Strategies for the Biofunctionalization of Straining Flow Spinning Regenerated Bombyx mori Fibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Functionalization with a Fluorophore
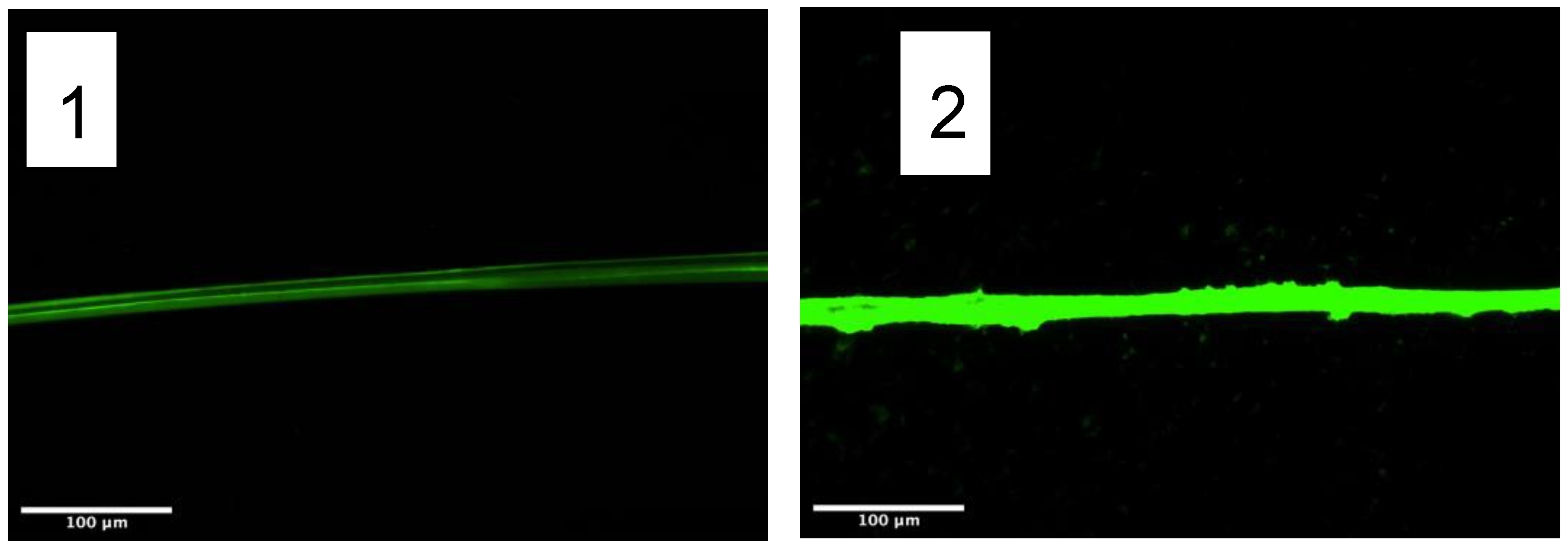
2.2. Functionalization with the System Streptavidin-Biotin
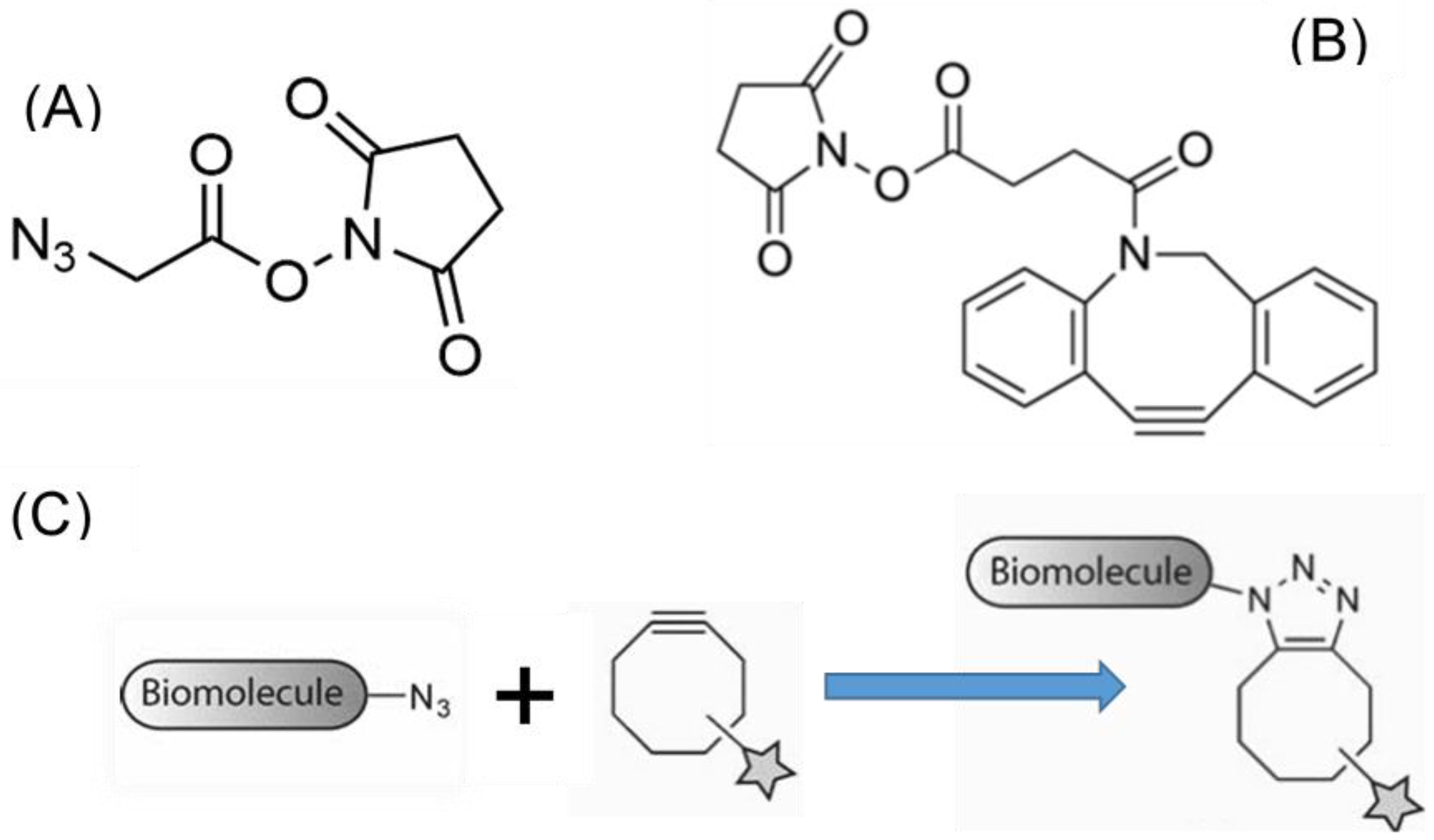
2.3. Functionalization with the Biorthogonal Alkyne-Azide Chemistry
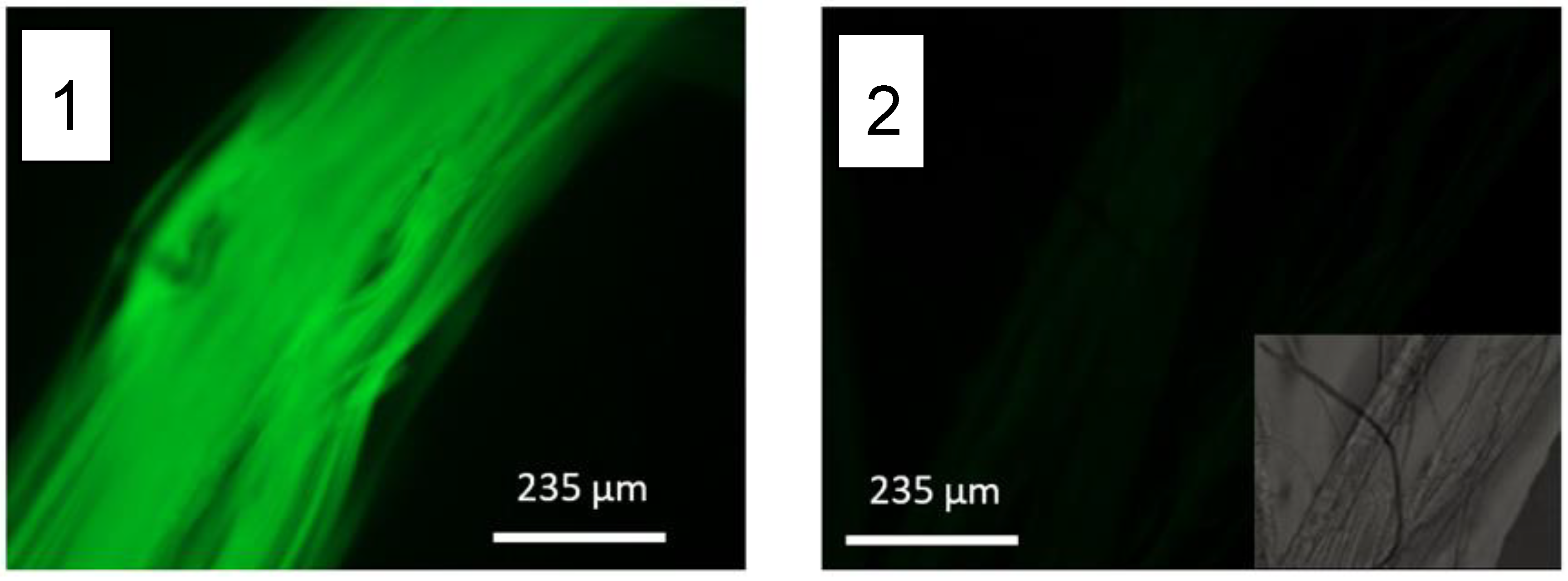
2.4. Functionalizatin with Cell-Adhesion Motifs
3. Materials and Methods
3.1. Preparation of the Silkworm Silk (Bombyx mori) Protein Solution
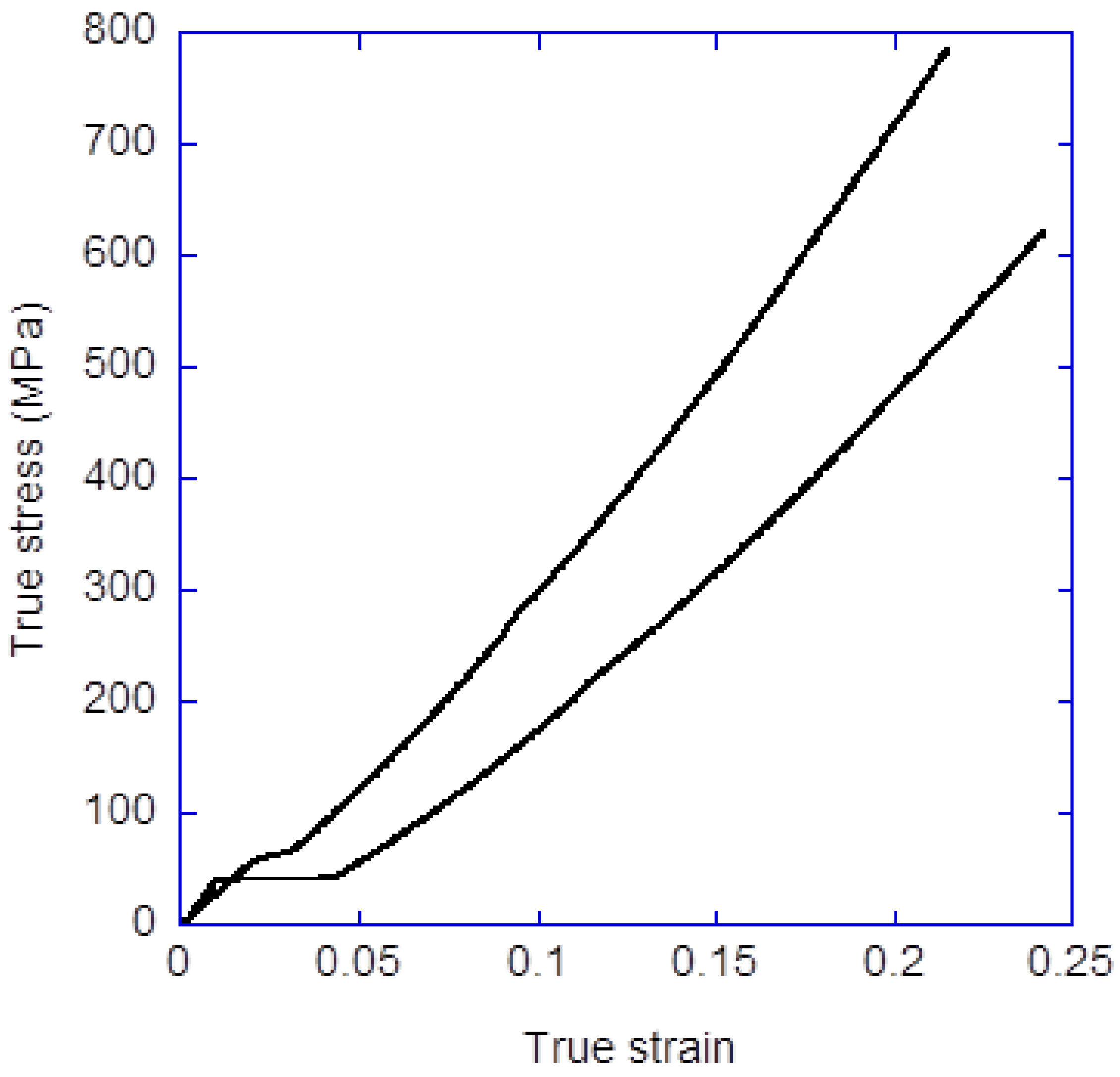
3.2. Spinning of High-Performance Regenerated Silk Fibers through Straining Flow Spinning
3.3. Measurement of Fluorescence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vepari, C.; Kaplan, D. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Riekel, C.; Branden, C.; Craig, C.; Ferrero, C.; Heidelbach, F.; Muller, M. Aspects of X-ray diffraction on single spider fibers. Int. J. Biol. Macromol. 1999, 24, 179–186. [Google Scholar] [CrossRef]
- Koyanagi, R.; Zhu, Z.; Asakura, T. Regenerated Bombyx mori silk fiber with enhanced biodegradability. J. Insect Biotechnol. Sericol. 2010, 79, 27–30. [Google Scholar]
- Heim, M.; Keerl, D.; Scheibel, T. Spider silk: From soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. Engl. 2009, 48, 3584–3596. [Google Scholar] [CrossRef]
