LncRNA MALAT1 Participates in Protection of High-Molecular-Weight Hyaluronan against Smoke-Induced Acute Lung Injury by Upregulation of SOCS-1
Abstract
:1. Introduction
2. Results
2.1. Intratracheal Nebulization with HA1600 Alleviated Smoke-Induced Acute Lung Injury
2.2. HA1600 Inhibited Smoke-Induced Pulmonary Inflammation
2.3. Intratracheal Nebulization with HA1600 Increased MALAT1 Expression in Smoke-Induced ALI
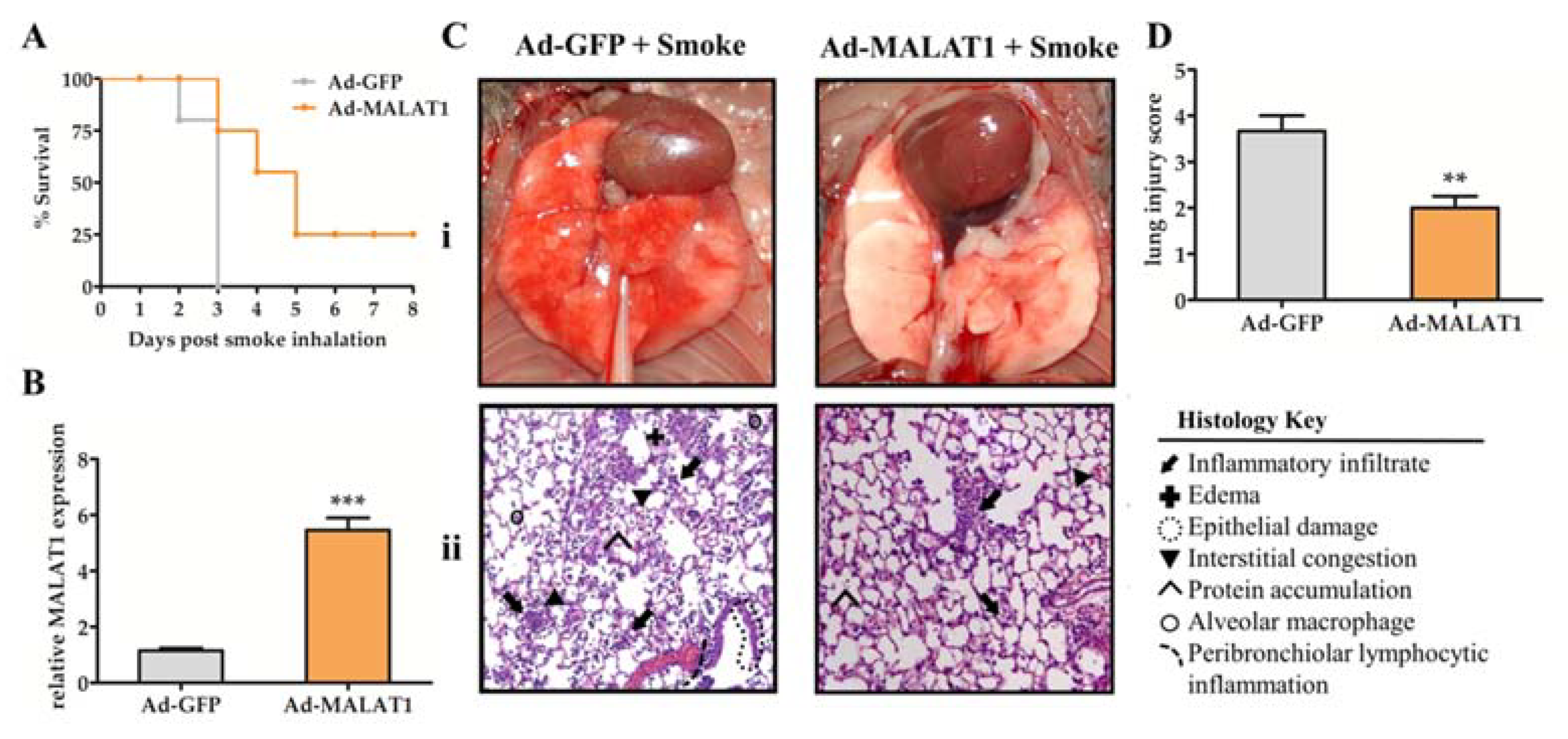
2.4. Overexpression of MALAT1 in Lung Tissue Prolonged Animal Survival and Alleviated Lung Damage after Smoke Exposure
2.5. Overexpression of MALAT1 Increases the Level of SOCS-1 after Smoke Exposure
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pretreatment with HA1600 and Smoke Inhalation
4.3. Bronchoalveolar Lavage Fluid (BALF) and Cell Coounts
4.4. Adenoviral Vectors
4.5. Isolation of RNA and Quantitative Real-Time RT-PCR
4.6. Lung Histology
4.7. Lung Injury Score
4.8. Enzyme-Linked Immunosorbent Assay (ELISA) and Bicinchoninic Acid (BCA) Assay
4.9. Western Blot Analysis
4.10. Statistical Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peck, M.; Molnar, J.; Swart, D. A global plan for burn prevention and care. Bull. World Health Organ. 2009, 87, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Saffle, J.R.; Davis, B.; Williams, P. Recent outcomes in the treatment of burn injury in the United States: A report from the American Burn Association Patient Registry. J. Burn Care Rehabil. 1995, 16, 219–289. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.M.; Schneider-Futschik, E.K.; Knibbs, L.D.; Irving, L.B. Health impacts of bushfire smoke exposure in Australia. Respirology 2020, 25, 495–501. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Jalaludin, B.B.; Morgan, G.G.; Hanigan, I.C.; Johnston, F.H. Bushfire smoke: Urgent need for a national health protection strategy. Med. J. Aust. 2020, 212, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, L.A.; White, A.R. The potential impact of bushfire smoke on brain health. Neurochem. Int. 2020, 139, 104796. [Google Scholar] [CrossRef]
- Enkhbaatar, P.; Pruitt, B.A., Jr.; Suman, O.; Mlcak, R.; Wolf, S.E.; Sakurai, H.; Herndon, D.N. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. Lancet 2016, 388, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Bai, Y.; Ma, Y.; Liu, C.; Wang, S.; Zhao, R.; Dong, J.; Ji, H.-L. Preclinical and clinical studies of smoke-inhalation-induced acute lung injury: Update on both pathogenesis and innovative therapy. Ther. Adv. Respir. Dis. 2019, 13, 1753466619847901. [Google Scholar] [CrossRef] [Green Version]
- Putensen, C.; Theuerkauf, N.; Zinserling, J.; Wrigge, H.; Pelosi, P. Meta-analysis: Ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann. Intern. Med. 2009, 151, 566–576. [Google Scholar] [CrossRef]
