soybean (Glycine max L., cv. Saedanbaek) for Different Roasting Conditions. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 605–612. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, J. Modification of Isoflavones by Processing and Photosensitization in Model and Food Systems. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 833–840. [Google Scholar] [CrossRef]
- Park, C.U.; Jeong, M.K.; Park, M.H.; Yeu, J.; Park, M.S.; Kim, M.-J.; Ahn, S.M.; Chang, P.-S.; Lee, J. Formation of Succinyl Genistin and Succinyl Daidzin by Bacillus Species. J. Food Sci. 2010, 75, C128–C133. [Google Scholar] [CrossRef]
- Kim, K.-M.; Park, J.-S.; Choi, H.; Kim, M.-S.; Seo, J.-H.; Pandey, R.P.; Kim, J.W.; Hyun, C.-G.; Kim, S.-Y. Biosynthesis of Novel Daidzein Derivatives Using Bacillus amyloliquefaciens Whole Cells. Biocatal. Biotransform. 2018, 36, 469–475. [Google Scholar] [CrossRef]
- Lee, J.; Doo, E.-H.; Kwon, D.Y.; Park, J.-B. Functionalization of Isoflavones with Enzymes. Food Sci. Biotechnol. 2008, 17, 228–233. [Google Scholar]
- Park, J.; Park, M.H.; Jeong, M.K.; Kim, M.-J.; Park, K.-M.; Lee, J. Changes of Isoflavone Profiles in Cheonggukjang with Lentinus edodes. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 115–119. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Han, Y.S.; Chang, P.-S.; Lee, J.-M.; Kim, Y.S.; Lee, J. Changes in Isoflavone Profiles during Cheongyukjang Preparation, A Traditional Banga Food. Korean J. Food Sci. Technol. 2009, 41, 141–145. [Google Scholar]
- Huang, R.-Y.; Chou, C.-C. Heating Affects the Content and Distribution Profile of Isoflavones in Steamed Black Soybeans and Black Soybean Koji. J. Agric. Food Chem. 2008, 56, 8484–8489. [Google Scholar] [CrossRef]
- Aguiar, C.L.; Haddad, R.; Eberlin, M.N.; Carrao-Panizzi, M.C.; Tsai, S.M.; Park, Y.K. Thermal Behavior of Malonylglucoside Isoflavone in Soybean Flour Analyzed by RPHPLC/DAD and Electrospray Ionization Mass Spectrometry. LWT-Food Sci. Technol. 2012, 48, 114–119. [Google Scholar] [CrossRef]
- Bao, K.; Chen, T.-L.; Zhang, S.; Huang, Z.-Z.; Huang, Y.-F.; Huang, Z.-H.; Zhu, Y.-Y.; Wu, Q.-N.; Duan, J.-A.; Zhang, Z.-Z.; et al. A Succinyl Isoflavone Identified in Natto Promotes Anti-Ischemic Effects in the Middle Cerebral Artery Occlusion Rats. J. Funct. Foods 2020, 73, 104104. [Google Scholar] [CrossRef]
- Jung, M.J.; Wang, M.H. Effect of Fermented Soybean-Derived Chungkookjang on Diet-Induced Hyperlipidemia in Bio F1B Hamsters. Food Biotechnol. 2009, 23, 74–82. [Google Scholar] [CrossRef]
- Yang, S.; Chang, P.-S.; Lee, J. Isoflavone Distribution and β-Glucosidase Activity in Cheonggukjang, a Traditional Korean Whole Soybean-Fermented Food. Food Sci. Biotechnol. 2006, 15, 96–101. [Google Scholar]
- Zhang, S.; Chen, G.; Chu, J.; Wu, B.; He, B. High Production of Succinyl Isoflavone Glycosides by Bacillus licheniformis ZSP01 Resting Cells in Aqueous Miscible Organic Medium. Biotechnol. Appl. Biochem. 2015, 62, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, R.H.; Kim, H.-W.; Lee, S.; Lee, S.-J.; Na, H.; Kim, J.H.; Wee, C.-D.; Yoo, S.M.; Lee, S.H. Isoflavone Characterization in Soybean Seed and Fermented Products Based on High-Resolution Mass Spectrometry. J. Korean Soc. Food Sci. Nutr. 2021, 50, 950–961. [Google Scholar] [CrossRef]