- Soong, H.; Kenyon, K. Adverse reactions to virgin silk sutures in cataract-surgery. Ophthalmology 1984, 91, 479–483. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Plaza, G.R.; Corsini, P.; Marsano, E.; Perez-Rigueiro, J.; Biancotto, L.; Elices, M.; Riekel, C.; Agullo-Rueda, F.; Gallardo, E.; Calleja, J.M.; et al. Old silks endowed with new properties. Macromolecules 2009, 42, 8977–8982. [Google Scholar] [CrossRef]
- Murphy, A.R.; John, P.S.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838, Erratum in Biomaterials 2008, 29, 4260. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Venkatesan, H.; Hu, J. Chemically modified silk proteins. Adv. Eng. Mater. 2018, 20, 1700961. [Google Scholar] [CrossRef]
- Katashima, T.; Malay, A.D.; Numata, K. Chemical modification and biosynthesis of silk-like polymers. Curr. Opin. Chem. Eng. 2019, 24, 61–68. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.; Gbureck, U.; Rackwitz, L.; Noeth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.; Pastor, B.C.; van Rijt, S.; Habibovic, P. Understanding interactions between biomaterials and biological systems using proteomics. Biomaterials 2018, 167, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [Green Version]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Punet, X.; Mauchauffe, R.; Giannotti, M.I.; Rodriguez-Cabello, J.C.; Sanz, F.; Engel, E.; Mateos-Timoneda, M.A.; Planell, J.A. Enhanced cell-material interactions through the biofunctionalization of polymeric surfaces with engineered peptides. Biomacromolecules 2013, 14, 2690–2702. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine I. Graft-polymerization onto fabric using ammonium persulfate and interaction between fabric and platelets. J. Appl. Polym. Sci. 1999, 73, 2541–2544. [Google Scholar] [CrossRef]
- Do, S.; Park, J.; Nam, H.; Kim, J.; Lee, J.; Oh, Y.; Suh, J. Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice. J. Vet. Sci. 2012, 13, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Anis, P.; Capar, G.; Toprak, T.; Yener, E. Sericin removal from silk fibers with eco-friendly alternative methods. Tekst. Ve Konfeksiyon 2016, 26, 368–374. [Google Scholar]
- Martel, A.; Burghammer, M.; Davies, R.J.; Riekel, C. Thermal behavior of Bombyx mori silk: Evolution of crystalline parameters, molecular structure, and mechanical properties. Biomacromolecules 2007, 8, 3548–3556. [Google Scholar] [CrossRef]
- Perez-Rigueiro, J.; Madurga, R.; Ganan-Calvo, A.M.; Elices, M.; Guinea, G.; Tasei, Y.; Nishimura, A.; Matsuda, H.; Asakura, T. Emergence of supercontraction in regenerated silkworm (Bombyx mori) silk fibers. Sci. Rep. 2019, 9, 2398. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Madurga, R.; Ganan-Calvo, A.M.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Perez-Rigueiro, J. Production of high performance bioinspired silk fibers by straining flow spinning. Biomacromolecules 2017, 18, 1127–1133. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [Green Version]
