Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway
Abstract
:1. Introduction
2. Results
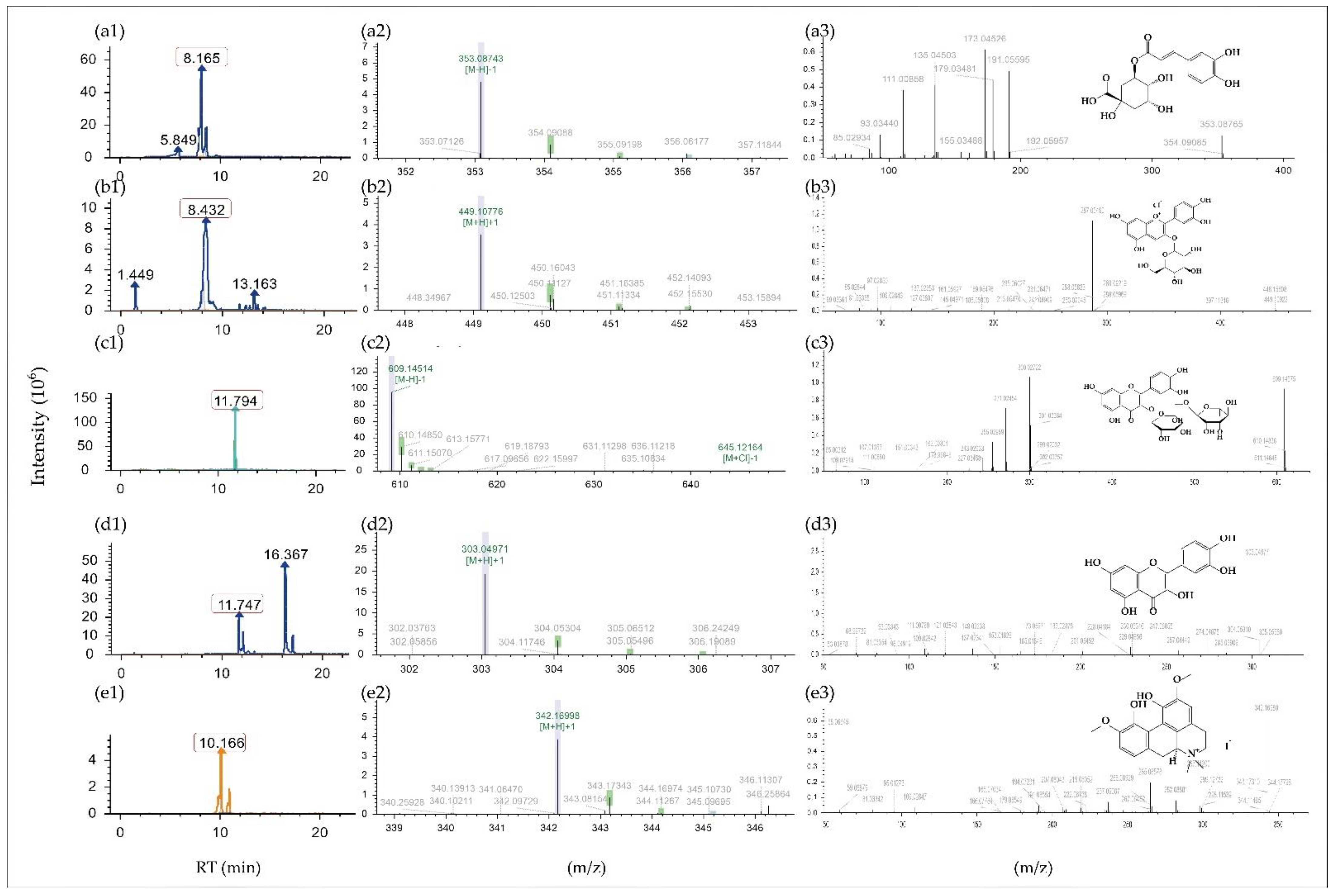
2.1. Composition Analysis and Identification of Phenolic Compounds in MBE
2.2. MBE and Rutin Reduced the Alcohol Damage to GES-1
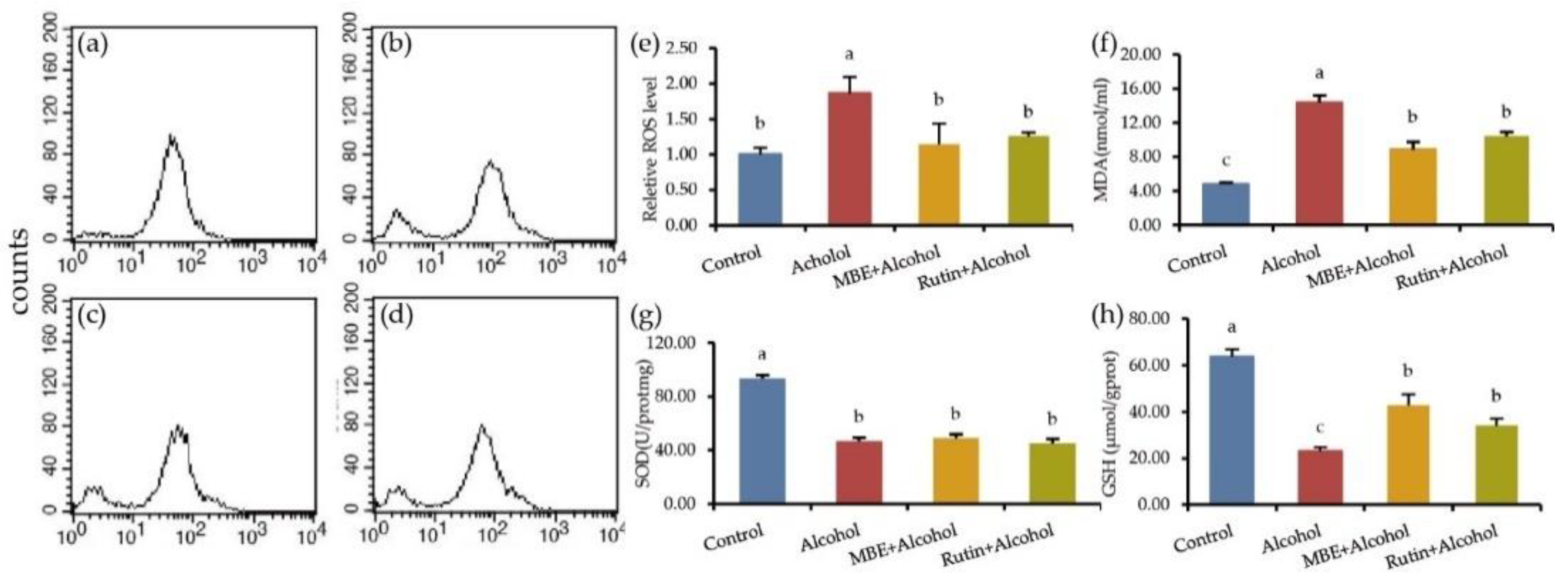
2.3. MBE and Rutin Can Improve the Antioxidant Capacity of GES-1 Cells
2.4. MBE and Rutin Can Reduce Alcohol-Damaged GES-1 Cell Apoptosis
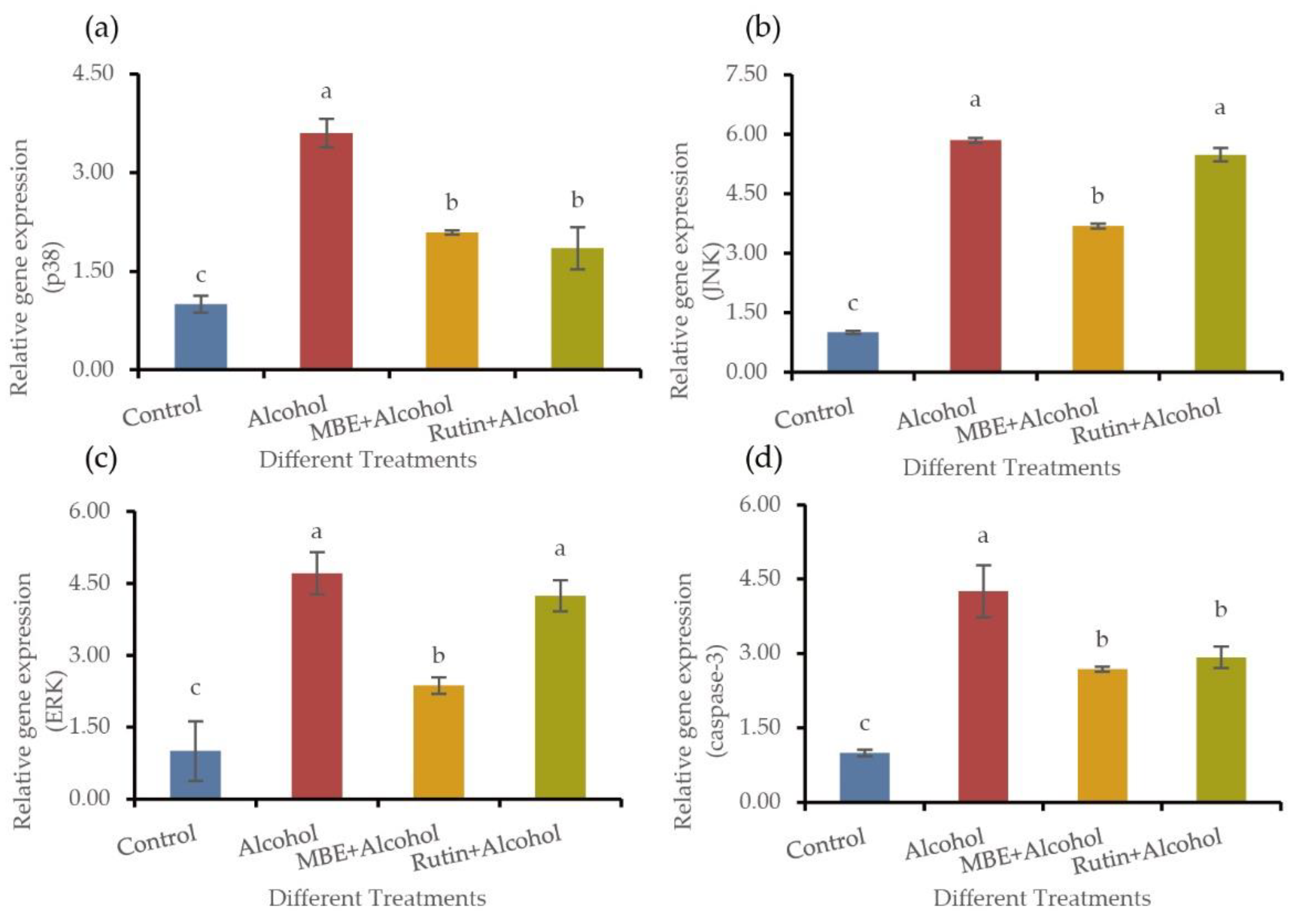
2.5. MBE and Rutin May Regulate the Antioxidant Capacity of GES-1 Cells by Regulating the Expression of MAPK Pathway Related Genes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Determination of Ethanol Extract from Mulberry by LC-MS/MS
4.3. Determination of Main Components of Mulberry Ethanol Extract
4.4. Cell Culture
4.5. MTT Assay Detected Cell Viability
4.6. Apoptosis Was Detected by the Annexin V Method
4.7. Determination of Reactive Oxygen Species (ROS)
4.8. Determination of SOD, MDA and GSH
4.8.1. Cell Protein Sample Collection
4.8.2. Determination of the Protein Concentration in the Sample
4.9. Quantitative Real-Time PCR (qPCR) Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Garaycoechea, J.I.; Crossan, G.P.; Langevin, F.; Mulderrig, L.; Louzada, S.; Yang, F.; Guilbaud, G.; Park, N.; Roerink, S.; Nik-Zainal, S.; et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature 2018, 553, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J. The protective activity of conyza blinii saponin against acute gastric ulcer induced by ethanol. J. Ethnopharmacol. 2014, 158 Pt A, 358–363. [Google Scholar] [CrossRef]
- Oates, P.J.; Hakkinen, J.P. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology 1988, 94, 10–21. [Google Scholar] [CrossRef]
- Balogun, S.O.; Damazo, A.S.; de Oliveira Martins, D.T. Helicteres sacarolha A. St.- Hil. et al.: Gastroprotective and possible mechanism of actions in experimental animals. J. Ethnopharmacol. 2015, 166, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.D.; Padmavathi, P.; Bulle, S.; Hebbani, A.V.; Marthadu, S.B.; Venugopalacharyulu, N.C.; Maturu, P.; Varadacharyulu, N.C. Association between alcohol-induced oxidative stress and membrane properties in synaptosomes: A protective role of vitamin E. Neurotoxicol. Teratol. 2017, 63, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, E.J.; Kim, S.H.; Lee, H.J. Gastroprotective effects of fermented lotus root against ethanol/hcl-induced gastric mucosal acute toxicity in rats. Nutrients 2020, 12, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, G.; Oliveira, A.P.; Noleto, I.; Araujo, A.K.; Lopes, A.L.F.; Sousa, F.B.M.; Chaves, L.S.; Alves, E.H.P.; Vasconcelos, D.F.P.; Araujo, A.R.; et al. Activation of transient receptor potential vanilloid channel 4 contributes to the development of ethanol-induced gastric injury in mice. Eur. J. Pharmacol. 