Choline Chloride/Urea Deep Eutectic Solvents: A Promising Reaction Medium for the Synthesis of Bio-Based Poly(hydroxyurethane)s
Abstract
:1. Introduction
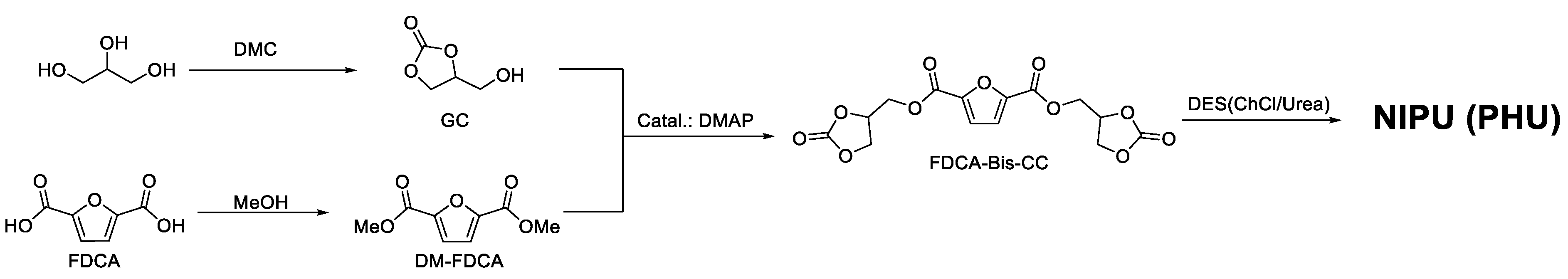
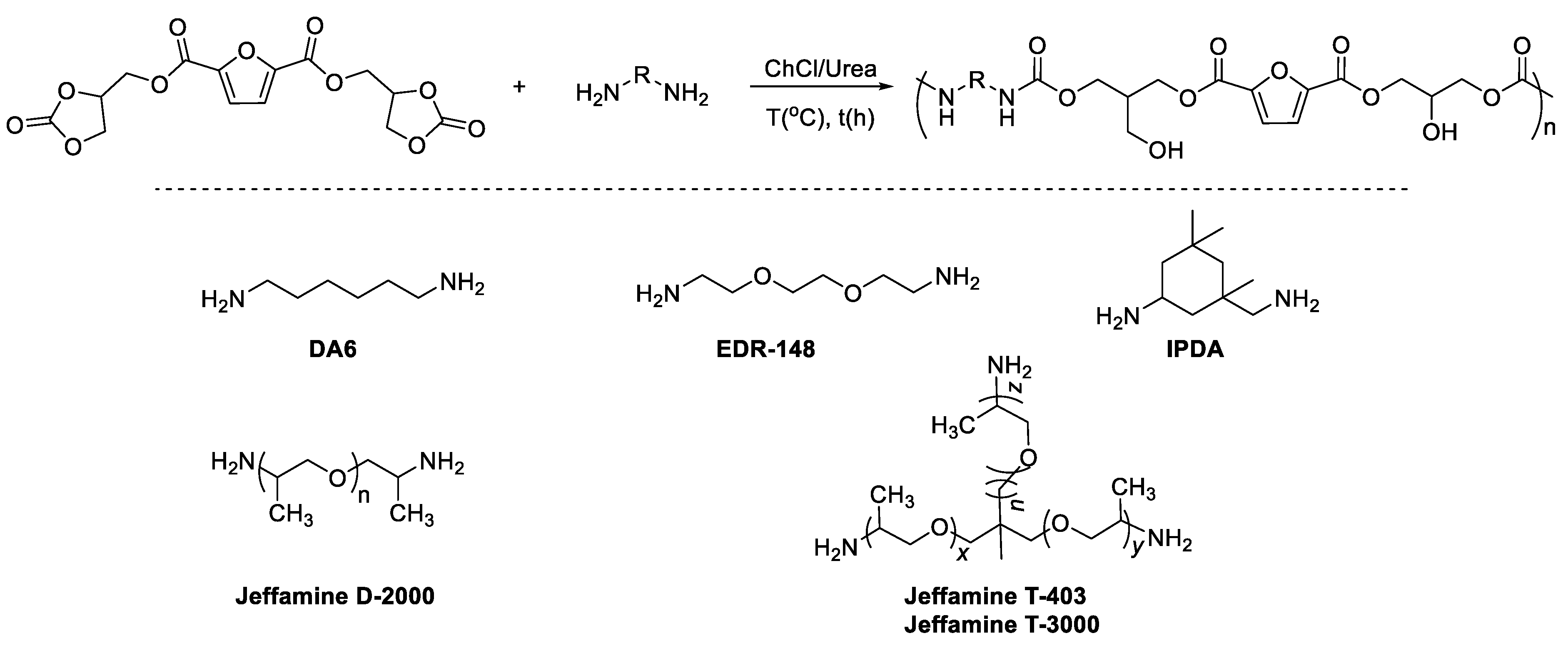
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Material
4.2. Synthesis of GC
4.3. Synthesis of Dimethyl Furan-2,5-dicarboxylate (DM-FDCA)
4.4. Synthesis of Furan-2,5-dicarboxylate Bis Cyclic Carbonate (FDCA-Bis-CC)
4.5. Synthesis of the DESs
4.6. ChCl/Urea
4.7. PHURs Syntheses
4.8. PHURs Synthesized from FDCA-Bis-CC and EDR-148 at 110 °C in ChCl/Urea
4.9. PHURs Synthesized from FDCA-Bis-CC and DA6 in CHCl/Urea
4.10. PHURs Synthesized from FDCA-Bis-CC and IPDA at 110 °C in ChCl/Urea
4.11. PHURs Synthesized from FDCA-Bis-CC and Jeffamine D-2000 in CHCl/Urea
4.12. PHURs Synthesized from FDCA-Bis-CC and Jeffamine T-403 in CHCl/Urea
4.13. PHURs Synthesized from FDCA-Bis-CC and Jeffamine T-3000 in CHCl/Urea
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bayer, O. Das Di-Isocyanat-Polyadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Shen, L.; Haufe, J.; Patel, M.K. Report of Utrecht University Commissioned by European Polysaccharide Network of Excellence and European Bioplastics; Utrecht University: Utrecht, The Netherlands, 2009. [Google Scholar]
- Singh, H.; Jain, A.K. Ignition, Combustion, Toxicity, and Fire Retardancy of Polyurethane Foams: A Comprehensive Review. J. Appl. Polym. Sci. 2009, 111, 1115–1143. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin Exposure to Isocyanates: Reasons for Concern. Environ. Health Perspect. 2007, 115, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Kreye, O.; Mutlu, H.; Meier, M.A.R. Sustainable Routes to Polyurethane Precursors. Green Chem. 2013, 15, 1431–1455. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-Isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439. [Google Scholar] [CrossRef] [Green Version]
- de Caro, P.; Bandres, M.; Urrutigoïty, M.; Cecutti, C.; Thiebaud-Roux, S. Recent Progress in Synthesis of Glycerol Carbonate and Evaluation of Its Plasticizing Properties. Front. Chem. 2019, 7, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Fujita, S.I.; Arai, M. Development in the Green Synthesis of Cyclic Carbonate from Carbon Dioxide Using Ionic Liquids. J. Organomet. Chem. 2005, 690, 3490–3497. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic Limonene Dicarbonate as a New Monomer for Non-Isocyanate Oligo- and Polyurethanes (NIPU) Based upon Terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Sonnati, M.O.; Amigoni, S.; Taffin de Givenchy, E.P.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol Carbonate as a Versatile Building Block for Tomorrow: Synthesis, Reactivity, Properties and Applications. Green Chem. 2013, 15, 283–306. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved Utilisation of Renewable Resources: New Important Derivatives of Glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Besse, V.; Camara, F.; Voirin, C.; Auvergne, R.; Caillol, S.; Boutevin, B. Synthesis and Applications of Unsaturated Cyclocarbonates. Polym. Chem. 2013, 4, 4545–4561. [Google Scholar] [CrossRef]
- Werpy, T.P.G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Ecochard, Y.; Caillol, S. Hybrid Polyhydroxyurethanes: How to Overcome Limitations and Reach Cutting Edge Properties? Eur. Polym. J. 2020, 137, 10915. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Qin, Y.; Li, Y. A Novel 2,5-Furandicarboxylic Acid-Based Bis(Cyclic Carbonate) for the Synthesis of Biobased Non-Isocyanate Polyurethanes. RSC Adv. 2017, 7, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Liu, X.; Li, C.; Jiang, Y.; Zhu, J. Synthesis and Properties of a Bio-Based Epoxy Resin from 2,5-Furandicarboxylic Acid (FDCA). RSC Adv. 2015, 5, 15930–15939. [Google Scholar] [CrossRef]
- Florek, J.; Mushtaq, A.; Larivière, D.; Cantin, G.; Fontaine, F.G.; Kleitz, F. Selective Recovery of Rare Earth Elements Using Chelating Ligands Grafted on Mesoporous Surfaces. RSC Adv. 2015, 5, 103782–103789. [Google Scholar] [CrossRef]
- Christoffers, J.; Önal, N. Azeotropic Transesterification of β-Keto Esters. Eur. J. Org. Chem. 2000, 2000, 1633–1635. [Google Scholar] [CrossRef]
- Otera, J.; Yano, T.; Kawabata, A.; Nozaki, H. Novel Distannoxane-Catalyzed Transesterification and a New Entry to α, β-Unsaturated Carboxylic Acids. Tetrahedron Lett. 1986, 27, 2383–2386. [Google Scholar] [CrossRef]
- Cornille, A.; Blain, M.; Auvergne, R.; Andrioletti, B.; Boutevin, B.; Caillol, S. A Study of Cyclic Carbonate Aminolysis at Room Temperature: Effect of Cyclic Carbonate Structures and Solvents on Polyhydroxyurethane Synthesis. Polym. Chem. 2017, 8, 592–604. [Google Scholar] [CrossRef]
- Diakoumakos, C.D.; Kotzev, D.L. Non-Isocyanate-Based Polyurethanes Derived upon the Reaction of Amines with Cyclocarbonate Resins. Macromol. Symp. 2004, 216, 37–46. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. Salt Effect on Polyaddition of Bifunctional Cyclic Carbonate and Diamine. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6282–6286. [Google Scholar] [CrossRef]
- Lambeth, R.H.; Henderson, T.J. Organocatalytic Synthesis of (Poly)Hydroxyurethanes from Cyclic Carbonates and Amines. Polymer 2013, 54, 5568–5573. [Google Scholar] [CrossRef]
- Blain, M.; Yau, H.; Jean-Gérard, L.; Auvergne, R.; Benazet, D.; Schreiner, P.R.; Caillol, S.; Andrioletti, B. Urea-and Thiourea-Catalyzed Aminolysis of Carbonates. ChemSusChem 2016, 9, 2269–2272. [Google Scholar] [CrossRef]
