Efficient Adsorption of Chromium Ions from Aqueous Solutions by Plant-Derived Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
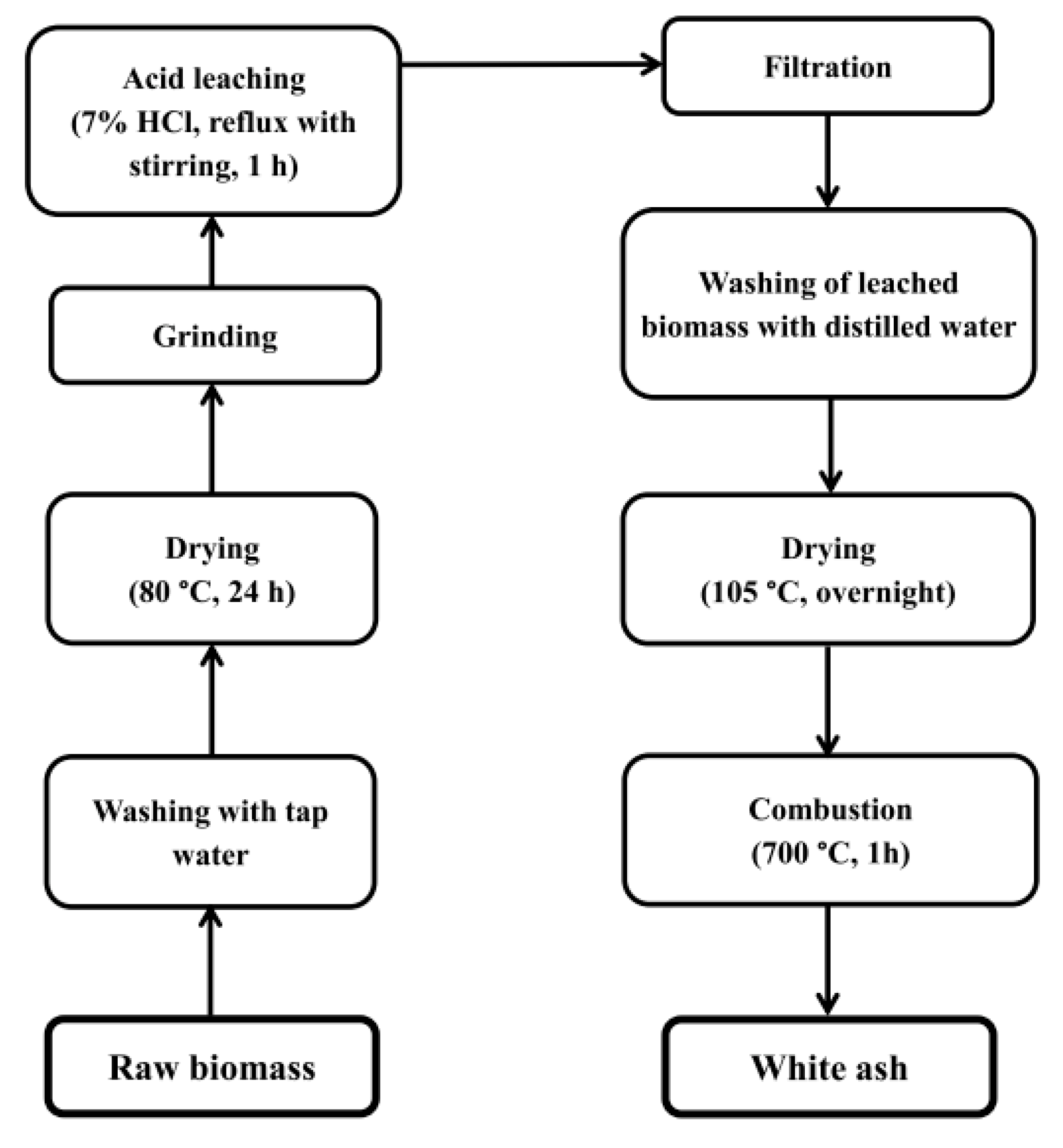
2.2. Preparation of Silica Samples
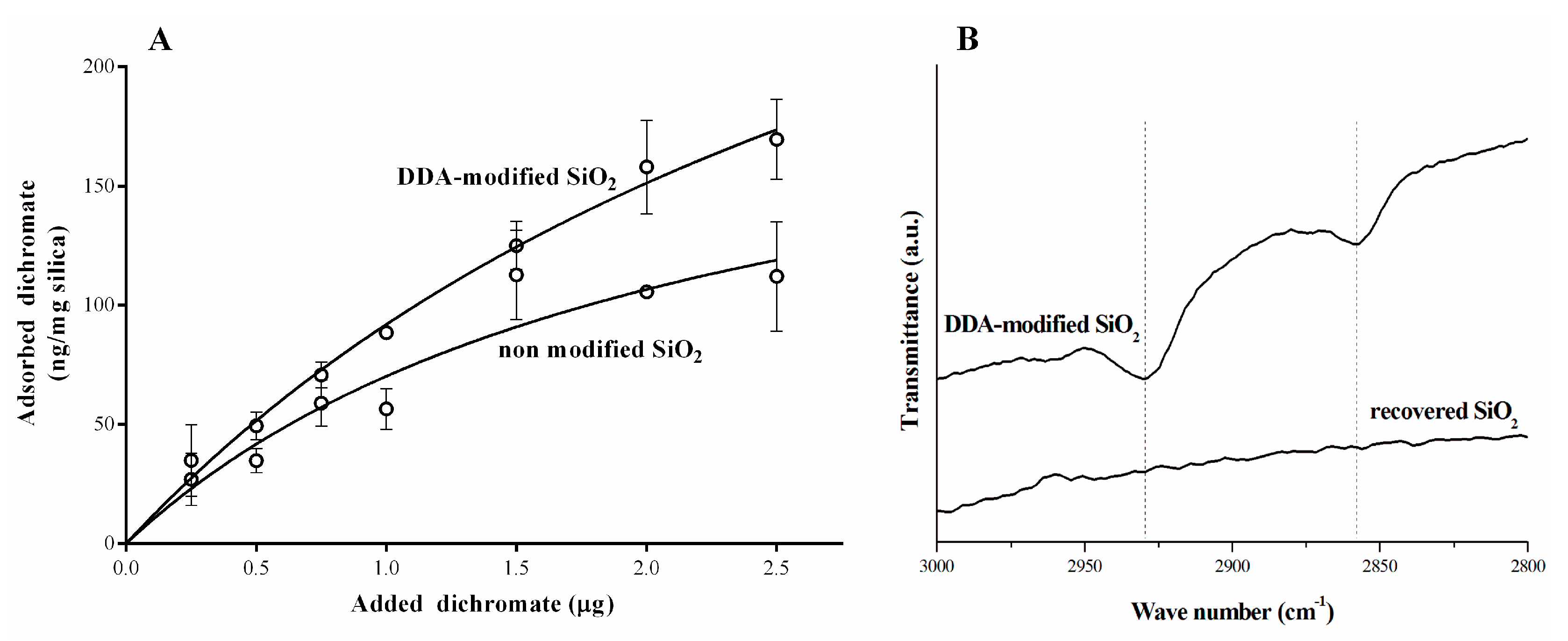
2.3. Modification of Silica Powder with Dodecylamine
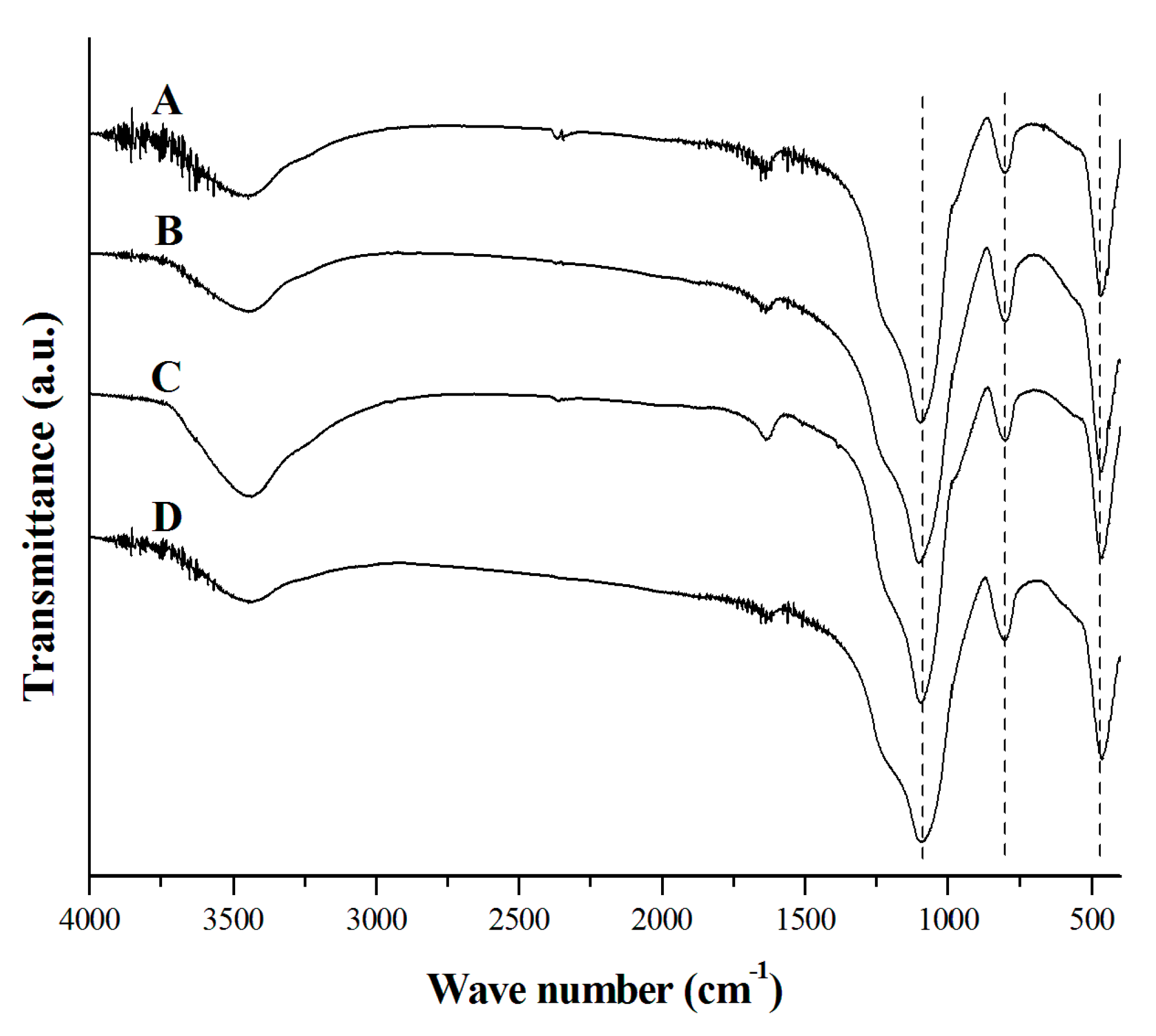
2.4. Characterization of Powders
2.5. Adsorption Study
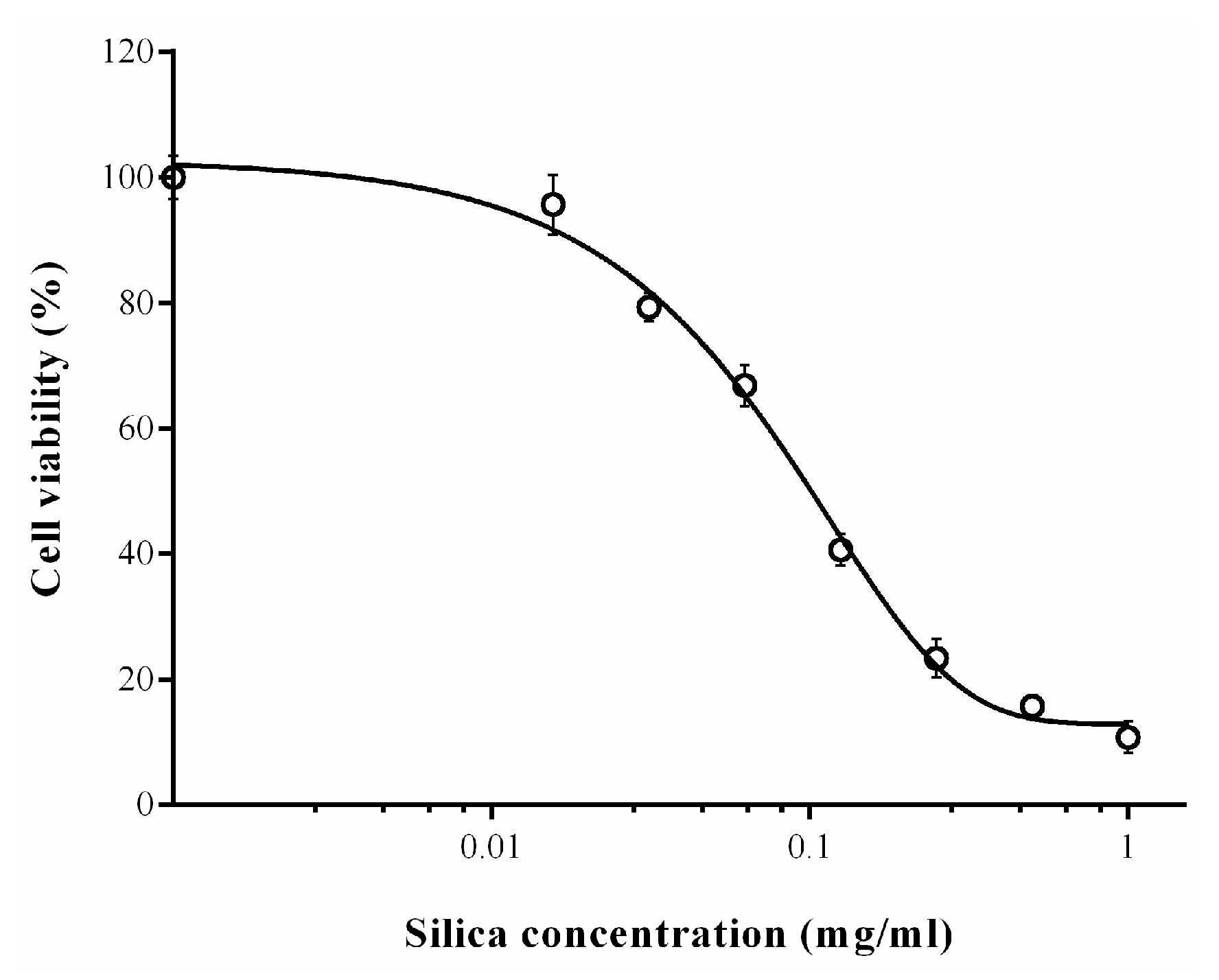
2.6. Cytotoxicity of Biosilica Powders
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World–New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [Green Version]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and opportunities in biomass ash management and its utilization in novel applications. Renew. Sust. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbanna, B.A.; Al-Najoli, E.; Alsherief, A.; Negm, S.; Abou El-Nour, E.; Nofal, A.; Sharabash, S. Agricultural waste management for climate change mitigation: Some implications to Egypt. In