- Jokerst, J.V.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Haldar, R.; Rao, K.V.; George, S.J.; Maji, T.K. Exciplex formation and energy transfer in a self-assembled metal-organic hybrid system. Chem.-A Eur. J. 2012, 18, 5848–5852. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-harvesting metal-organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef]
- Zhang, X.; Ballem, M.A.; Ahren, M.; Suska, A.; Bergman, P.; Uvdal, K. Nanoscale Ln(III)-carboxylate coordination polymers (Ln = Gd, Eu, Yb): Temperature-controlled guest encapsulation and light harvesting. J. Am. Chem. Soc. 2010, 132, 10391–10397. [Google Scholar] [CrossRef]
- Zhang, X.; Ballem, M.A.; Hu, Z.; Bergman, P.; Uvdal, K. Nanoscale light-harvesting metal-organic frameworks. Angew. Chem. 2011, 50, 5728–5732. [Google Scholar]
- Weber, P.; Ohlendorf, D.; Wendoloski, J.; Salemme, F. Structural origins of high-affinity biotin binding to streptavidin. Science 1989, 243, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, A.; Blomstergren, A.; Nord, O.; Lukacs, M.; Lundeberg, J.; Uhlen, M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 2005, 26, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem.-Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [Green Version]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The controlled display of biomolecules on nanoparticles: A challenge suited to bioorthogonal chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.; Dobersen, M.J.; Quarles, R.H. A novel procedure for labeling immunoglobulins by conjugation to oligosaccharide moieties. Immunol. Lett. 1984, 8, 273–277. [Google Scholar] [CrossRef]
- Rostovtsev, V.; Green, L.; Fokin, V.; Sharpless, K. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem.-Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Wolbers, F.; ter Braak, P.; Le Gac, S.; Luttge, R.; Andersson, H.; Vermes, I.; van den Berg, A. Viability study of HL60 cells in contact with commonly used microchip materials. Electrophoresis 2006, 27, 5073–5080. [Google Scholar] [CrossRef]
- Devaraj, N.K. The future of bioorthogonal chemistry. ACS Cent. Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Márquez Calvo, J.; Elices, M.; Guinea, G.V.; Perez-Rigueiro, J.; Arroyo-Hernandez, M. Stability and activity of lactate dehydrogenase on biofunctional layers deposited by activated vapor silanization (AVS) and immersion silanization (IS). Appl. Surf. Sci. 2017, 416, 965–970. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudre, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. Npj Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Lopez, A.; Colchero, L.; Elices, M.; Guinea, G.V.; Perez-Rigueiro, J.; Gonzalez-Nieto, D. Improved cell adhesion to activated vapor silanization-biofunctionalized Ti-6Al-4V surfaces with ECM-derived oligopeptides. Biomater. Adv. 2022, 133, 112614. [Google Scholar] [CrossRef]