- Hsu, C.; Wu, B.-Y.; Chang, Y.-C.; Chang, C.-F.; Chiou, T.-Y.; Su, N.-W. Phosphorylation of Isoflavones by Bacillus subtilis BCRC 80517 May Represent Xenobiotic Metabolism. J. Agric. Food Chem. 2018, 66, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-H.; Noh, H.; Kim, H.-W.; Cho, S.-Y.; Kim, H.-J.; Lee, S.-H.; Lee, S.-H.; Gunter, M.J.; Ferrari, P.; Scalbert, A.; et al. Metabolic Tracking of Isoflavones in Soybean Products and Biosamples from Healthy Adults After Fermented Soybean Consumption. Food Chem. 2020, 330, 127317. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, H.-W.; Lee, S.; Na, H.; Kwon, R.H.; Kim, J.H.; Yoon, H.; Choi, Y.-M.; Wee, C.-D.; Yoo, S.M.; et al. Characterization of Isoflavones from Seed of Selected Soybean (Glycine max L.) Resources Using High-Resolution Mass Spectrometry. Korean J. Food Nutr. 2020, 33, 655–665. [Google Scholar]
- Kim, H.-W.; Lee, S.H.; Yoo, S.M.; Chung, M.-N.; Kim, J.B.; Kehraus, S.; Konig, G.M. Identification and Quantification of Hydroxybenzoyl and Hydroxycinnamoyl Derivatives from Korean Sweet Potato Cultivars by UPLC-DAD-QToF/MS. J. Food Compos. Anal. 2021, 100, 103905. [Google Scholar] [CrossRef]
- Gu, L.; Gu, W. Characterisation of Soy Isoflavones and Screening for Novel Malonyl Glycosides Using High-Performance Liquid Chromatography-Electrospray Ionisation-Mass Spectrometry. Phytochem. Anal. 2001, 12, 377–382. [Google Scholar] [CrossRef]
- Yerramsetty, V.; Mathias, K.; Bunzel, M.; Ismail, B. Detection and Structural Characterization of Thermally Generated Isoflavone Malonylglucoside Derivatives. J. Agric. Food Chem. 2011, 59, 174–183. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Z.-P.; Zeng, M.-M.; He, Z.-Y.; Tao, G.-J.; Qin, F.; Chen, J. A Novel Isoflavone Profiling Method Based on UPLC-PDA-ESI-MS. Food Chem. 2017, 219, 40–47. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, S.; Cao, D.; Tang, D. Steam Flash Explosion Pretreatment Enhances Soybean Seed Coat Phenolic Profiles and Antioxidant Activity. Food Chem. 2020, 319, 126552. [Google Scholar] [CrossRef] [PubMed]
- Gasparetto, J.C.; Smolarek, F.S.F.; de Francisco, T.M.G.; Miranda, L.C.; Pontarolo, R.; Siqueira, P.F. Development and Validation of an HPLC-DAD Method for Analysis of the Six Major Isoflavones in Extracts from Soybean Processing. J. Am. Oil Chem. Soc. 2012, 89, 1211–1222. [Google Scholar] [CrossRef]
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonyl Isoflavone Glycosides in Soybean Seeds (Glycine max Merrill). Agric. Biol. Chem. 1991, 55, 2227–2233. [Google Scholar] [CrossRef] [Green Version]
- Ohta, N.; Kuwata, G.; Akahori, H.; Watanabe, T. Isoflavonoid Constituents of Soybeans and Isolation of a New Acetyl Dadizin. Agric. Biol. Chem. 1979, 43, 1415–1419. [Google Scholar]
- Kudou, S.; Shimoyamada, M.; Imura, T.; Uchida, T.; Okubo, K. A New Isoflavone Glycosides in Soybean Seeds (Glycine max Merrill), Glycitein 7-O-β-D-(6”-O-Acetyl)-Glucopyranoside. Agric. Biol. Chem. 1991, 55, 859–860. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, B.; Hwang, S.-R.; Kim, K.; Lee, J.H. Rapid Characterization of Metabolites in Soybean Using Ultra High Performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry (UPLC-ESI-Q-TOF-MS/MS) and Screening for α-Glucosidase Inhibitory and Antioxidant Properties Through Different Solvent Systems. J. Food Drug Anal. 2018, 26, 277–291. [Google Scholar]
- Lee, M.J.; Chung, I.-M.; Kim, H.; Jung, M.Y. High Resolution LC–ESI-TOF-Mass Spectrometry Method for Fast Separation, Identification, and Quantification of 12 Isoflavones in Soybeans and Soybean Products. Food Chem. 2015, 176, 254–262. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, W.S.; Ahn, J.K.; Chung, I.M. Analysis of Isoflavone Concentration and Composition in Soybean [Glycine max (L.)] Seeds between the Cropping Year and Storage for 3 Years. Eur. Food Res. Technol. 2005, 220, 207–214. [Google Scholar] [CrossRef]
- Park, M.; Jeong, M.K.; Kim, M.; Lee, J. Modification of Isoflavone Profiles in a Fermented Soy Food with Almond Powder. J. Food Sci. 2012, 71, C128–C134. [Google Scholar] [CrossRef]