Waste Management in MENA Regions; Negm, A.M., Shareef, N., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 149–168. [Google Scholar] [CrossRef]
- Lohan, S.K.; Jat, H.S.; Yadav, A.K.; Sidhu, H.S.; Jat, M.L.; Choudhary, M.; Peter, J.K.; Sharma, P.C. Burning issues of paddy residue management in north-west states of India. Renew. Sust. Energy Rev. 2018, 81, 693–706. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Ou, L.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors. J. Environ. Manag. 2020, 261, 109879. [Google Scholar] [CrossRef]
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation possibilities of exhausted biosorbents loaded with metal ions—A review. J. Environ. Manag. 2018, 224, 288–297. [Google Scholar] [CrossRef]
- Bulgariu, L.; Ferţu, D.I.; Cara, I.G.; Gavrilescu, M. Efficacy of alkaline-treated soy waste biomass for the removal of heavy-metal ions and opportunities for their recovery. Materials 2021, 14, 7413. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Lisa, G.; Mocanu, A.M.; Bulgariu, L. Potential use of biochar from various waste biomass as biosorbent in Co(II) removal processes. Water 2019, 11, 1565. [Google Scholar] [CrossRef] [Green Version]
- Chiew, Y.L.; Cheong, K.Y. A review on the synthesis of SiC from plant-based biomasses. Mater. Sci. Eng. B-Adv. 2011, 176, 951–964. [Google Scholar] [CrossRef]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef]
- Patel, K.G.; Shettigar, R.R.; Misra, N.M. Recent advance in silica production technologies from agricultural waste stream–Review. J. Adv. Agr. Technol. 2017, 4, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.H.; da Silva Reis, T.V.; Rovani, S.; Alves Fungaro, D. Green synthesis and characterization of biosilica produced from sugarcane waste ash. Hindawi J. Chem. 2017, 2017, 6129035. [Google Scholar] [CrossRef]