- Goligher, E.C.; Costa, E.L.V.; Yarnell, C.J.; Brochard, L.J.; Stewart, T.E.; Tomlinson, G.; Brower, R.G.; Slutsky, A.S.; Amato, M.P.B. Effect of Lowering Vt on Mortality in Acute Respiratory Distress Syndrome Varies with Respiratory System Elastance. Am. J. Respir. Crit. Care Med. 2021, 203, 1378–1385. [Google Scholar] [CrossRef]
- Miller, A.C.; Ferrada, P.A.; Kadri, S.S.; Nataraj-Bhandari, K.; Vahedian-Azimi, A.; Quraishi, S.A. High-Frequency Ventilation Modalities as Salvage Therapy for Smoke Inhalation-Associated Acute Lung Injury: A Systematic Review. J. Intensive Care Med. 2018, 33, 335–345. [Google Scholar] [CrossRef]
- Reper, P.; Heijmans, W. High-frequency percussive ventilation and initial biomarker levels of lung injury in patients with minor burns after smoke inhalation injury. Burns 2015, 41, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Ferguson, N.D.; Brochard, L.; Fan, E.; Mercat, A.; Combes, A.; Pellegrino, V.; Schmidt, M.; Slutsky, A.S.; Brodie, D. ECMO for ARDS: From salvage to standard of care? Lancet Respir. Med. 2019, 7, 108–110. [Google Scholar] [CrossRef]
- Miranda, J.J.; Bernabe-Ortiz, A.; Diez-Canseco, F.; Malaga, G.; Cardenas, M.K.; Carrillo-Larco, R.M.; Lazo-Porras, M.; Moscoso-Porras, M.; Pesantes, M.A.; Ponce, V.; et al. Towards sustainable partnerships in global health: The case of the CRONICAS Centre of Excellence in Chronic Diseases in Peru. Global Health 2016, 12, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, D.F.; Cross, L.M.; Hamid, U.; Gardner, E.; Elborn, J.S.; Cullen, K.M.; Dushianthan, A.; Grocott, M.P.; Matthay, M.A.; O’Kane, C.M. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir. Med. 2017, 5, 484–491. [Google Scholar] [CrossRef] [Green Version]
- National Heart, L.; Blood Institute, A.C.T.N.; Truwit, J.D.; Bernard, G.R.; Steingrub, J.; Matthay, M.A.; Liu, K.D.; Albertson, T.E.; Brower, R.G.; Shanholtz, C.; et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014, 370, 2191–2200. [Google Scholar] [CrossRef] [Green Version]
- McAuley, D.F.; Laffey, J.G.; O’Kane, C.M.; Perkins, G.D.; Mullan, B.; Trinder, T.J.; Johnston, P.; Hopkins, P.A.; Johnston, A.J.; McDowell, C.; et al. Simvastatin in the acute respiratory distress synfrome. N. Engl. J. Med. 2014, 371, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.R.; Pritchard, M.W.; Thomas, C.M.; Smith, A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2019, 7, CD004477. [Google Scholar] [CrossRef]
- Garantziotis, S.; Matalon, S. Sugarcoating Lung Injury: A Novel Role for High-Molecular-Weight Hyaluronan in Pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, 1197–1198. [Google Scholar] [CrossRef]