2021, 902, 174113. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, T. Experimental production of peptic ulcer, gastric damage and cancer models and their use in pathophysiological studies and pharmacological treatment--polish achievements. J. Physiol. Pharmacol. 2003, 54 (Suppl. 3), 99–126. [Google Scholar]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Cho, E.; Chung, E.Y.; Jang, H.Y.; Hong, O.Y.; Chae, H.S.; Jeong, Y.J.; Kim, S.Y.; Kim, B.S.; Yoo, D.J.; Kim, J.S.; et al. Anti-cancer effect of cyanidin-3-glucoside from mulberry via caspase-3 cleavage and DNA fragmentation in vitro and in vivo. Anticancer Agents Med. Chem. 2017, 17, 1519–1525. [Google Scholar] [CrossRef]
- Radojkovic, M.; Moreira, M.M.; Soares, C.; Barroso, M.F.; Cvetanovic, A.; Svarc-Gajic, J.; Morais, S.; Delerue-Matos, C. Microwave-assisted extraction of phenolic compounds from morus nigra leaves: Optimization and characterization of the antioxidant activity and phenolic composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Jin, J.; Shi, L. Protective function of cis-mulberroside a and oxyresveratrol from ramulus mori against ethanol-induced hepatic damage. Environ. Toxicol. Pharmacol. 2008, 26, 325–330. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. Map kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arab, H.H.; Salama, S.A.; Eid, A.H.; Kabel, A.M.; Shahin, N.N. Targeting mapks, nf-kappab and pi3k/akt pathways by methyl palmitate ameliorates ethanol-induced gastric mucosal injury in rats. J. Cell Physiol. 2019, 234, 22424–22438. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, L.; Zhang, H.; Su, L.; Qin, L.P. Triggering of p38 mapk and jnk signaling is important for oleanolic acid-induced apoptosis via the mitochondrial death pathway in hypertrophic scar fibroblasts. Phytother. Res. 2014, 28, 1468–1478. [Google Scholar] [CrossRef]
- Duan, F.; Yu, Y.; Guan, R.; Xu, Z.; Liang, H.; Hong, L. Vitamin k2 induces mitochondria-related apoptosis in human bladder cancer cells via ros and jnk/p38 mapk signal pathways. PLoS ONE 2016, 11, e0161886. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, Y.M.; Jin, W.Y.; Sung, Y.Y.; Choi, G.; Kim, H.K. Antioxidant activities and polyphenol content of morus alba leaf extracts collected from varying regions. Biomed Rep. 2014, 2, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Punithavathi, V.R.; Shanmugapriya, K.; Prince, P.S. Protective effects of rutin on mitochondrial damage in isoproterenol-induced cardiotoxic rats: An in vivo and in vitro study. Cardiovasc. Toxicol. 2010, 10, 181–189. [Google Scholar] [CrossRef]
