Ultrastructural and Morphological Effects in T-Lymphoblastic Leukemia CEM-SS Cells Following Treatment with Nordamnacanthal and Damnacanthal from Roots of Morinda elliptica
Abstract
:1. Introduction
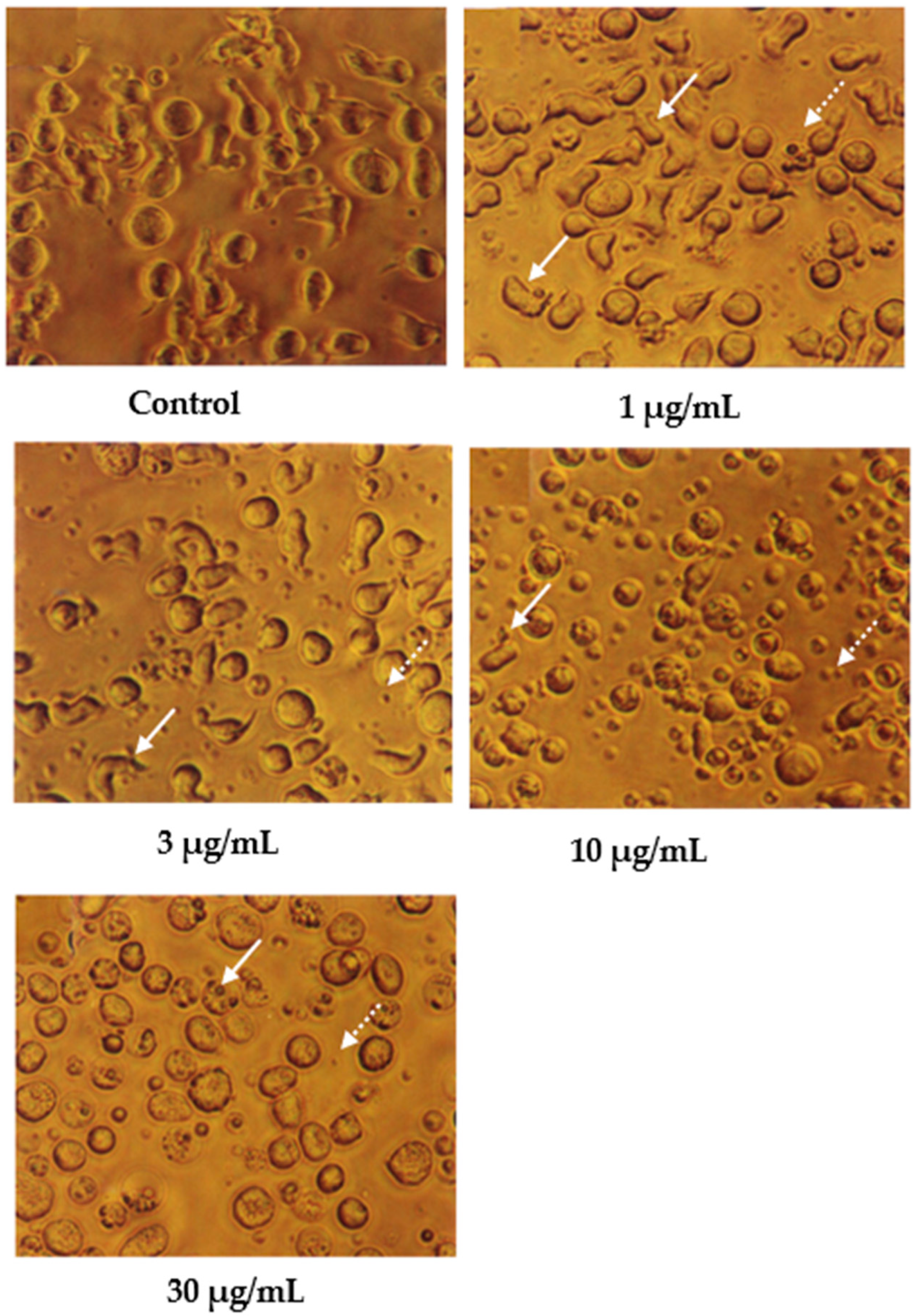
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Cells and Compounds
4.1.2. Compounds
4.2. Methods
4.2.1. Cell Lines
4.2.2. 3-[4,5-dimethylthizol-2-yl]-2,5-diphenyltetrazolium Bromide (MTT) Assay
4.2.3. Trypan Blue Dye Exclusion Method
4.2.4. Giemsa Staining
4.2.5. Wright’s Staining
4.2.6. Scanning Electron Microscopy
4.2.7. Transmission Electron Microscopy
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- CDC. Deaths and Mortality. 2017. Available online: https://www.cdc.gov/nchs/fastats/deaths.htm (accessed on 16 November 2020).