- Huang, P.M.; Syrkina, O.; Yu, L.; Dedaj, R.; Zhao, H.; Shiedlin, A.; Liu, Y.Y.; Garg, H.; Quinn, D.A.; Hales, C.A. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology 2010, 15, 1131–1139. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lee, C.H.; Dedaj, R.; Zhao, H.; Mrabat, H.; Sheidlin, A.; Syrkina, O.; Huang, P.M.; Garg, H.G.; Hales, C.A.; et al. High-molecular-weight hyaluronan--a possible new treatment for sepsis-induced lung injury: A preclinical study in mechanically ventilated rats. Crit. Care 2008, 12, R102. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Shi, Q.; Zhang, L.; Zhao, H. High molecular weight hyaluronan attenuates fine particulate matter-induced acute lung injury through inhibition of ROS-ASK1-p38/JNK-mediated epithelial apoptosis. Environ. Toxicol. Pharmacol. 2018, 59, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhao, L.; Xu, C.; Zhang, L.; Zhao, H. High Molecular Weight Hyaluronan Suppresses Macrophage M1 Polarization and Enhances IL-10 Production in PM2.5-Induced Lung Inflammation. Molecules 2019, 24, 1776. [Google Scholar] [CrossRef] [Green Version]
- Matsui, M.; Corey, D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttenhofer, A.; Schattner, P.; Polacek, N. Non-coding RNAs: Hope or hype? Trends Genet. 2005, 21, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Jae, N.; Holdt, L.; Dimmeler, S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J. Am. Coll. Cardiol. 2016, 67, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Hämmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Puthanveetil, P.; Chen, S.; Feng, B.; Gautam, A.; Chakrabarti, S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J. Cell Mol. Med. 2015, 19, 1418–1425. [Google Scholar] [CrossRef]
- Zhou, H.J.; Wang, L.Q.; Wang, D.B.; Yu, J.B.; Zhu, Y.; Xu, Q.S.; Zheng, X.J.; Zhan, R.Y. Long noncoding RNA MALAT1 contributes to inflammatory response of microglia following spinal cord injury via the modulation of a miR-199b/IKKbeta/NF-kappaB signaling pathway. Am. J. Physiol. Cell Physiol. 2018, 315, C52–C61. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Q.; Wu, Y.; Hu, F.; Gu, L.; Chen, T.; Wang, W. lncRNA Malat1 modulates the maturation process, cytokine secretion and apoptosis in airway epithelial cell-conditioned dendritic cells. Exp. Ther. Med. 2018, 16, 3951–3958. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shi, H.; Ma, N.; Zi, P.; Liu, Q.; Sun, R. BML-111 alleviates acute lung injury through regulating the expression of lncRNA MALAT1. Arch. Biochem. Biophys. 2018, 649, 15–21. [Google Scholar] [CrossRef]