- Tasli, N.G.; Cimen, F.K.; Karakurt, Y.; Ucak, T.; Mammadov, R.; Suleyman, B.; Kurt, N.; Suleyman, H. Protective effects of rutin against methanol induced acute toxic optic neuropathy: An experimental study. Int. J. Ophthalmol. 2018, 11, 780–785. [Google Scholar]
- Luan, S.; Yun, X.; Rao, W.; Xiao, C.; Xu, Z.; Lang, J.; Huang, Q. Emamectin benzoate induces ros-mediated DNA damage and apoptosis in trichoplusia tn5b1-4 cells. Chem. Biol. Interact. 2017, 273, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Aredia, F.; Czaplinski, S.; Fulda, S.; Scovassi, A.I. Molecular features of the cytotoxicity of an nhe inhibitor: Evidence of mitochondrial alterations, ros overproduction and DNA damage. BMC Cancer 2016, 16, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.W.; Ko, W.M.; Park, J.H.; Seo, K.H.; Oh, E.J.; Lee, D.Y.; Lee, D.S.; Kim, Y.C.; Lim, D.W.; Han, D.; et al. Isoprenylated flavonoids from the root bark of morus alba and their hepatoprotective and neuroprotective activities. Arch. Pharm. Res. 2015, 38, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Tatsumi, M.; Ishimori, T.; Lilien, D.L.; Engles, J.M.; Wahl, R.L. Effect of nicotine and ephedrine on the accumulation of f-18-fdg in brown adipose tissue. J. Nucl. Med. 2007, 48, 981–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.L.; Yang, L.; Zheng, H.Y. Hypolipidemic and antioxidant effects of mulberry (morus alba l.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef]
- Chang, L.W.; Juang, L.J.; Wang, B.S.; Wang, M.Y.; Tai, H.M.; Hung, W.J.; Chen, Y.J.; Huang, M.H. Antioxidant and antityrosinase activity of mulberry (morus alba l.) twigs and root bark. Food Chem. Toxicol. 2011, 49, 785–790. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.M.; Zhang, J.; Zhang, Y.Q. An efficient preparation of mulberroside a from the branch bark of mulberry and its effect on the inhibition of tyrosinase activity. PLoS ONE 2014, 9, e109396. [Google Scholar] [CrossRef]
- Wan, L.; Chen, G.; Jian, S.; Yin, X.J.; Zhu, H. Antioxidant and xanthine oxidase inhibitory properties and lc-ms/ms identification of compoundsof ethanolic extract from mulberry leaves. Acta Sci. Pol. Technol. Aliment. 2018, 17, 313–319. [Google Scholar]
- Qin, C.G.; Li, Y.; Niu, W.N.; Ding, Y.; Zhang, R.J.; Shang, X.Y. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J. Food Sci. 2010, 28, 117–126. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Compos. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (morus alba l.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Kawvised, S.; Wattanathorn, J.; Thukham-Mee, W. Neuroprotective and cognitive-enhancing effects of microencapsulation of mulberry fruit extract in animal model of menopausal women with metabolic syndrome. Oxid. Med. Cell Longev. 