- GLOBOCAN. New Global Cancer Data. 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 18 May 2022).
- Fattizzo, B.; Rosa, J.; Giannotta, J.A.; Baldini, L.; Fracchiolla, N.S. The Physiopathology of T- Cell Acute Lymphoblastic Leukemia: Focus on Molecular Aspects. Front. Oncol. 2020, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Quirke, V. Targeting the American market for medicines, ca. 1950s–1970s: ICI and Rhône-Poulenc compared. Bull. Hist. Med. 2014, 88, 654–696. [Google Scholar] [CrossRef] [Green Version]
- Anttila, J.V.; Shubin, M.; Cairns, J.; Borse, F.; Guo, Q.; Mononen, T.; Vázquez-García, I.; Pulkkinen, O.; Mustonen, V. Contrasting the impact of cytotoxic and cytostatic drug therapies on tumour progression. PLoS Comput. Biol. 2019, 15, e1007493. [Google Scholar] [CrossRef] [Green Version]
- Ponticelli, C.; Moroni, G. Fetal Toxicity of Immunosuppressive Drugs in Pregnancy. J. Clin. Med. 2018, 7, 552. [Google Scholar] [CrossRef] [Green Version]
- Swift, L.H.; Golsteyn, R.M. Genotoxic anti-cancer agents and their relationship to DNA damage, mitosis, and checkpoint adaptation in proliferating cancer cells. Int. J. Mol. Sci. 2014, 15, 3403–3431. [Google Scholar] [CrossRef] [Green Version]
- Demoor-Goldschmidt, C.; de Vathaire, F. Review of risk factors of secondary cancers among cancer survivors. Br. J. Radiol. Suppl. 2019, 92, 20180390. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 17, 1941. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Neuss, N.; Neuss, M.N. Chapter 6 Therapeutic Use of Bisindole Alkaloids from Catharanthus. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Suffness, M., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 37, pp. 229–240. [Google Scholar]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef]
- Gimlette, J.D. A Dictionary of Malayan Medicine; Oxford University Press: Kuala Lumpur, India, 1971. [Google Scholar]
- Abu Bakar, F.I.; Abu Bakar, M.F.; Rahmat, A.; Abdullah, N.; Sabran, S.F.; Endrini, S. Anti-gout Potential of Malaysian Medicinal Plants. Front. Pharmacol. 2018, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Mohamad, H.; Mohidin, A.; Lajis, N.H. Antioxidant activity of anthraquinones from Morinda elliptica. Nat. Prod. Sci. 2002, 8, 48–51. Available online: https://www.koreascience.or.kr/article/JAKO200203041137061.pdf (accessed on 3 December 2021).
- Jasril; Lajis, N.H.; Mooi, L.Y.; Abdullah, M.A.; Sukari, M.A.; Ali, A.M. Antitumor promoting and actioxidant activities of anthraquinones isolated from the cell suspension culture of Morinda elliptica. Asia-Pac. J. Mol. Biol. Biotechnol. 2003, 11, 3–7. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.512.743&rep=rep1&type=pdf (accessed on 3 December 2021).
- Shami, A.M.M. Antibacterial and antioxidant properties of anthraquinones fractions from Morinda Citrifolia fruit. J. Rep. Pharm. Sci. 2018, 7, 379–388. Available online: http://www.jrpsjournal.com/article.asp?issn=2322-1232;year=2018;volume=7;issue=3;spage=379;epage=388;aulast=M (accessed on 3 December 2021).