- Nan, C.-C.; Zhang, N.; Cheung, K.C.P.; Zhang, H.-D.; Li, W.; Hong, C.-Y.; Chen, H.-S.; Liu, X.-Y.; Li, N.; Cheng, L. Knockdown of lncRNA MALAT1 Alleviates LPS-Induced Acute Lung Injury via Inhibiting Apoptosis Through the miR-194-5p/FOXP2 Axis. Front. Cell Dev. Biol. 2020, 8, 586869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hamblin, M.H.; Yin, K.-J. The long noncoding RNA Malat1: Its physiological and pathophysiological functions. RNA Biol. 2017, 14, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Severgnini, M.; Takahashi, S.; Tu, P.; Perides, G.; Homer, R.J.; Jhung, J.W.; Bhavsar, D.; Cochran, B.H.; Simon, A.R. Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2005, 171, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Galam, L.; Parthasarathy, P.T.; Cho, Y.; Cho, S.H.; Lee, Y.C.; Lockey, R.F.; Kolliputi, N. Adenovirus-mediated transfer of the SOCS-1 gene to mouse lung confers protection against hyperoxic acute lung injury. Free Radic. Biol. Med. 2015, 84, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xu, C.; Ma, Y.; Zhu, K.; Chen, X.; Shi, Q.; Su, W.; Zhao, H. SOCS-1 ameliorates smoke inhalation-induced acute lung injury through inhibition of ASK-1 activity and DISC formation. Clin. Immunol. 2018, 191, 94–99. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Chen, X.; Shi, Q.; Su, W.; Zhao, H. SOCS-1 Suppresses Inflammation Through Inhibition of NALP3 Inflammasome Formation in Smoke Inhalation-Induced Acute Lung Injury. Inflammation 2018, 41, 1557–1567. [Google Scholar] [CrossRef]
- Zhao, G.; Su, Z.Y.; Song, D.; Mao, Y.M.; Mao, X.H. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-kappa B. FEBS Lett. 2016, 590, 2884–2895. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Qiu, J.; Qiu, G.; Chen, Y.; Song, Z.; Li, J.; Gong, X. Long non-coding RNA MALAT1 protects preterm infants with bronchopulmonary dysplasia by inhibiting cell apoptosis. BMC Pulm. Med. 2017, 17, 199. [Google Scholar] [CrossRef] [Green Version]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. CurR Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zornig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef]
- Cremer, S.; Michalik, K.M.; Fischer, A.; Pfisterer, L.; Jae, N.; Winter, C.; Boon, R.A.; Muhly-Reinholz, M.; John, D.; Uchida, S.; et al. Hematopoietic Deficiency of the Long Noncoding RNA MALAT1 Promotes Atherosclerosis and Plaque Inflammation. Circulation 2019, 139, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Zeng, X.Y.; Jiang, S.L.; Tan, H.Y.; Yan, M.Y.; Yang, H.Z. Long non-coding RNA MALAT1 sponges miR-149 to promote inflammatory responses of LPS-induced acute lung injury by targeting MyD88. Cell Biol. Int. 2019, 42, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Zhong, L.; Xiao, Z.; Yang, M.; Chen, M.; Wang, C.; Xie, X.; Chen, X. The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs by long non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Y.; Zhang, L.; Zhao, H. MicroRNA-155 Participates in Smoke-Inhalation-Induced Acute Lung Injury through Inhibition of SOCS-1. Molecules 2020, 25, 1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazzam-Jazi, M.; Lanjanian, H.; Maleknia, S.; Hedayati, M.; Daneshpour, M.S. Interplay between SARS-CoV-2 and human long non-coding RNAs. J. Cell Mol. Med. 2021, 25, 5823–5827. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Shaath, H.; Alajez, N.M. Protein Coding and Long Noncoding RNA (lncRNA) Transcriptional Landscape in SARS-CoV-2 Infected Bronchial Epithelial Cells Highlight a Role for Interferon and Inflammatory Response. Genes 2020, 11, 760. [Google Scholar] [CrossRef]
- Leist, S.R.; Dinnon, K.H.; Schäfer, A.; Tse, L.V.; Okuda, K.; Hou, Y.J.; West, A.; Edwards, C.E.; Sanders, W.; Fritch, E.J.; et al. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 2020, 183, 1070–1085. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Enkhbaatar, P.; Sousse, L.E.; Sakurai, H.; Rehberg, S.W.; Asmussen, S.; Kraft, E.R.; Wright, C.L.; Bartha, E.; Cox, R.A.; et al. Nebulization with γ-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock 2012, 37, 408–414. [Google Scholar] [CrossRef] [Green Version]




| Tissue | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Thickening of the alveolar wall | Normal | Thickening in less than 1/3 area | Thickening in 1/3 to 2/3 area | Thickening in more than 2/3 area |
| Lung hemorrhage | Normal | 1–5 alveoli exist at least 5 erythrocytes | 5–10 alveoli exist at least 5 erythrocytes | More than 10 alveoli exist at least 5 erythrocytes |
| Interstitial fibroblasts | No fibroblasts | Fibrin was observed in less than 1/3 area | Fibrin was observed in 1/3 to 2/3 area | Fibrin was observed in more than 2/3 area |
| Inflammatory infiltration | Infiltrate in less than 5 cells | Infiltrate in 5–10 cells | Infiltrate in 5–10 cells | Infiltrate in more than 20 cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, B.; Lang, K.; Gong, Y.; Cheng, X.; Deng, S.; Shi, Q.; Zhao, H. LncRNA MALAT1 Participates in Protection of High-Molecular-Weight Hyaluronan against Smoke-Induced Acute Lung Injury by Upregulation of SOCS-1. Molecules 2022, 27, 4128. https://doi.org/10.3390/molecules27134128
Li S, Li B, Lang K, Gong Y, Cheng X, Deng S, Shi Q, Zhao H. LncRNA MALAT1 Participates in Protection of High-Molecular-Weight Hyaluronan against Smoke-Induced Acute Lung Injury by Upregulation of SOCS-1. Molecules. 2022; 27(13):4128. https://doi.org/10.3390/molecules27134128
Chicago/Turabian StyleLi, Shaoguang, Bin Li, Ke Lang, Yubei Gong, Xiang Cheng, Shufen Deng, Qiwen Shi, and Hang Zhao. 2022. "LncRNA MALAT1 Participates in Protection of High-Molecular-Weight Hyaluronan against Smoke-Induced Acute Lung Injury by Upregulation of SOCS-1" Molecules 27, no. 13: 4128. https://doi.org/10.3390/molecules27134128