- Choi, Y.-M.; Yoon, H.; Lee, S.; Ko, H.-C.; Shin, M.-J.; Lee, M.C.; Hur, O.S.; Ro, N.Y.; Desta, K.T. Isoflavones, Anthocyanins, Phenolic Content, and Antioxidant Activities of Black Soybeans (Glycine max (L.) Merrill) as Affected by Seed Weight. Sci. Rep. 2020, 10, 19960. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, H.-W.; Lee, S.-H.; Kim, Y.J.; Asamenew, G.; Choi, J.; Lee, J.-W.; Jung, H.-A.; Yoo, S.M.; Kim, J.-B. Characterization of Catechins, Theaflavins, and Flavonols by Leaf Processing Step in Green and Black Teas (Camellia sinensis) Using UPLC-DAD-QToF/MS. Eur. Food Res. Technol. 2019, 245, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Ahn, J.K.; Kim, S.H.; Kim, J.T.; Han, S.J.; Jung, M.Y.; Chung, I.M. Variation in Isoflavone of Soybean Cultivars with Location and Storage Duration. J. Agric. Food Chem. 2003, 51, 3382–3389. [Google Scholar] [CrossRef] [PubMed]



| Acylated Group | Peak No. | Isoflavone Derivatives | Abbreviation | RT (min) | Molecular Formula | Observed m/z [M + H]+ | Error (3) (ppm) | ESI(+)-QToF-MS (Fragmented Ions of [M + H]+, m/z) | References |
|---|---|---|---|---|---|---|---|---|---|
| Daidzein Derivatives (14) | |||||||||
| Aglycone | 33 (2) | Daidzein | D | 20.19 | C15H10O4 | 255.0652 | 0.0 | 277[M + Na]+, 255[M + H]+ | [8,19,24,26,27,29,33] |
| Glu | 4 (2) | Daidzein 7-O-glucoside (Daidzin) | D7G | 11.26 | C21H20O9 | 417.1176 | −1.0 | 455[M + K]+, 439[M + Na]+, 417[M + H]+, 255[M + H-Glu]+ | [19,24,26,27,29,33] |
| Mal-Glu | 11 | Daidzein 4′-O-(6″-O-malonyl)glucoside (6″-O-Malonylisodaidzin) | D4′(6″M)G | 14.65 | C24H22O12 | 503.1184 | 0.0 | 541[M + K]+, 525[M + Na]+, 503[M + H]+, 255[M + H-Mal-Glu]+ | [24,26,27,31] |
| 13 | Daidzein 7-O-(4″-O-malonyl)glucoside (4″-O-Malonyldaidzin) | D7(4″M)G | 15.17 | C24H22O12 | 503.1184 | 0.0 | 541[M + K]+, 525[M + Na]+, 503[M + H]+, 255[M + H-Mal-Glu]+ | [24,26,27,30,31] | |
| 17 (2) | Daidzein 7-O-(6″-O-malonyl)glucoside (6″-O-Malonyldaidzin) | D7(6″M)G | 16.07 | C24H22O12 | 503.1181 | −0.6 | 541[M + K]+, 525[M + Na]+, 503[M + H]+, 255[M + H-Mal-Glu]+ | [24,26,27,29,30,31,34] | |
| Ac-Glu | 16 | Daidzein 4′-O-(6″-O-acetyl)glucoside (6″-O-Acetylisodaidzin) | D4′(6″Ac)G | 15.80 | C23H22O10 | 459.1285 | −0.2 | 497[M + K]+, 481[M + Na]+, 459[M + H]+, 255[M + H-Ac-Glu]+ | [24] |
| 24 (2) | Daidzein 7-O-(6″-O-acetyl)glucoside (6″-O-Acetyldaidzin) | D7′(6″Ac)G | 18.02 | C23H22O10 | 459.1285 | −0.2 | 497[M + K]+, 481[M + Na]+, 459[M + H]+, 255[M + H-Ac-Glu]+ | [8,24,26,29,35] | |
| Suc-Glu | 15 1) | Daidzein 4′-O-(4″-O-succinyl)glucoside (4″-O-Succinylisodaidzin) | D4′(4″S)G | 15.74 | C25H24O12 | 517.1344 | 0.7 | 555[M + K]+, 539[M + Na]+, 517[M + H]+, 255[M + H-Suc-Glu]+ | |
| 18 (1) | Daidzein 4′-O-(6″-O-succinyl)glucoside (6″-O-Succinylisodaidzin) | D4′(6″S)G | 16.16 | C25H24O12 | 517.1343 | 0.5 | 555[M + K]+, 539[M + Na]+, 517[M + H]+, 255[M + H-Suc-Glu]+ | ||
| 20 | Daidzein 7-O-(4″-O-succinyl)glucoside (4″-O-Succinyldaidzin) | D7(4″S)G | 16.52 | C25H24O12 | 517.1333 | −1.7 | 555[M + K]+, 539[M + Na]+, 517[M + H]+, 255[M + H-Suc-Glu]+ | [24] | |
| 21 | Daidzein 7-O-(6″-O-succinyl)glucoside (6″-O-Succinyldaidzin) | D7(6″S)G | 17.10 | C25H24O12 | 517.1339 | −0.5 | 555[M + K]+, 539[M + Na]+, 517[M + H]+, 255[M + H-Suc-Glu]+ | [8,9,20,23,24,26] | |
| 29 | Daidzein succinyl glucoside isomer | DSG | 19.14 | C25H24O12 | 517.1342 | 0.3 | 555[M + K]+, 539[M + Na]+, 517[M + H]+, 255[M + H-Suc-Glu]+ | ||
| Phos | 1 (1) | Daidzein 7-O-phosphate | D7P | 8.51 | C15H11O7P | 335.0315 | 0.0 | 335[M + H]+, 317[M + H − H2O]+, 255[M + H-Phos]+ | [14,25] |