- Abu, N.; Zamberi, N.R.; Yeap, S.K.; Nordin, N.; Mohamad, N.E.; Romli, M.F.; Rasol, N.E.; Subramani, T.; Ismail, N.H.; Alitheen, N.B. Subchronic toxicity, immunoregulation and anti-breast tumor effect of Nordamnacantal, an anthraquinone extracted from the stems of Morinda citrifolia L. BMC Complement Altern Med. 2018, 18, 31. [Google Scholar] [CrossRef] [Green Version]
- Kanokmedhakul, K.; Kanokmedhakul, S.; Phatchana, R. Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J. Ethnopharmacol. 2005, 100, 284–288. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Imoto, M.; Koyano, T.; Umezawa, K. Induction of normal phenotypes in ras-transformed cells by damnacanthal from Morinda citrifolia. Cancer Lett. 1993, 73, 161–166. [Google Scholar] [CrossRef]
- Ali, A.; Ismail, N.; Mackeen, M.; Yazan, L.S.; Mohamed, S.M.; Ho, A.S.; Lajis, N.H. Antiviral, cyototoxic and antimicrobial activities of anthraquinones isolated from the roots of Morinda elliptica. Pharm. Biol. 2000, 38, 298–301. [Google Scholar] [CrossRef]
- Latifah, S.Y.; Gopalsamy, B.; Abdul Rahim, R.; Manaf Ali, A.; Haji Lajis, N. Anticancer Potential of Damnacanthal and Nordamnacanthal from Morinda elliptica Roots on T-lymphoblastic Leukemia Cells. Molecules 2021, 26, 1554. [Google Scholar] [CrossRef]
- Hu, X.M.; Li, Z.X.; Lin, R.H.; Shan, J.Q.; Yu, Q.W.; Wang, R.X.; Liao, L.S.; Yan, W.T.; Wang, Z.; Shang, L.; et al. Guidelines for Regulated Cell Death Assays: A Systematic Summary, A Categorical Comparison, A Prospective. Front. Cell Dev. Biol. 2021, 9, 368. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sun, R.; Geng, S.; Shan, Y.; Li, X.; Fang, W. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J. Virol. 2019, 93, e01784-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayarathna, S.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Standardized Polyalthia longifolia leaf extract (PLME) inhibits cell proliferation and promotes apoptosis: The anti-cancer study with various microscopy methods. Biomed. Pharm. 2017, 91, 366–377. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, K.; Parent, A.; Zhang, J. Mechanism of damnacanthal-induced [Ca2+]i elevation in human dermal fibroblasts. Eur. J. Pharmacol. 2000, 387, 119–124. [Google Scholar] [CrossRef]
- Berridge, M.J.; Irvine, R.F. Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, T.; Das, S.; Mondal, M.; Roychoudhury, S.; Chakraborti, S. Oxidant, mitochondria and calcium: An overview. Cell. Signal 1999, 11, 77–85. [Google Scholar] [CrossRef]
- Takeyama, N.; Matsuo, N.; Tanaka, T. Oxidative damage to mitochondria is mediated by the Ca2+-dependent inner-membrane permeability transition. Biochem. J. 1993, 294, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Siman, R.; Noszek, J.C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron 1988, 1, 279–287. [Google Scholar] [CrossRef]
- Verity, M.A. Mechanisms of phospholipase A2 activation and neuronal injury. Ann. N. Y. Acad. Sci. 1993, 679, 110–120. [Google Scholar] [CrossRef]
- Granville, D.J.; Carthy, C.M.; Hunt, D.W.C.; McManus, B.M. Apoptosis: Molecular aspects of cell death and disease. Lab. Investig. 1998, 78, 893–913. [Google Scholar]