- Garipov, R.M.; Sysoev, V.A.; Mikheev, V.V.; Zagidullin, A.I.; Deberdeev, R.Y.; Irzhak, V.I.; Berlin, A.A. Reactivity of Cyclocarbonate Groups in Modified Epoxy-Amine Compositions. Dokl. Phys. Chem. 2003, 393, 289–292. [Google Scholar] [CrossRef]
- Prömpers, G.; Keul, H.; Höcker, H. Polyurethanes with Pendant Hydroxy Groups: Polycondensation of 1,6-Bis-O-Phenoxycarbonyl-2,3:4,5-Di-O-Isopropylidenegalactitol and 1,6-Di-O-Phenoxycarbonylgalactitol with Diamines. Green Chem. 2006, 8, 467–478. [Google Scholar] [CrossRef]
- Andrioletti, B. Techniques de l’Ingénieur, 2016, CHV4000, 1–7. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/voies-de-synthese-et-solvants-alternatifs-42492210/solvants-verts-chv4000/ (accessed on 29 May 2022).
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.W.; Zong, M.H.; Li, N.; Lou, W.Y. Recent Progress on Deep Eutectic Solvents in Biocatalysis. Bioresour. Bioprocess. 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Vatanpour, V.; Ganjali, M.R.; Saeb, M.R. Deep Eutectic Solvents in Membrane Science and Technology: Fundamental, Preparation, Application, and Future Perspective. Sep. Purif. Technol. 2021, 258, 118015. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Wang, J.; Han, J.; Khan, M.Y.; He, D.; Peng, H.; Chen, D.; Xie, X.; Xue, Z. Deep Eutectic Solvents for Green and Efficient Iron-Mediated Ligand-Free Atom Transfer Radical Polymerization. Polym. Chem. 2017, 8, 1616–1627. [Google Scholar] [CrossRef]
- Zhu, A.; Jiang, T.; Han, B.; Zhang, J.; Xie, Y.; Ma, X. Supported Choline Chloride/Urea as a Heterogeneous Catalyst for Chemical Fixation of Carbon Dioxide to Cyclic Carbonates. Green Chem. 2007, 9, 169–172. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal Degradation of Segmented Polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Han, E.; Ke, W. Effect of Acrylic Polymer and Nanocomposite with Nano-SiO2 on Thermal Degradation and Fire Resistance of APP-DPER-MEL Coating. Polym. Degrad. Stab. 2006, 91, 1937–1947. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chemie. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Wu, B.; Xu, Y.; Bu, Z.; Wu, L.; Li, B.G.; Dubois, P. Biobased Poly(Butylene 2,5-Furandicarboxylate) and Poly(Butylene Adipate-Co-Butylene 2,5-Furandicarboxylate)s: From Synthesis Using Highly Purified 2,5-Furandicarboxylic Acid to Thermo-Mechanical Properties. Polymer 2014, 55, 3648–3655. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Kim, T.K.; Lee, S.D.; Ahn, B.S.; Kim, H.S.; Lee, H. CaO-Catalyzed Synthesis of Glycerol Carbonate from Glycerol and Dimethyl Carbonate: Isolation and Characterization of an Active Ca Species. Appl. Catal. A Gen. 2011, 401, 220–225. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Tao, H.; Sun, D.; Xiao, D.; Liu, C. The Investigation of Thermal Pyrolysis of Glycerol Carbonate Derivatives by TG-FTIR. Thermochim. Acta 2016, 624, 76–81. [Google Scholar] [CrossRef]