- Fernandez-Garcia, L.; Mari-Buye, N.; Barios, J.A.; Madurga, R.; Elices, M.; Perez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Gonzalez-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef] [Green Version]
- Perez-Rigueiro, J.; Madurga, R.; Ganan-Calvo, A.M.; Plaza, G.R.; Elices, M.; Lopez, P.A.; Daza, R.; Gonzalez-Nieto, D.; Guinea, G.V. Straining flow spinning of artificial silk fibers: A review. Biomimetics 2018, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Gonska, N.; Lopez, P.A.; Lozano-Picazo, P.; Thorpe, M.; Guinea, G.V.; Johansson, J.; Barth, A.; Perez-Rigueiro, J.; Rising, A. Structure-function relationship of artificial spider silk fibers produced by straining flow spinning. Biomacromolecules 2020, 21, 2116–2124. [Google Scholar] [CrossRef]
- Madurga, R.; Gañán-Calvo, A.M.; Plaza, G.R.; Atienza, J.M.; Guinea, G.V.; Elices, M.; López, P.A.; Daza, R.; González-Nieto, D.; Pérez-Rigueiro, J. Comparison of the effects of post-spinning drawing and wet stretching on regenerated silk fibers produced through straining flow spinning. Polymer 2018, 150, 311–317. [Google Scholar] [CrossRef]
- Madurga, R.; Ganan-Calvo, A.M.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Perez-Rigueiro, J. Straining flow spinning: Production of regenerated silk fibers under a wide range of mild coagulating chemistries. Green Chem. 2017, 19, 3380–3389. [Google Scholar] [CrossRef]