2017, 2017, 2962316. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Bai, J.; Tian, S.; Ma, M.; Li, W.; Yin, Y.; Deng, R.; Cui, J.; Li, J.; Wang, G.; et al. Autophagy protects gastric mucosal epithelial cells from ethanol-induced oxidative damage via mtor signaling pathway. Exp. Biol Med. 2017, 242, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, A.Q.; Chen, M.H.; Gao, J.; Wang, L.; Yang, H.Y.; Li, L.; Zhang, B.; He, H.K.; Wang, S.J. A tri-o-bridged diels-alder adduct from cortex mori radicis. Molecules 2018, 23, 133. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Choi, E.J. Pathological roles of mapk signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Akanda, M.R.; Park, B.Y. Involvement of mapk/nf-kappab signal transduction pathways: Camellia japonica mitigates inflammation and gastric ulcer. Biomed. Pharmacother. 2017, 95, 1139–1146. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rexroad, P.R.; Cathey, R.D. Pollution-reduced kjeldahl method for crude protein. J. Assoc. Off. Anal. Chem. 1976, 59, 1213–1217. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, R.A.; Rexroad, P.R. Comparison of leco fp-228 “nitrogen determinator” with aoac copper catalyst kjeldahl method for crude protein. J. Assoc. Off. Anal. Chem. 1987, 70, 1028–1030. [Google Scholar] [CrossRef]
- Abul-Fadl, M.A. Colorimetric estimation of manganese by means of the folin-ciocalteu phenol reagent. Biochem. J. 1948, 42, xxxvii. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]


| Composition | Quantification (mg/g) |
|---|---|
| Carbohydratesugar | 65.9 ± 4.82 |
| Protein | 28 ± 1.69 |
| Fats | 94 ± 1.72 |
| Water | 84.3 ± 2.98 |
| Anthocyanins (cyanidin-3-glucoside) | 101.4 ± 8.14 |
| Phenols (gallic acid) | 308.6 ± 23.51 |
| Rutin | 19 ± 0.63 |
| Category | N | RT | Accurate Mass | Molecular Ion [M−H]−/[M + H]+ | Polarity | Molecular Formula | Putative ID | Fragment Ions [M−H]−/[M + H]+ | |
|---|---|---|---|---|---|---|---|---|---|
| Polyphenols | Flavones | 1 | 8.139 | 448.1006 | 449.1082 | + | C21H20O11 | Cynaroside | 287.05484 |
| 2 | 12.778 | 594.1585 | 593.1509 | − | C27H30O15 | Kaempferol-3-O-gulcorhamnoside | 285.03983/111.00861 | ||
| 3 | 12.778 | 594.1585 | 595.1653 | − | C27H30O15 | Lonicerin | 285.03983/111.00861 | ||
| 4 | 12.980 | 624.1690 | 623.1616 | − | C28H32O16 | Isorhamnetin-3-O-nehesperidine | 111.00859/314.04282 | ||
| 5 | 12.980 | 624.1690 | 623.1615 | − | C28H32O16 | Narcissoside | 111.00859/315.05096 | ||
| 6 | 13.339 | 448.1006 | 447.0930 | + | C21H20O11 | Kaempferol-7-O-β-D-glucopyranoside | 285.03970/447.09253 | ||