- Li, F.; Li, X.L.; Li, C.; Shi, J.; Fu, Y. Aerobic Oxidative Esterification of 5-Hydroxymethylfurfural to Dimethyl Furan-2,5-Dicarboxylate by Using Homogeneous and Heterogeneous PdCoBi/C Catalysts under Atmospheric Oxygen. Green Chem. 2018, 20, 3050–3058. [Google Scholar] [CrossRef]
- Schumann, N.C.; Bruning, J.; Marshall, A.C.; Abell, A.D. The Role of N-Terminal Heterocycles in Hydrogen Bonding to α-Chymotrypsin. Bioorganic Med. Chem. Lett. 2019, 29, 396–399. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]


| Entry | Cat. | Yield (%) |
|---|---|---|
| 1 | CaO | Traces |
| 2 | NaOCH3 | Traces |
| 3 | KOH | Not detected |
| 4 | Ti(OBu)4 | Not detected |
| 5 | DMAP | 52 |
| Entry | Temp. (°C) | Catal. | Yield (%) |
|---|---|---|---|
| 1 | 80 | 10% | 45 |
| 2 | 90 | 10% | 52 |
| 3 | 100 | 10% | 65 |
| 4 | 90 | 5% | 50 |
| 5 | 90 | 2.5% | 17 |
| 6 a | 90 | 5% | 67 |
| 7 b | 90 | 5% | 69 |
| 8 c | 90 | 5% | 58 |
| Entry | Solvent | Results |
|---|---|---|
| 1 | DMF | Many by-products |
| 2 | glycerol | n.r. |
| 3 | ChCl/urea = 1:2 | Yes |
| 4 a | ChCl/urea = 1:2 | n.r. |
| 5 | ChCl/AcNH2 = 1:2 | n.r. |
| 6 | ChCl/glycerol = 1:2 | n.r. |
| 7 | AcNH2/urea = 1:2 | Yes * |
| Entry | Diamines | T (°C) | t (h) | Mn (g/mol) | D |
|---|---|---|---|---|---|
| 1 | EDR-148 | 110 | 24 | 24,578 | 1.00 |
| 2 | DA6 | 110 | 40 | 18,137 | 1.08 |
| 3 | EDR-148 | 65 | 48 | 25,320 | 1.03 |
| 4 | IPDA | 110 | 40 | 27,374 | 1.08 |
| 5 | Jeff D-2000 | 110 | 40 | 6784 | 1.01 |
| 6 | Jeff T-403 | 110 | 40 | 32,113 | 1.04 |
| 7 | Jeff T-3000 | 110 | 40 | 7386 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, G.; Andrioletti, B. Choline Chloride/Urea Deep Eutectic Solvents: A Promising Reaction Medium for the Synthesis of Bio-Based Poly(hydroxyurethane)s. Molecules 2022, 27, 4131. https://doi.org/10.3390/molecules27134131
Shen G, Andrioletti B. Choline Chloride/Urea Deep Eutectic Solvents: A Promising Reaction Medium for the Synthesis of Bio-Based Poly(hydroxyurethane)s. Molecules. 2022; 27(13):4131. https://doi.org/10.3390/molecules27134131
Chicago/Turabian StyleShen, Guanfei, and Bruno Andrioletti. 2022. "Choline Chloride/Urea Deep Eutectic Solvents: A Promising Reaction Medium for the Synthesis of Bio-Based Poly(hydroxyurethane)s" Molecules 27, no. 13: 4131. https://doi.org/10.3390/molecules27134131
APA StyleShen, G., & Andrioletti, B. (2022). Choline Chloride/Urea Deep Eutectic Solvents: A Promising Reaction Medium for the Synthesis of Bio-Based Poly(hydroxyurethane)s. Molecules, 27(13), 4131. https://doi.org/10.3390/molecules27134131