| 2 (1) | Daidzein 4′-O-phosphate | D4′P | 10.36 | C15H11O7P | 335.0323 | 2.4 | 335[M + H]+, 317[M + H − H2O]+, 255[M + H-Phos]+ | [25] | |
| Genistein Derivatives (18) | |||||||||
| Aglycone | 38 (2) | Genistein | Gn | 23.58 | C15H10O5 | 271.0599 | 0.7 | 293[M + Na]+, 271[M + H]+ | [19,24,26,27,29,33] |
| Glu | 3 | Genistein 5-O-glucoside | Gn5G | 10.76 | C21H20O10 | 433.1131 | 0.4 | 471[M + K]+, 455[M + Na]+, 433[M + H]+, 271[M + H-Glu]+ | [24,26,27] |
| 10 (2) | Genistein 7-O-glucoside (Genistin) | Gn7G | 14.65 | C21H20O10 | 433.1124 | −1.2 | 471[[M + K]+, 455[M + Na]+, 433[M + H]+, 271[M + H-Glu]+ | [19,24,26,27,29,33] | |
| Api-Glu | 8 | Genistein 7-O-(6″-O-apiosyl)glucoside (Ambocin) | Gn7(6″Ap)G | 13.73 | C26H28O14 | 565.1553 | 0.2 | 587[M + Na]+, 565[M + H]+, 433[M + H-Api]+, 271[M + H-Api-Glu]+ | [24,27] |
| 9 | Genistein 7-O-(2″-O-apiosyl)glucoside | Gn7(6″Ap)G | 14.38 | C26H28O14 | 565.1552 | 0.0 | 587[M + Na]+, 565[M + H]+, 433[M + H-Api]+, 271[M + H-Api-Glu]+ | [24,27] | |
| Mal-Glu | 12 | Genistein 5-O-(6″-O-malonyl)glucoside | Gn5(6″M)G | 15.10 | C24H22O13 | 519.1133 | 0.0 | 557[M + K]+, 541[M + Na]+, 519[M + H]+, 271[M + H-Mal-Glu]+ | [24,26,27] |
| 25 | Genistein 4′-O-(6″-O-malonyl)glucoside (6″-O-Malonylsophoricoside) | Gn4′(6″M)G | 18.15 | C24H22O13 | 519.1136 | 0.5 | 557[M + K]+, 541[M + Na]+, 519[M + H]+, 271[M + H-Mal-Glu]+ | [24,26,27] | |
| 26 | Genistein 7-O-(4″-O-malonyl)glucoside (4″-O-Malonylgenistin) | Gn7(4″M)G | 18.69 | C24H22O13 | 519.1128 | −1.0 | 557[M + K]+, 541[M + Na]+, 519[M + H]+, 271[M + H-Mal-Glu]+ | [24,26,27,30] | |
| 28 (2) | Genistein 7-O-(6″-O-malonyl)glucoside (6″-O-Malonylgenistin) | Gn7(6″M)G | 19.14 | C24H22O13 | 519.1130 | −0.6 | 557[M + K]+, 541[M + Na]+, 519[M + H]+, 271[M + H-Mal-Glu]+ | [8,24,26,27,29,30,34] | |
| Ac-Glu | 22 | Genistein 5-O-(6″-O-acetyl)glucoside | Gn5(6″Ac)G | 17.22 | C23H22O11 | 475.1237 | −0.4 | 513[M + K]+, 497[M + Na]+, 475[M + H]+, 271[M + H-Ac-Glu]+ | [24] |
| 30 | Genistein 4′-O-(6″-O-acetyl)glucoside (6″-O-Acetylsophoricoside) | Gn4′(6″Ac)G | 19.34 | C23H22O11 | 475.1240 | 0.2 | 513[M + K]+, 497[M + Na]+, 475[M + H]+, 271[M + H-Ac-Glu]+ | [24,26] | |
| 32 | Genistein 7-O-(4″-O-acetyl)glucoside (4″-O-Acetylgenistin) | Gn7(4″Ac)G | 19.71 | C23H22O11 | 475.1239 | 0.9 | 513[M + K]+, 497[M + Na]+, 475[M + H]+, 271[M + H-Ac-Glu]+ | [24] | |
| 36 (2) | Genistein 7-O-(6″-O-acetyl)glucoside (6″-O-Acetylgenistin) | Gn7(6″Ac)G | 21.79 | C23H22O11 | 475.1238 | 0.7 | 513[M + K]+, 497[M + Na]+, 475[M + H]+, 271[M + H-Ac-Glu]+ | [24,26,27,29] | |
| Suc-Glu | 31 | Genistein 7-O-(4″-O-succinyl)glucoside (4″-O-Succinylgenistin) | Gn7(4″S)G | 19.62 | C25H24O13 | 533.1289 | −0.1 | 571[M + K]+, 555[M + Na]+, 533[M + H]+, 271[M + H-Suc-Glu]+ | [24] |
| 34 | Genistein 7-O-(6″-O-succinyl)glucoside (6″-O-Succinylgenistin) | Gn7(6″S)G | 20.31 | C25H24O13 | 533.1288 | −0.3 | 571[M + K]+, 555[M + Na]+, 533[M + H]+, 271[M + H-Suc-Glu]+ | [8,9,23,24,26] | |
| 37 | Genistein succinyl glucoside isomer | GnSG | 22.40 | C25H24O13 | 533.1286 | −0.7 | 571[M + K]+, 555[M + Na]+, 533[M + H]+, 271[M + H-Suc-Glu]+ | ||
| Phos | 5 (1) | Genistein 7-O-phosphate | Gn7P | 11.31 | C15H11O8P | 351.0267 | 0.8 | 351[M + H]+, 333[M + H − H2O]+, 271[M + H-Phos]+ | [25] |
| 7 (1) | Genistein 4′-O-phosphate | Gn4′P | 12.77 | C15H11O8P | 351.0268 | 1.1 | 351[M + H]+, 333[M + H − H2O]+, 271[M + H-Phos]+ | [25] | |
| Glycitein Derivatives (6) | |||||||||
| Aglycone | 35 (2) | Glycitein | Gy | 21.36 | C16H12O5 | 285.0759 | 0.5 | 323[M + K]+, 307[M + Na]+, 285[M + H]+, 270[M + H-CH3]+, | [19,24,26,27,29,33] |