- Madurga, R.; Gañán-Calvo, A.; Mariscal, T.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Perez-Rigueiro, J. Production of regenerated silkworm silk fibers from aqueous dopes through straining flow spinning. Text. Res. J. 2018, 89, 4554–4567. [Google Scholar] [CrossRef]
- Perez-Rigueiro, J.; Viney, C.; Llorca, J.; Elices, M. Silkworm silk as an engineering material. J. Appl. Polym. Sci. 1998, 70, 2439–2447. [Google Scholar] [CrossRef]
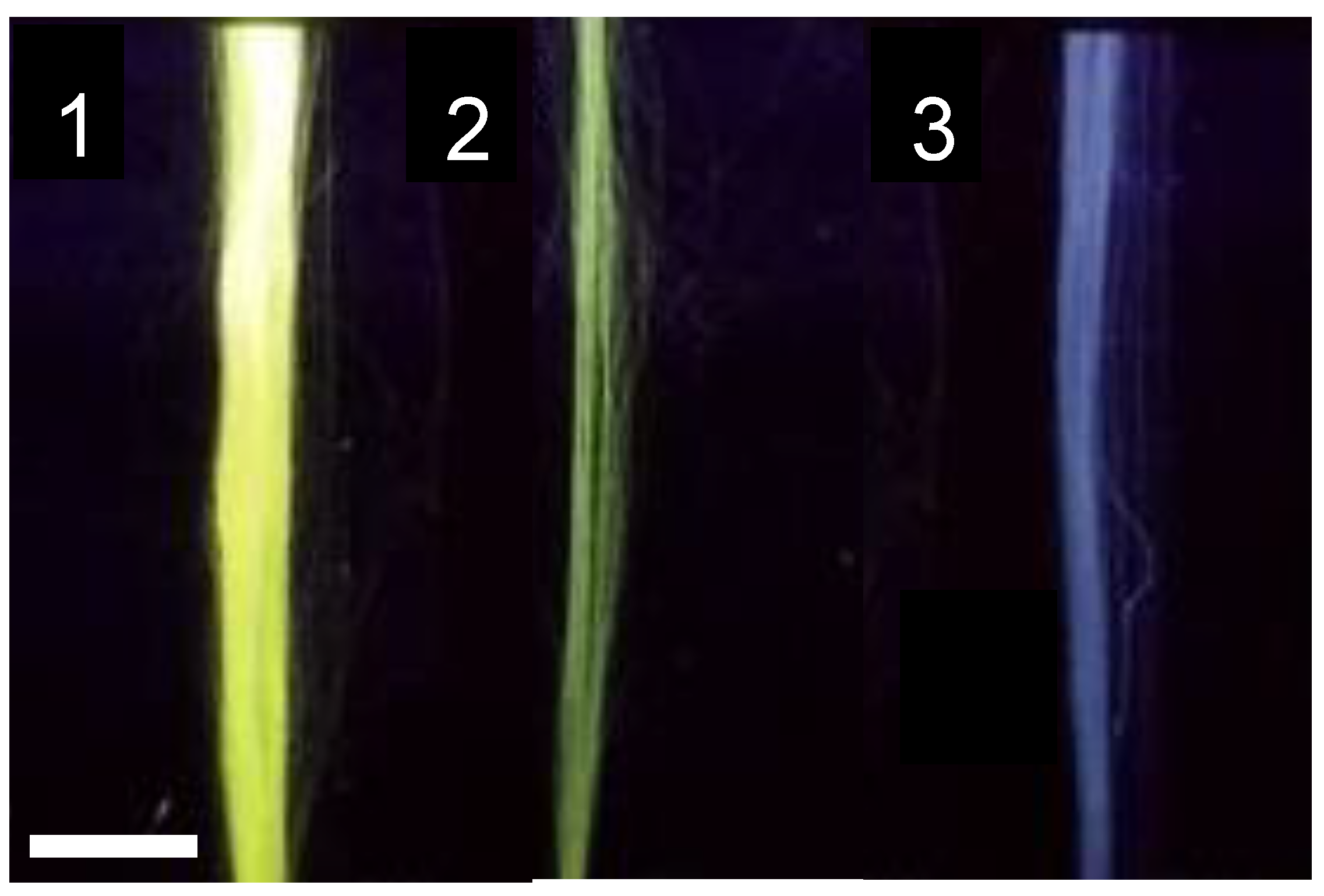
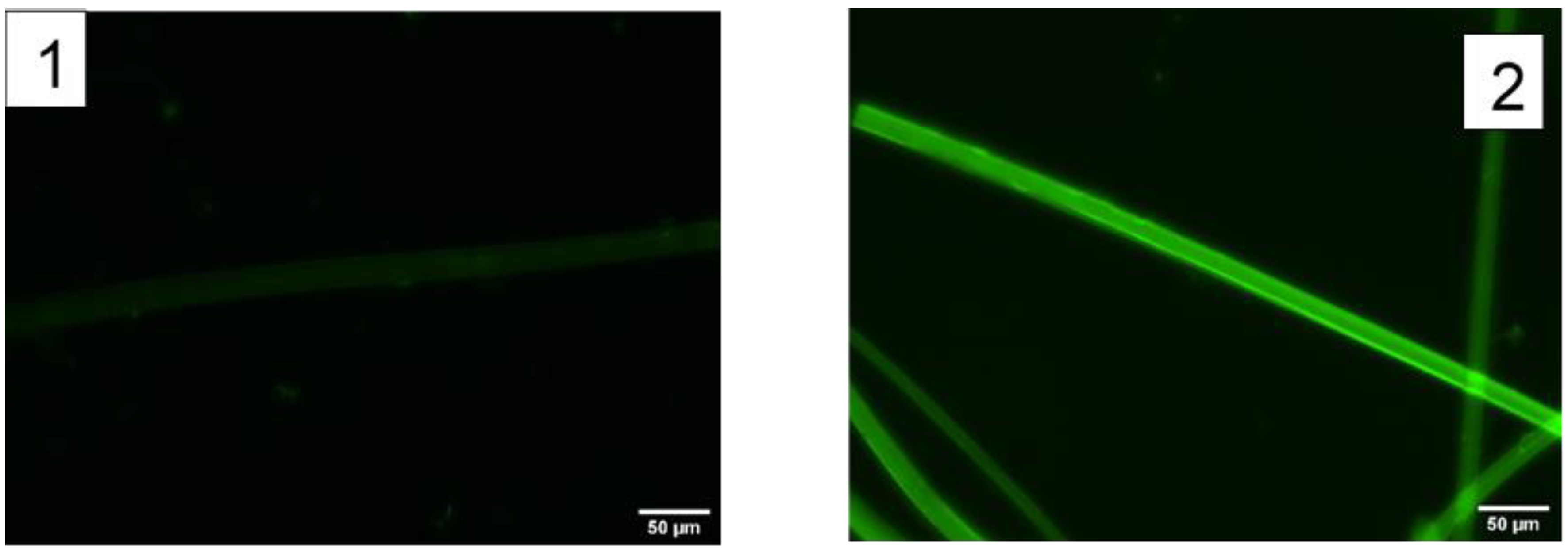
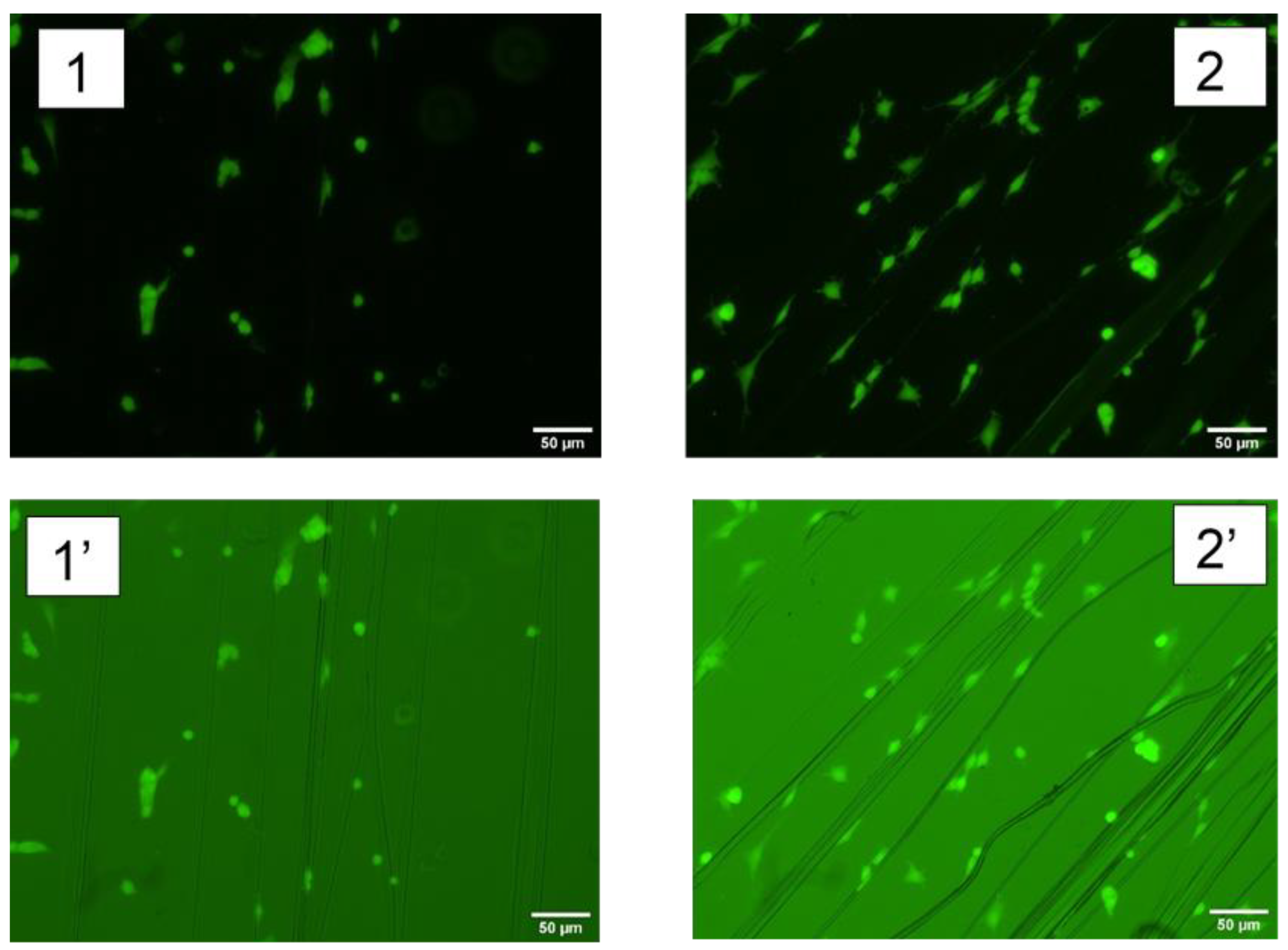
| Protocol | Fluorescein/Fibroin (μg/mg) |
|---|---|
| A | 0.060 |
| B | 0.042 |
| SFS Fiber | Fluorescence Intensity (a.u.) |
|---|---|
| Control (Non-biotinylized) | 49 ± 6 |
| Biotin-functionalized | 130 ± 20 |
| Protein | Specific Activity (μmolNADH/min.mgprot) |
|---|---|
| LDH (Stock) | 220 ± 30 |
| Alkyne-functionalized LDH | 1.3 ± 0.9 |
| Azide-functionalized LDH | 180 ± 30 |
| Fiber | Fluorescence Intensity (a.u.) |
|---|---|
| Alkyne functionalized + fluorescein azide | 42 ± 3 |
| Control fiber + fluorescein azide | 8 ± 2 |
| Fiber | pmoleNADH/min.mgfiber |
|---|---|
| Alkyne-functionalized | 420 ± 70 |
| Control | 70 ± 50 |
| Fibers | Number of Cells Attached per Fiber | Number of Cells Attached to Fibers/Total Number of Cells |
|---|---|---|
| Control | 1.6 ± 0.2 | 0.27 ± 0.05 |