| 7 | 16.301 | 286.0477 | 285.0403 | − | C15H10O6 | Luteolin | 133.02939 | ||
| 8 | 18.111 | 270.0528 | 269.0457 | − | C15H10O5 | Apigenin | 269.04526 | ||
| 9 | 18.873 | 316.0583 | 315.0510 | − | C16H12O7 | Eupafolin | 300.02695 | ||
| 10 | 22.143 | 552.1057 | 551.0979 | − | C31H20O10 | Bilobetin | 519.07239/551.09796 | ||
| 11 | 22.810 | 254.0579 | 253.0504 | − | C15H10O4 | Chrysin | 63.02395/143.05013 | ||
| 12 | 26.021 | 566.1213 | 567.1284 | + | C32H22O10 | Isoginkgetin | 135.04411 | ||
| 13 | 26.021 | 566.1213 | 567.1284 | + | C32H22O10 | Ginkgetin | 135.04411/567.12842 | ||
| 14 | 30.097 | 580.1370 | 581.1437 | + | C33H24O10 | Sciadopitysin | 135.04413 | ||
| Flavonols | 15 | 11.747 | 302.0427 | 301.0352 | + | C15H10O7 | Quercetin | 151.00360/178.99858 | |
| 16 | 11.747 | 302.0427 | 301.0354 | + | C15H10O7 | Morin | 151.00363 | ||
| 17 | 11.794 | 610.1534 | 609.1467 | − | C27H30O16 | Rutin | 300.02722/271.02454 | ||
| 18 | 12.054 | 464.0955 | 463.0881 | − | C21H20O12 | Hyperoside | 301.03476 | ||
| 19 | 12.330 | 304.0583 | 303.0507 | − | C15H12O7 | Taxifolin | 125.02422/285.04004 | ||
| 20 | 12.778 | 594.1585 | 593.1509 | − | C27H30O15 | Kaempferol-3-O-rutinoside | 285.03983/111.00861 | ||
| 21 | 13.339 | 448.1006 | 447.0928 | − | C21H20O11 | Quercitrin | 300.02713/301.03433 | ||
| 22 | 13.339 | 448.1006 | 447.0928 | − | C21H20O11 | Quercetin 7-rhamnoside | 300.02713/301.03433 | ||
| 23 | 14.074 | 318.0376 | 317.0300 | − | C15H10O8 | Myricetin | 137.02414/151.00339 | ||
| 24 | 18.528 | 286.0477 | 285.0404 | − | C15H10O6 | Kaempferol | 285.04016 | ||
| 25 | 18.873 | 316.0583 | 315.0508 | − | C16H12O7 | Isorhamnetin | 300.02695 | ||
| Flavanols | 26 | 8.078 | 290.0790 | 289.0715 | − | C15H14O6 | Epicatechin | 80.96500 | |
| 27 | 8.078 | 290.0790 | 289.0717 | − | C15H14O6 | (+)−Catechin hydrate | 80.96500 | ||
| 28 | 8.078 | 290.0790 | 289.0717 | − | C15H14O6 | Cianidanol | 80.96500 | ||
| 29 | 12.054 | 464.0955 | 463.0880 | − | C21H20O12 | Isoquercitrin | 301.03476 | ||
| 30 | 15.867 | 256.0736 | 255.0657 | − | C15H12O4 | Liquiritigenin | 119.05003 | ||
| 31 | 16.139 | 288.0634 | 287.0558 | − | C15H12O6 | Eriodictyol | 135.04501/151.00346 | ||
| 32 | 28.617 | 324.1362 | 323.1284 | − | C20H20O4 | Isobavachin | 119.05003 | ||
| Isoflavones | 33 | 16.425 | 284.0685 | 285.0756 | + | C16H12O5 | Calycosin | 114.96126/225.05447 | |
| Anthocyanines | 34 | 6.941 | 340.0794 | 339.0717 | − | C15H16O9 | Esculin | 177.0191 | |
| 35 | 8.139 | 448.1006 | 449.1078 | + | C21H20O11 | Cyanidin-3-O-glucoside chloride | 287.05484 | ||
| Anthocyanidins | 36 | 11.707 | 192.0423 | 193.0495 | + | C10H8O4 | Isoscopoletin | 133.02841/178.02605 | |
| 37 | 11.707 | 192.0423 | 193.0496 | + | C10H8O4 | Scopoletin | 133.02841/178.02605 | ||