| Glu | 6 (2) | Glycitein 7-O-glucoside (Glycitin) | Gy7G | 12.10 | C22H22O10 | 447.1285 | −0.2 | 485[M + K]+, 469[M + Na]+, 447[M + H]+, 285[M + H-Glu]+ | [19,24,26,27,29,33] |
| Mal-Glu | 14 | Glycitein 4′-O-(6″-O-malonyl)glucoside | Gy4′(6″M)G | 15.42 | C25H24O13 | 533.1279 | −2.0 | 571[M + K]+, 555[M + Na]+, 533[M + H]+, 285[M + H-Mal-Glu]+ | [24,27] |
| 19 (2) | Glycitein 7-O-(6″-O-malonyl)glucoside (6″-O-Malonylglycitin) | Gy7(6″M)G | 16.49 | C25H24O13 | 533.1288 | −0.3 | 571[M + K]+, 555[M + Na]+, 533[M + H]+, 285[M + H-Mal- Glu]+ | [8,26,27,29,34] | |
| Ac-Glu | 27 (2) | Glycitein 7-O-(6″-O-acetyl)glucoside (6″-O-Acetylglycitin) | Gy7(6″Ac)G | 18.69 | C24H24O11 | 489.1394 | 0.5 | 527[M + K]+, 511[M + Na]+, 489[M + H]+, 285[M + H-Ac-Glu]+ | [24,26,29,36] |
| Suc-Glu | 23 | Glycitein 7-O-(6″-O-succinyl)glucoside (6″-O-Succinylglycitin) | Gy7(6″S)G | 17.58 | C26H26O13 | 547.1450 | 0.7 | 585[M + K]+, 569[M + Na]+, 547[M + H]+, 285[M + H-Suc-Glu]+ | [9,24] |
| Acylated Group | Peak No. | Daewon (Control Variety) | KLS 87248 (Korean Landrace) | ||||||||
| Raw | Steamed | 21 h | 36 h | 60 h | Raw | Steamed | 21 h | 36 h | 60 h | ||
| Aglycone | 33 (2) | 0.2 ± 0.1 h | 1.7 ± 0.2 ef | 1.6 ± 0.3 efg | 10.2 ± 2.5 c | 15.8 ± 0.7 b | 1.2 ± 0.3 efgh | 1.6 ± 0.1 efg | 1.7 ± 0.1 ef | 15.3 ± 0.3 b | 31.7 ± 0.8 a |
| Glu | 4 (2) | 7.0 ± 0.6 m | 62.2 ± 2.5 c | 49.8 ± 0.9 e | 25.0 ± 3.8 i | 15.3 ± 1.6 k | 24.3 ± 1.7 j | 107.4 ± 2.2 a | 92.9 ± 0.6 b | 45.8 ± 0.6 f | 23.2 ± 0.6 ij |
| Mal-Glu | 11 | 4.6 ± 0.2 c | 1.8 ± 0.0 ij | 1.4 ± 0.1 kl | 1.2 ± 0.0 lm | 0.9 ± 0.1 m | 9.5 ± 0.3 a | 4.6 ± 0.5 c | 3.7 ± 0.2 d | 3.1 ± 0.2 fg | 2.3 ± 0.1 h |
| 13 | 0.6 ± 0.1 g | 0.7 ± 0.1 fg | 0.7 ± 0.1 fg | 0.9 ± 0.2 ef | 1.1 ± 0.2 e | 1.5 ± 0.1 d | 2.9 ± 0.3 a | 2.9 ± 0.2 a | 2.9 ± 0.1 a | 2.5 ± 0.2 b | |
| 17 (2) | 57.5 ± 2.0 c | 12.7 ± 0.5 h | 13.3 ± 0.6 h | 13.3 ± 0.5 h | 12.4 ± 1.1 h | 116.1 ± 6.1 a | 30.8 ± 2.2 ef | 30.8 ± 2.1 ef | 31.4 ± 1.5 ef | 28.4 ± 2.0 f | |
| Ac-Glu | 16 | ND | 0.7 ± 0.0 ab | 0.7 ± 0.0 abc | 0.8 ± 0.2 a | 0.6 ± 0.1 cd | ND | ND | 0.5 ± 0.0 de | ND | ND |
| 24 (2) | ND | 5.5 ± 0.3 c | 4.0 ± 0.3 d | 2.2 ± 0.2 f | 1.4 ± 0.1 ghi | 0.8 ± 0.1 jk | 9.8 ± 0.6 a | 8.0 ± 0.6 b | 4.4 ± 0.1 d | 1.9 ± 0.3 fg | |
| Suc-Glu | 15 | ND | ND | 0.3 ± 0.1 e | 0.9 ± 0.1 b | 1.0 ± 0.0 b | ND | ND | 0.3 ± 0.1 de | 1.4 ± 0.1 a | 1.4 ± 0.1 a |
| 18 | ND | ND | 0.5 ± 0.1 h | 1.8 ± 0.5 ef | 1.9 ± 0.1 de | ND | ND | 1.6 ± 0.2 ef | 3.4 ± 0.1 b | 4.7 ± 0.3 a | |
| 20 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 21 | ND | ND | 11.1 ± 0.6 gh | 14.1 ± 0.5 d | 11.7 ± 0.9 fg | ND | ND | 13.3 ± 1.0 de | 25.7 ± 1.2 a | 23.9 ± 1.6 b | |
| 29 | 3.6 ± 0.4 bcd | 0.8 ± 0.5 e | 0.8 ± 0.6 e | 0.8 ± 0.5 e | 0.7 ± 0.5 e | 13.8 ± 2.2 a | 3.3 ± 0.1 bcde | 3.2 ± 0.0 bcde | 2.9 ± 0.1 cde | 2.6 ± 0.1 de | |
| Phos | 1 (1) | ND | ND | 1.0 ± 0.0 fg | 6.7 ± 0.1 de | 19.0 ±1.1 b | ND | ND | 1.2 ± 0.2 fg | 12.2 ± 0.4 | 34.6 ± 3.8 a |
| 2 (1) | ND | ND | ND | ND | ND | ND | ND | ND | 0.8 ± 0.0 c | 3.6 ± 0.5 a | |
| Total Daidzein (14) | 73.6 ± 3.2 h | 86.1 ± 2.7 f | 85.1 ± 1.6 f | 77.9 ± 2.1 gh | 81.9 ± 3.3 fg | 167.0 ± 5.5 a | 160.4 ± 4.4 a | 160.1 ± 3.8 a | 149.4 ± 0.9 b | 160.9 ± 1.7 a | |
| Aglycone | 38 (2) | 0.3 ± 0.1 efg | 2.0 ± 0.2 ef | 1.9 ± 0.0 ef | 7.1 ± 2.5 c | 15.5 ± 0.7 b | 0.7 ± 0.1 fg | 1.2 ± 0.1 fg | 1.3 ± 0.0 efg | 6.9 ± 0.2 c | 16.9 ± 0.2 a |
| Glu | 3 | 0.5 ± 0.0 hi | 3.2 ± 0.1 c | 3.0 ± 0.1 d | 1.6 ± 0.2 f | 1.1 ± 0.1 g | 1.6 ± 0.4 f | 9.0 ± 0.2 a | 8.9 ± 0.1 a | 4.6 ± 0.1 b | 2.5 ± 0.1 e |
| 10 (2) | 9.1 ± 0.7 m | 108.0 ± 4.2 b | 88.8 ± 2.2 d | 57.2 ± 6.2 g | 40.6 ± 1.4 i | 26.7 ± 0.9 k | 122.0 ± 2.4 a | 106.0 ± 0.3 b | 64.6 ± 0.9 f | 39.9 ± 1.1 i | |