- Li, W.; Shi, X.; Xu, Y.; Wan, J.; Wei, S.; Zhu, R. Tamoxifen promotes apoptosis and inhibits invasion in estrogen-positive breast cancer MCF-7 cells. Mol. Med. Rep. 2017, 16, 478–484. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hung, M.-H.; Wang, D.-S.; Chu, P.-Y.; Su, J.-C.; Teng, T.-H.; Huang, C.T.; Chao, T.T.; Wang, C.Y.; Shiau, C.W.; et al. Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2A-dependent phospho-Akt inactivation in estrogen receptor-negative human breast cancer cells. Breast Cancer Res. 2014, 16, 431. [Google Scholar] [CrossRef] [Green Version]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Nakajima, W.; Seike, M.; Gemma, A.; Tanaka, N. Cisplatin-induced apoptosis in non-small-cell lung cancer cells is dependent on Bax- and Bak-induction pathway and synergistically activated by BH3-mimetic ABT-263 in p53 wild-type and mutant cells. Biochem. Biophys. Res. Commun. 2016, 473, 490–496. [Google Scholar] [CrossRef]
- Ismail, N.H.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H.; Lajis, N.H. Anthraquinones from Morinda elliptica. Phytochemistry 1997, 45, 1723–1725. [Google Scholar] [CrossRef] [Green Version]
- Hirose, Y. Syntheses of Damnacanthal, Damnacanthol, Norjuzunal and Norjuzunol, the Coloring Matters of Damnacanthus spp. Chem. Pharm. Bull. 1960, 8, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Adnan, N.E. Isolation and photophysical properties of Di- and Tri-substituted natural anthraquinones from Malaysian Morinda citrifolia. Sains Malays. 2018, 47, 903–906. [Google Scholar] [CrossRef]
- Lillie, R.D.H.J. Conn’s Biological Stains, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1977. [Google Scholar]
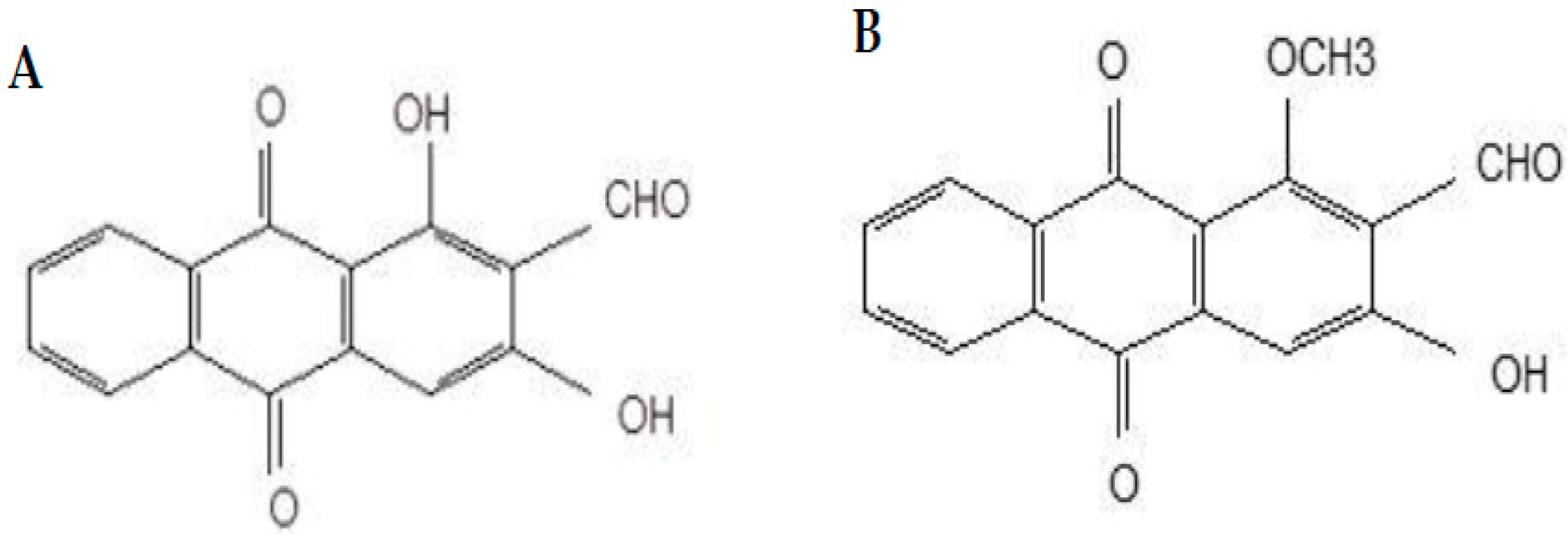












| Cell Line | Nordamnacanthal | IC50 (μg/mL) | Damnacanthal |
|---|---|---|---|
| T-lymphoblastic leukemia (CEM-SS) | 1.7 ± 0.4 | 10 ± 0.5 | |
| Human peripheral blood mononuclear cells (PBMCs) | >30 | >30 | |
| Mouse embryo (3T3) | >30 | >30 | |
| Monkey kidney fibroblast (Vero) | >30 | >30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latifah, S.Y.; Gopalsamy, B.; Abdul Rahim, R.; Manaf Ali, A.; Haji Lajis, N. Ultrastructural and Morphological Effects in T-Lymphoblastic Leukemia CEM-SS Cells Following Treatment with Nordamnacanthal and Damnacanthal from Roots of Morinda elliptica. Molecules 2022, 27, 4136. https://doi.org/10.3390/molecules27134136
Latifah SY, Gopalsamy B, Abdul Rahim R, Manaf Ali A, Haji Lajis N. Ultrastructural and Morphological Effects in T-Lymphoblastic Leukemia CEM-SS Cells Following Treatment with Nordamnacanthal and Damnacanthal from Roots of Morinda elliptica. Molecules. 2022; 27(13):4136. https://doi.org/10.3390/molecules27134136
Chicago/Turabian StyleLatifah, Saiful Yazan, Banulata Gopalsamy, Raha Abdul Rahim, Abdul Manaf Ali, and Nordin Haji Lajis. 2022. "Ultrastructural and Morphological Effects in T-Lymphoblastic Leukemia CEM-SS Cells Following Treatment with Nordamnacanthal and Damnacanthal from Roots of Morinda elliptica" Molecules 27, no. 13: 4136. https://doi.org/10.3390/molecules27134136
APA StyleLatifah, S. Y., Gopalsamy, B., Abdul Rahim, R., Manaf Ali, A., & Haji Lajis, N. (2022). Ultrastructural and Morphological Effects in T-Lymphoblastic Leukemia CEM-SS Cells Following Treatment with Nordamnacanthal and Damnacanthal from Roots of Morinda elliptica. Molecules, 27(13), 4136. https://doi.org/10.3390/molecules27134136