| Chalcones | 38 | 14.605 | 436.1370 | 435.1290 | − | C21H24O10 | Phloridzin | 167.03477/273.07651 | |
| 39 | 14.605 | 436.1370 | 435.1290 | − | C21H24O10 | Trilobatin | 167.03477/273.07651 | ||
| 40 | 18.154 | 272.0685 | 271.0612 | − | C15H12O5 | Naringenin Chalcone | 119.05000/151.00345 | ||
| 41 | 18.154 | 272.0685 | 271.0611 | − | C15H12O5 | Naringenin Chalcone | 119.05000/151.00345 | ||
| 42 | 28.617 | 324.1362 | 323.1284 | − | C20H20O4 | Isobavachalcone | 119.05006 | ||
| Polyphenols | Resveratrol | 43 | 8.139 | 448.1006 | 449.1078 | + | C21H20O11 | Astragalin | 287.05484 |
| 44 | 17.757 | 228.0786 | 229.0856 | + | C14H12O3 | Resveratrol | 107.04910/135.04399/183.08055 | ||
| Terpenoids | 45 | 12.054 | 326.1002 | 325.0928 | − | C15H18O8 | Bilobalide | 163.11269 | |
| 46 | 12.720 | 440.1319 | 439.1242 | − | C20H24O11 | Ginkgolide C | 125.02421/38313443 | ||
| 47 | 16.025 | 408.1420 | 409.1483 | + | C20H24O9 | Ginkgolide A | 345.13315 | ||
| 48 | 16.150 | 424.1370 | 423.1294 | − | C20H24O10 | Ginkgolide B | 113.02425/367.13931 | ||
| 49 | 17.481 | 448.2309 | 493.2287 | − | C21H36O10 | Atractyloside A | 59.01380/447.22342 | ||
| 50 | 20.238 | 406.1264 | 405.1191 | − | C20H22O9 | Ginkgolide K | 72.99297 | ||
| 51 | 22.993 | 244.1099 | 245.1171 | + | C15H16O3 | Linderalactone | 199.11176 | ||
| 52 | 30.420 | 384.2301 | 385.2364 | + | C24H32O4 | Resibufogenin | 109.02851/275.20062 | ||
| 53 | 30.568 | 472.3553 | 471.3477 | − | C30H48O4 | Echinocystic acid | 407.33185/471.34732 | ||
| 54 | 30.691 | 470.3396 | 471.3471 | + | C30H46O4 | 18 β-Glycyrrhetintic acid | 189.16389/235.16933/317.21124 | ||
| 55 | 31.275 | 454.3447 | 455.3516 | + | C30H46O3 | Ursolic acid | 205.15874 | ||
| 56 | 31.320 | 302.2246 | 303.2316 | + | C20H30O2 | Abietic acid | 257.22626 | ||
| 57 | 32.329 | 424.3705 | 425.3775 | + | C30H48O | Lupenone | 95.08546/109.10110 | ||
| 58 | 33.739 | 472.3553 | 473.3625 | + | C30H48O4 | Maslinic acid | 203.17943/409.34622/427.35706 | ||
| 59 | 37.369 | 454.3447 | 455.3519 | + | C30H46O3 | Wilforlide A | 205.15874 | ||
| 60 | 37.369 | 234.1620 | 235.1691 | + | C15H22O2 | Curcumenol | 189.16383/217.15863 | ||
| 61 | 37.369 | 234.1620 | 235.1694 | + | C15H22O2 | Artemisinic acid | 189.16383/217.15884 | ||
| 62 | 37.369 | 454.3447 | 455.3520 | + | C30H46O3 | Oleanonic acid | 205.15874 | ||
| 63 | 37.369 | 454.3447 | 455.3502 | + | C30H46O3 | Liquidambaric acid | 205.15874 | ||
| Polyphenols | 64 | 37.369 | 454.3447 | 455.3515 | + | C30H46O3 | β-Elemonic acid | 205.15874 | |
| 65 | 37.369 | 456.3603 | 457.3668 | + | C30H48O3 | Ursolic acid | 411.362 | ||
| 66 | 41.687 | 440.3654 | 441.3733 | + | C30H48O2 | Roburic acid | 95.08572/109.10140 | ||