| Api-Glu | 8 | 0.2 ± 0.0 j | 0.4 ± 0.0 hi | 0.4 ± 0.0 gh | 0.5 ± 0.0 g | 0.5 ± 0.0 g | 1.3 ± 0.1 a | 0.8 ± 0.0 de | 0.8 ± 0.0 d | 0.9 ± 0.0 c | 1.0 ± 0.1 b |
| 9 | 0.4 ± 0.1 i | 0.5 ± 0.0 hi | 0.6 ± 0.0 ghi | 0.6 ± 0.0 ghi | 0.6 ± 0.1 gh | 1.2 ± 0.0 f | 1.3 ± 0.0 ef | 1.2 ± 0.0 ef | 1.3 ± 0.1 ef | 1.4 ± 0.1 e | |
| Mal-Glu | 12 | 3.5 ± 0.2 b | 0.8 ± 0.1 e | 0.8 ± 0.0 e | 0.5 ± 0.1 e | 0.5 ± 0.1 e | 9.6 ± 0.7 a | 1.8 ± 0.3 d | 1.8 ± 0.2 d | 2.1 ± 0.2 cd | 1.8 ± 0.2 d |
| 25 | 5.7 ± 0.5 d | 1.8 ± 0.4 klm | 1.6 ± 0.3 mn | 1.1 ± 0.1 n | 1.1 ± 0.1 n | 10.7 ± 0.8 a | 3.7 ± 0.3 e | 3.1 ± 0.2 efg | 2.5 ± 0.3 hij | 1.7 ± 0.2 lm | |
| 26 | 0.8 ± 0.1 e | 0.5 ± 0.0 f | 0.5 ± 0.0 f | 0.6 ± 0.1 f | 0.6 ± 0.1 f | 0.3 ± 0.0 g | 0.9 ± 0.1 e | 0.8 ± 0.1 e | 1.0 ± 0.0 de | 1.0 ± 0.1 de | |
| 28 (2) | 89.3 ± 3.9 c | 23.3 ± 1.0 g | 24.7 ± 1.5 g | 26.1 ± 1.2 g | 25.1 ± 2.1 g | 123.8 ± 5.1 b | 34.8 ± 3.4 f | 35.5 ± 3.5 f | 38.0 ± 1.7 f | 37.3 ± 2.8 f | |
| Ac-Glu | 22 | ND | 1.1 ± 0.1 b | 0.7 ± 0.2 d | ND | ND | ND | 1.2 ± 0.1 a | 1.1 ± 0.0 b | 0.8 ± 0.1 c | 0.4 ± 0.1 e |
| 30 | ND | 0.7 ± 0.1 cd | 0.2 ± 0.1 e | ND | ND | ND | 1.0 ± 0.2 a | 0.7 ± 0.0 bc | 0.9 ± 0.2 b | ND | |
| 32 | ND | 0.9 ± 0.1 def | ND | 1.6 ± 0.2 b | 1.0 ± 0.1 cd | ND | 0.9 ± 0.1 def | 1.1 ± 0.1 cd | 2.0 ± 0.1 a | 1.0 ± 0.0 cde | |
| 36 (2) | 0.1 ± 0.0 l | 11.1 ± 0.1 a | 9.3 ± 0.3 c | 6.9 ± 0.5 e | 5.2 ± 0.2 g | 1.1 ± 0.2 k | 11.5 ± 0.2 a | 10.1 ± 1.0 b | 7.7 ± 0.3 d | 4.7 ± 0.6 g | |
| Suc-Glu | 31 | ND | ND | ND | 1.3 ± 0.6 de | 3.7 ± 0.3 b | ND | ND | ND | 1.3 ± 0.1 d | 7.1 ± 0.5 a |
| 34 | ND | ND | 17.0 ± 1.0 e | 27.0 ± 2.3 c | 31.3 ± 2.3 b | ND | ND | 14.4 ± 1.5 f | 31.0 ± 2.0 b | 48.6 ± 3.9 a | |
| 37 | 1.4 ± 0.1 abc | 1.0 ± 0.5 c | 0.8 ± 0.5 bc | 0.7 ± 0.2 bc | 0.9 ± 0.0 bc | 2.7 ± 0.0 ab | 2.0 ± 0.0 abc | 1.4 ± 0.7 abc | 1.6 ± 0.0 abc | 1.6 ± 0.1 abc | |
| Phos | 5 (1) | ND | ND | ND | 4.7 ± 0.7 cd | 13.2 ± 3.1 b | ND | ND | ND | 6.0 ± 0.3 c | 14.7 ± 2.5 a |
| 7 (1) | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.5 ± 0.1 | |
| Total Genistein (18) | 111.6 ± 5.3 f | 155.4 ± 4.0 d | 150.3 ± 2.7 d | 137.5 ± 3.7 e | 140.1 ± 7.3 e | 179.7 ± 3.6 bc | 192.1 ± 4.7 a | 188.4 ± 5.4 a | 173.2 ± 2.8 c | 183.3 ± 6.6 ab | |
| Aglycone | 35 (2) | ND | 0.8 ± 0.1 a | ND | ND | 0.6 ± 0.1 bcd | ND | 0.9 ± 0.1 abc | 0.7 ± 0.0 ab | 0.4 ± 0.1 cd | 0.7 ± 0.1 ab |
| Glu | 6 (2) | 3.5 ± 0.3 l | 15.1 ± 0.5 c | 14.2 ± 0.1 d | 9.3 ± 0.5 f | 7.6 ± 0.2 h | 8.7 ± 0.5 g | 22.3 ± 0.4 a | 21.4 ± 0.3 b | 14.8 ± 0.1 c | 9.2 ± 0.2 fg |
| Mal-Glu | 14 | 0.9 ± 0.1 b | 0.4 ± 0.1 fgh | 0.4 ± 0.0 fg | 0.3 ± 0.0 ghi | 0.3 ± 0.1 ghi | 1.2 ± 0.2 a | 0.5 ± 0.1 efg | 0.5 ± 0.1 cde | 0.4 ± 0.1 efg | 0.2 ± 0.0 i |
| 19 (2) | 9.5 ± 0.9 c | 2.3 ± 0.1 g | 2.5 ± 0.1 g | 2.6 ± 0.1 g | 2.5 ± 0.1 g | 17.7 ± 1.4 a | 4.9 ± 0.3 f | 4.8 ± 0.4 f | 5.1 ± 0.2 f | 4.8 ± 0.3 f | |
| Ac-Glu | 27 (2) | ND | 1.2 ± 0.1 c | 0.9 ± 0.1 d | 0.6 ± 0.1 f | 0.3 ± 0.0 ijk | 0.1 ± 0.0 lm | 1.8 ± 0.2 a | 1.4 ± 0.1 b | 0.9 ± 0.1 d | 0.4 ± 0.0 hij |
| Suc-Glu | 23 | ND | ND | 1.9 ± 0.1 gh | 5.8 ± 0.7 d | 7.5 ± 0.5 b | ND | ND | 1.5 ± 0.1 hi | 7.0 ± 0.2 bc | 11.8 ± 1.5 a |
| Total Glycitein (6) | 13.8 ± 1.3 g | 19.8 ± 0.4 d | 19.9 ± 0.1 d | 18.6 ± 0.2 de | 18.8 ± 1.0 de | 27.8 ± 1.2 bc | 30.4 ± 0.4 a | 30.3 ± 0.9 a | 28.6 ± 0.5 b | 27.0 ± 1.9 c | |
| Total Isoflavones | 199.0 ± 9.7 h | 261.2 ± 6.7 f | 255.3 ± 4.2 f | 234.0 ± 5.6 g | 241.7 ± 11.0 g | 374.5 ± 7.9 b | 382.9 ± 9.5 a | 378.8 ± 10.1 ab | 351.3 ± 4.2 c | 371.2 ± 9.1 ab | |
| Acylated Group | Peak No. | Nongrim 51 (Japanese Breeding Line) | GNU-2007-14613 (Korean Landrace) | ||||||||
| Raw | Steamed | 21 h | 36 h | 60 h | Raw | Steamed | 21 h | 36 h | 60 h | ||