| RGD-functionalized | 2.5 ± 0.3 | 0.61 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Picazo, P.; Castro-Domínguez, C.; Bruno, A.L.; Baeza, A.; Martínez, A.S.; López, P.A.; Castro, Á.; Lakhal, Y.; Montero, E.; Colchero, L.; et al. Strategies for the Biofunctionalization of Straining Flow Spinning Regenerated Bombyx mori Fibers. Molecules 2022, 27, 4146. https://doi.org/10.3390/molecules27134146
Lozano-Picazo P, Castro-Domínguez C, Bruno AL, Baeza A, Martínez AS, López PA, Castro Á, Lakhal Y, Montero E, Colchero L, et al. Strategies for the Biofunctionalization of Straining Flow Spinning Regenerated Bombyx mori Fibers. Molecules. 2022; 27(13):4146. https://doi.org/10.3390/molecules27134146
Chicago/Turabian StyleLozano-Picazo, Paloma, Cristina Castro-Domínguez, Augusto Luis Bruno, Alejandro Baeza, Adelia S. Martínez, Patricia A. López, Ángela Castro, Yassmin Lakhal, Elena Montero, Luis Colchero, and et al. 2022. "Strategies for the Biofunctionalization of Straining Flow Spinning Regenerated Bombyx mori Fibers" Molecules 27, no. 13: 4146. https://doi.org/10.3390/molecules27134146
APA StyleLozano-Picazo, P., Castro-Domínguez, C., Bruno, A. L., Baeza, A., Martínez, A. S., López, P. A., Castro, Á., Lakhal, Y., Montero, E., Colchero, L., González-Nieto, D., Rojo, F. J., Panetsos, F., Ramos, M., Daza, R., Gañán-Calvo, A. M., Elices, M., Guinea, G. V., & Pérez-Rigueiro, J. (2022). Strategies for the Biofunctionalization of Straining Flow Spinning Regenerated Bombyx mori Fibers. Molecules, 27(13), 4146. https://doi.org/10.3390/molecules27134146