| Phenolic acid | 67 | 1.368 | 192.0634 | 191.0561 | − | C7H12O6 | Quinic acid | 85.02938 | |
| 68 | 4.549 | 154.0266 | 153.0193 | − | C7H6O4 | Protocatechuic acid | 109.02932 | ||
| 69 | 4.549 | 154.0266 | 153.0192 | − | C7H6O4 | Gentisic acid | 109.02932 | ||
| 70 | 8.457 | 180.0423 | 181.0491 | + | C9H8O4 | Caffeic acid | 163.03886 | ||
| 71 | 8.475 | 354.0951 | 353.0876 | − | C16H18O9 | Cryptochlorogenic acid | 173.04517/191.05585 | ||
| 72 | 8.475 | 354.0951 | 353.0879 | − | C16H18O9 | Chlorogenic acid | 173.04517/191.05585 | ||
| 73 | 8.475 | 354.0951 | 353.0878 | − | C16H18O9 | 1-Caffeoylquinic acid | 173.04517/191.05585 | ||
| 74 | 13.916 | 516.1268 | 515.1193 | − | C25H24O12 | 1,3-Dicaffeoylquinic acid | 173.04526/353.08749 | ||
| 75 | 13.916 | 516.1268 | 515.1192 | − | C25H24O12 | Isochlorogenic acid C | 173.04526/353.08749 | ||
| 76 | 13.916 | 516.1268 | 515.1187 | − | C25H24O12 | Isochlorogenic acid B | 173.04526/353.08749 | ||
| 77 | 13.916 | 516.1268 | 515.1186 | − | C25H24O12 | Isochlorogenic acid A | 173.04526/353.08749 | ||
| 78 | 15.867 | 256.0736 | 255.0657 | − | C15H12O4 | Isoliquiritigenin | 119.05003 | ||
| 79 | 17.266 | 208.0736 | 207.0664 | − | C11H12O4 | Ethyl Caffeate | 135.04500/207.06589 | ||
| 80 | 43.102 | 320.2351 | 319.2277 | − | C20H32O3 | Ginkgolic Acid (C13:0) | 275.23758 | ||
| 81 | 43.546 | 346.2508 | 345.2430 | − | C22H34O3 | Ginkgolic Acid C15:1 | 301.25305 | ||
| 82 | 46.176 | 374.2821 | 373.2745 | − | C24H38O3 | Ginkgolic acid C17-1 | 329.28458 | ||
| Other Phenols | 83 | 4.237 | 182.0579 | 183.0653 | + | C9H10O4 | 3,5-Dimethoxy-4-hydroxybenzaldehyde | 81.03349/123.04411 | |
| 84 | 6.970 | 138.0317 | 137.0242 | − | C7H6O3 | Protocatechualdehyde | 137.02423 | ||
| 85 | 9.459 | 492.1268 | 493.1342 | + | C23H24O12 | Aurantio-obtusin β-D-glucoside | 331.08093 | ||
| 86 | 16.425 | 284.0685 | 285.0756 | + | C16H12O5 | Emodin-3-methyl ether/Physcion | 114.96126/270.05191 | ||
| 87 | 38.380 | 178.0630 | 179.0702 | + | C10H10O3 | Ferulaldehyde | 147.04407/161.05971 | ||
| Non- polyphenols | Amino acid | 88 | 1.239 | 176.0432 | 174.9559 | − | C5H8N2O5 | 3-[(Carboxycarbonyl) amino]-L-alanine | 118.96609/146.96106 |
| 89 | 1.702 | 115.0633 | 116.0707 | + | C5H9NO2 | L-Proline | 70.0652 | ||
| 90 | 1.893 | 181.0738 | 182.0812 | + | C9H11NO3 | L-Tyrosine | 91.04922/119.04924/136.07571 | ||
| 91 | 2.198 | 131.0946 | 132.1021 | + | C6H13NO2 | L-Leucine | 86.09642 | ||
| 92 | 3.123 | 165.0789 | 166.0862 | + | C9H11NO2 | L-Phenylalanine | 130.08076 | ||
| carbohydrate | 93 | 1.358 | 342.1162 | 387.1141 | − | C12H22O11 | Lactose | 89.02428/179.05602 | |
| 94 | 8.647 | 518.1636 | 517.1561 | − | C22H30O14 | Sibiricose A5 | 175.03986 | ||