| Aglycone | 33 (2) | 0.2 ± 0.1 gh | 1.8 ± 0.0 ef | 1.4 ± 0.2 efgh | 6.0 ± 0.3 d | 16.1 ± 1.0 b | 1.0 ± 0.0 fgh | 1.3 ± 0.1 efgh | 0.9 ± 0.1 fgh | 2.5 ± 0.3 e | 9.9 ± 0.1 c |
| Glu | 4 (2) | 13.1 ± 0.5 kl | 64.3 ± 1.1 c | 55.0 ± 0.3 d | 36.5 ± 0.8 g | 24.2 ± 1.0 ij | 10.1 ± 2.1 l | 30.8 ± 1.4 h | 25.1 ± 0.6 i | 15.7 ± 0.7 k | 12.0 ± 0.3 l |
| Mal-Glu | 11 | 5.5 ± 0.3 b | 4.6 ± 0.2 c | 3.5 ± 0.2 de | 3.3 ± 0.2 ef | 2.8 ± 0.2 g | 3.8 ± 0.1 d | 2.8 ± 0.2 g | 2.3 ± 0.1 h | 2.1 ± 0.1 hi | 1.6 ± 0.2 jk |
| 13 | 0.7 ± 0.0 fg | 2.0 ± 0.1 c | 2.0 ± 0.4 c | 2.4 ± 0.2 b | 2.6 ± 0.4 ab | 0.5 ± 0.1 g | 1.7 ± 0.1 d | 1.6 ± 0.1 d | 1.6 ± 0.2 d | 1.7 ± 0.1 cd | |
| 17 (2) | 92.0 ± 2.0 b | 31.1 ± 1.5 ef | 30.1 ± 1.8 ef | 33.0 ± 2.3 e | 29.9 ± 0.8 ef | 48.3 ± 2.1 d | 21.7 ± 1.5 g | 22.2 ± 1.1 g | 22.9 ± 1.1 g | 19.7 ± 1.6 g | |
| Ac-Glu | 16 | ND | 0.4 ± 0.0 ef | ND | ND | ND | ND | 0.3 ± 0.1 fg | 0.3 ± 0.0 g | 0.6 ± 0.0 bcd | ND |
| 24 (2) | ND | 3.3 ± 0.2 e | 2.8 ± 0.3 e | 2.2 ± 0.2 f | 1.4 ± 0.2 ghi | 0.3 ± 0.0 kl | 1.5 ± 0.2 gh | 1.3 ± 0.2 hi | 1.0 ± 0.1 ij | 0.6 ± 0.1 jk | |
| Suc-Glu | 15 | ND | ND | 0.4 ± 0.2 d | 0.9 ± 0.0 b | 0.9 ± 0.1 b | ND | ND | ND | ND | 0.7 ± 0.1 c |
| 18 | ND | 1.0 ± 0.1 g | 1.5 ± 0.3 f | 2.3 ± 0.2 d | 2.6 ± 0.3 c | ND | ND | 1.1 ± 0.2 g | 1.7 ± 0.1 ef | 2.3 ± 0.2 d | |
| 20 | ND | ND | ND | 0.2 ± 0.0 b | 0.5 ± 0.0 a | ND | ND | ND | ND | ND | |
| 21 | ND | ND | 9.3 ± 0.6 i | 15.8 ± 1.0 c | 12.8 ± 0.7 ef | ND | ND | 5.8 ± 0.3 j | 10.0 ± 0.5 hi | 10.1 ± 1.0 hi | |
| 29 | 5.4 ± 0.1 bc | 3.7 ± 0.2 bcde | 3.6 ± 0.1 bcde | 3.4 ± 0.1 bcde | 3.0 ± 0.1 bcde | 5.9 ± 0.2 b | 2.6 ± 0.1 de | 2.1 ± 0.9 de | 2.4 ± 0.2 de | 1.5 ± 0.6 de | |
| Phos | 1 (1) | ND | ND | 0.5 ± 0.1 fg | 7.8 ± 0.3 d | 19.0 ± 1.1 b | ND | ND | ND | 1.9 ± 0.3 f | 6.0 ± 0.2 e |
| 2 (1) | ND | ND | ND | ND | 1.3 ± 0.1 b | ND | ND | ND | ND | ND | |
| Total Daidzein (14) | 116.9 ± 2.8 cd | 112.2 ± 0.5 de | 110.2 ± 1.7 e | 113.7 ± 3.0 cde | 117.1 ± 3.4 c | 69.8 ± 2.4 i | 62.6 ± 2.3 j | 62.6 ± 0.5 j | 62.5 ± 1.1 j | 66.1 ± 2.2 ij | |
| Aglycone | 38 (2) | 0.2 ± 0.0 fg | 2.2 ± 0.2 e | 2.2 ± 0.3 e | 4.4 ± 0.2 d | 14.8 ± 0.2 b | 0.9 ± 0.1 efg | 1.4 ± 0.1 efg | 1.4 ± 0.1 efg | 2.0 ± 0.1 ef | 6.3 ± 0.1 c |
| Glu | 3 | 0.3 ± 0.0 i | 1.0 ± 0.1 g | 1.0 ± 0.1 g | 0.6 ± 0.0 h | 0.4 ± 0.1 hi | 0.4 ± 0.1 hi | 1.4 ± 0.0 f | 1.4 ± 0.1 f | 0.9 ± 0.0 g | 0.6 ± 0.1 h |
| 10 (2) | 18.7 ± 0.9 l | 104.4 ± 2.1 b | 95.1 ± 1.4 c | 72.1 ± 1.5 e | 53.7 ± 2.3 gh | 15.0 ± 3.4 l | 49.8 ± 2.7 h | 41.7 ± 1.3 i | 31.8 ± 1.5 j | 28.2 ± 0.4 j | |
| Api-Glu | 8 | 0.5 ± 0.0 g | 0.5 ± 0.0 g | 0.7 ± 0.0 f | 0.7 ± 0.0 ef | 0.8 ± 0.0 de | 0.3 ± 0.1 ij | ND | ND | ND | ND |
| 9 | 5.5 ± 0.1 d | 5.5 ± 0.1 d | 5.7 ± 0.1 c | 5.9 ± 0.3 b | 6.2 ± 0.3 a | 0.6 ± 0.0 gh | 0.6 ± 0.0 gh | 0.6 ± 0.0 gh | 0.7 ± 0.0 gh | 0.8 ± 0.0 g | |
| Mal-Glu | 12 | 2.0 ± 0.1 cd | 0.9 ± 0.1 e | 0.9 ± 0.0 e | 0.8 ± 0.1 e | 0.6 ± 0.1 e | 2.3 ± 0.1 c | 0.8 ± 0.1 e | 0.8 ± 0.1 e | 0.9 ± 0.1 e | 0.8 ± 0.1 e |
| 25 | 8.5 ± 0.2 b | 3.5 ± 0.4 ef | 3.0 ± 0.3 fgh | 3.1 ± 0.4 fgh | 2.9 ± 0.2 ghi | 6.3 ± 0.4 c | 2.7 ± 0.2 ghij | 2.4 ± 0.2 ijk | 2.2 ± 0.2 jkl | 1.8 ± 0.2 klm | |
| 26 | 1.6 ± 0.1 c | 1.8 ± 0.1 b | 1.6 ± 0.3 bc | 2.0 ± 0.2 a | 2.2 ± 0.1 a | 0.1 ± 0.0 g | 1.0 ± 0.1 de | 1.0 ± 0.1 de | 1.0 ± 0.1 de | 1.1 ± 0.2 d | |
| 28 (2) | 135.5 ± 8.1 a | 53.5 ± 3.8 e | 53.0 ± 4.1 e | 59.2 ± 5.0 e | 55.9 ± 4.0 e | 81.1 ± 5.4 d | 40.2 ± 1.5 f | 39.3 ± 2.9 f | 41.2 ± 2.7 f | 35.4 ± 0.4 f | |
| Ac-Glu | 22 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 30 | ND | 0.8 ± 0.1 b | 0.6 ± 0.1 cd | 0.5 ± 0.1 d | ND | ND | ND | ND | ND | ND | |
| 32 | ND | 0.5 ± 0.0 g | 0.6 ± 0.0 f | 1.2 ± 0.3 c | 0.8 ± 0.1 ef | ND | 0.3 ± 0.1 h | ND | 0.5 ± 0.1 g | 0.3 ± 0.0 gh | |