| Alkaloids | 95 | 1.385 | 137.0476 | 138.0551 | + | C7H7NO2 | Trigonelline HCl | 94.06523 | |
| 96 | 10.152 | 341.1627 | 342.1701 | + | C20H23NO4 | (+)-Magnoflorine | 58.06545/342.16980 | ||
| 97 | 16.940 | 365.1627 | 366.1699 | + | C22H23NO4 | Dehydrocorydaline | 59.04945/32214359 | ||
| Other Compounds | 98 | 1.312 | 182.0790 | 181.0721 | − | C6H14O6 | Mannitol | 71.01373/101.02425 | |
| 99 | 1.895 | 192.0270 | 191.0197 | − | C6H8O7 | Citric acid | 111.00856 | ||
| 100 | 2.996 | 126.0317 | 127.0389 | + | C6H6O3 | 5-hydroxymethyl furfural | 109.02843 | ||
| 101 | 7.638 | 376.1370 | 375.1294 | − | C16H24O10 | Loganic acid | 213.07661 | ||
| 102 | 7.652 | 460.1217 | 459.1143 | − | C19H24O13 | Parishin E | 111.00859 | ||
| 103 | 16.087 | 264.1362 | 263.1286 | − | C15H20O4 | Abscisic acid | 204.13876/219.13876 | ||
| 104 | 17.757 | 228.0786 | 229.0858 | + | C14H12O3 | Demethoxyyangonin | 81.03352/183.08055/211.07530 | ||
| 105 | 25.065 | 278.2246 | 279.2318 | + | C18H30O2 | α-Linolenic acid | 149.02338 | ||
| 106 | 26.121 | 486.3345 | 487.3416 | + | C30H46O5 | Quillaic acid | 187.1481 | ||
| 107 | 28.617 | 324.1362 | 323.1284 | − | C20H20O4 | Bavachin A | 119.05013 | ||
| 108 | 35.288 | 460.3916 | 461.3990 | + | C30H52O3 | 20(R)-Protopanaxadiol | 119.08558 | ||
| GENE | PRIMER (5′-3′) | SOURCE |
|---|---|---|
| β-actin | F: CTCCATCCTGGCCTCGCTGT R: GCTGTCACCTTCACCGTTCC | Sangon Biotech |
| caspase-3 | F: GTGAGGCGGTTGTAGAAGAGTT R: CTCACGGCCTGGGATTTCAA | Sangon Biotech |
| p38 | F: CCCACCCATATCTGGAGCAG R: GCCCTTGTCCTGACAAATTTAAGA | Sangon Biotech |
| JNK | F: CTGAAGCAGAAGCTCCACCA R: GCTGCCCCCGTATAACTCC | Sangon Biotech |
| ERK | F: TCAGACTCCAAAGCCCTTGAC R: TCAGCCGCTCCTTAGGTAGG | Sangon Biotech |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-Y.; Liang, J.; Ai, J.-Y.; Cui, J.-L.; Huang, W.-D.; You, Y.-L.; Zhan, J.-C. Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules 2022, 27, 4266. https://doi.org/10.3390/molecules27134266
Wu T-Y, Liang J, Ai J-Y, Cui J-L, Huang W-D, You Y-L, Zhan J-C. Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules. 2022; 27(13):4266. https://doi.org/10.3390/molecules27134266
Chicago/Turabian StyleWu, Tian-Yang, Juan Liang, Jing-Ya Ai, Jing-Long Cui, Wei-Dong Huang, Yi-Lin You, and Ji-Cheng Zhan. 2022. "Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway" Molecules 27, no. 13: 4266. https://doi.org/10.3390/molecules27134266
APA StyleWu, T.-Y., Liang, J., Ai, J.-Y., Cui, J.-L., Huang, W.-D., You, Y.-L., & Zhan, J.-C. (2022). Mulberry Ethanol Extract and Rutin Protect Alcohol-Damaged GES-1 Cells by Inhibiting the MAPK Pathway. Molecules, 27(13), 4266. https://doi.org/10.3390/molecules27134266