| 36 (2) | 0.2 ± 0.0 l | 7.3 ± 0.1 de | 7.0 ± 0.2 e | 6.2 ± 0.3 f | 4.7 ± 0.2 g | 1.0 ± 0.0 kl | 3.5 ± 0.1 h | 3.2 ± 0.2 hi | 2.8 ± 0.0 i | 2.0 ± 0.0 j | |
| Suc-Glu | 31 | ND | ND | ND | 0.9 ± 0.2 ef | 3.4 ± 0.4 bc | ND | ND | ND | 0.6 ± 0.2 f | 3.3 ± 0.4 c |
| 34 | ND | ND | 13.4 ± 0.8 f | 26.0 ± 2.1 c | 30.7 ± 1.4 b | ND | ND | 7.2 ± 0.4 g | 13.8 ± 1.4 f | 22.4 ± 2.0 d | |
| 37 | 1.1 ± 0.4 abc | 3.0 ± 0.1 a | 2.9 ± 0.2 a | 2.5 ± 0.1 ab | 2.4 ± 0.2 abc | 1.6 ± 0.1 abc | 2.2 ± 0.1 abc | 2.0 ± 0.1 abc | 1.8 ± 0.1 abc | 1.5 ± 0.0 abc | |
| Phos | 5 (1) | ND | ND | ND | 4.2 ± 0.2 d | 12.0 ± 0.6 b | ND | ND | ND | 0.9 ± 0.1 f | 2.6 ± 0.2 e |
| 7 (1) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Total Genistein (18) | 174.1 ± 8.0 c | 184.8 ± 1.7 a | 187.7 ± 4.0 a | 190.4 ± 7.7 a | 191.6 ± 3.7 a | 109.6 ± 1.2 fg | 103.9 ± 2.2 g | 101.0 ± 2.0 g | 101.2 ± 3.1 g | 107.2 ± 5.3 fg | |
| Aglycone | 35 (2) | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.4 ± 0.0 d |
| Glu | 6 (2) | 2.0 ± 0.2 m | 5.9 ± 0.1 i | 6.1 ± 0.0 i | 4.4 ± 0.1 k | 3.80 ± 0.1 l | 4.7 ± 0.6 jk | 10.0 ± 0.5 e | 8.8 ± 0.2 fg | 6.1 ± 0.2 i | 5.1 ± 0.1 j |
| Mal-Glu | 14 | 0.6 ± 0.1 c | 0.2 ± 0.0 hi | ND | ND | ND | 0.9 ± 0.1 b | 0.6 ± 0.0 cd | 0.5 ± 0.0 cdef | 0.6 ± 0.1 cde | 0.5 ± 0.1 defg |
| 19 (2) | 5.3 ± 0.5 ef | 2.2 ± 0.1 g | 2.3 ± 0.1 g | 2.3 ± 0.1 g | 2.5 ± 0.0 g | 11.5 ± 0.6 b | 6.0 ± 0.1 de | 5.9 ± 0.4 de | 6.3 ± 0.3 d | 5.2 ± 0.3 f | |
| Ac-Glu | 27 (2) | ND | 0.6 ± 0.0 fg | 0.4 ± 0.0 ghi | 0.3 ± 0.0 jk | 0.2 ± 0.0 lm | 0.1 ± 0.0 mn | 0.9 ± 0.0 d | 0.7 ± 0.0 e | 0.5 ± 0.1 fgh | 0.2 ± 0.0 kl |
| Suc-Glu | 23 | ND | ND | ND | 2.2 ± 0.2 g | 3.1 ± 0.2 f | ND | ND | 1.1 ± 0.1 i | 4.0 ± 0.2 e | 6.7 ± 0.6 c |
| Total Glycitein (6) | 8.0 ± 0.7 i | 8.9 ± 0.1 hi | 8.8 ± 0.2 hi | 9.2 ± 0.4 hi | 9.7 ± 0.2 h | 17.1 ± 0.7 f | 17.5 ± 0.4 ef | 17.0 ± 0.4 f | 17.5 ± 0.5 ef | 18.0 ± 1.0 ef | |
| Total Isoflavones | 299.0 ± 10.4 e | 305.9 ± 2.0 de | 306.6 ± 5.8 de | 313.3 ± 11.0 d | 318.3 ± 6.1 d | 196.5 ± 2.5 hi | 184.0 ± 4.8 ij | 180.6 ± 2.6 j | 181.2 ± 4.3 j | 191.3 ± 8.6 hij | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kwon, R.H.; Kim, J.H.; Na, H.; Lee, S.-J.; Choi, Y.-M.; Yoon, H.; Kim, S.Y.; Kim, Y.-S.; Lee, S.H.; et al. Changes in Isoflavone Profile from Soybean Seeds during Cheonggukjang Fermentation Based on High-Resolution UPLC-DAD-QToF/MS: New Succinylated and Phosphorylated Conjugates. Molecules 2022, 27, 4120. https://doi.org/10.3390/molecules27134120
Lee S, Kwon RH, Kim JH, Na H, Lee S-J, Choi Y-M, Yoon H, Kim SY, Kim Y-S, Lee SH, et al. Changes in Isoflavone Profile from Soybean Seeds during Cheonggukjang Fermentation Based on High-Resolution UPLC-DAD-QToF/MS: New Succinylated and Phosphorylated Conjugates. Molecules. 2022; 27(13):4120. https://doi.org/10.3390/molecules27134120
Chicago/Turabian StyleLee, Suji, Ryeong Ha Kwon, Ju Hyung Kim, Hyemin Na, So-Jeong Lee, Yu-Mi Choi, Hyemyeong Yoon, So Young Kim, Yong-Suk Kim, Sang Hoon Lee, and et al. 2022. "Changes in Isoflavone Profile from Soybean Seeds during Cheonggukjang Fermentation Based on High-Resolution UPLC-DAD-QToF/MS: New Succinylated and Phosphorylated Conjugates" Molecules 27, no. 13: 4120. https://doi.org/10.3390/molecules27134120
APA StyleLee, S., Kwon, R. H., Kim, J. H., Na, H., Lee, S.-J., Choi, Y.-M., Yoon, H., Kim, S. Y., Kim, Y.-S., Lee, S. H., Yoo, S. M., Kim, H.-W., & Wee, C.-D. (2022). Changes in Isoflavone Profile from Soybean Seeds during Cheonggukjang Fermentation Based on High-Resolution UPLC-DAD-QToF/MS: New Succinylated and Phosphorylated Conjugates. Molecules, 27(13), 4120. https://doi.org/10.3390/molecules27134120