- Carneiro, M.E.; Magalhães, W.L.E.; de Muñiz, G.I.B.; Nisgoski, S.; Satyanarayana, K.G. Preparation and characterization of nano silica from Equisetum arvenses. J. Bioproc. Biotech. 2015, 5, 205. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Mohtasham, N.H.; Gholizadeh, M. Nano silica extracted from horsetail plant as a natural silica support for the synthesis of H3PW12O40 immobilized on aminated magnetic nanoparticles (Fe3O4@SiO2-EP-NH-HPA): A novel and efficient heterogeneous nanocatalyst for the green one-pot synthesis of pyrano [2,3-c] pyrazole derivatives. Res. Chem. Intermediat. 2020, 46, 3037–3066. [Google Scholar] [CrossRef]
- Nazaripour, M.; Reshadi, M.A.M.; Mirbagheri, S.A.; Nazaripour, M.; Bazargan, A. Research trends of heavy metal removal from aqueous environments. J. Environ. Manag. 2021, 287, 112322. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Micropor. Mesopor. Mat. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, X.; Liu, C.; Ran, X.; Wang, C.; Lu, B. Mercapto-functionalized ordered mesoporous silica-modified PVDF membrane for efficiently scavenging Cd2+ from water. J. Envir. Manag. 2019, 302, 114103. [Google Scholar] [CrossRef]
- Yantasee, W.; Rutledge, R.D.; Chouyyok, W.; Sukwarotwat, V.; Orr, G.; Warner, C.L.; Warner, M.G.; Fryxell, G.E.; Wiacek, R.J.; Timchalk, C.; et al. Functionalized nanoporous silica for the removal of heavy metals from biological systems: Adsorption and application. ACS Appl. Mater. Interfaces 2010, 2, 2749–2758. [Google Scholar] [CrossRef] [Green Version]
- Kothalawala, N.; Blitz, J.P.; Gun’ko, V.M.; Jaroniec, M.; Grabicka, B.; Semeniuc, R.F. Post-synthesis surface-modified silicas as adsorbents for heavy metal ion contaminants Cd(II), Cu(II), Cr(III), and Sr(II) in aqueous solutions. J. Colloid. Interf. Sci. 2013, 392, 57–64. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Wisniewska, M.; Wołowicz, A.; Gun’ko, V.M.; Zarko, V.I. Mixed silica-alumina oxide as sorbent for dyes and metal ions removal from aqueous solutions and wastewaters. Micropor. Mesopor. Mat. 2017, 250, 128–147. [Google Scholar] [CrossRef]
- Soares, M.C.F.; Viana, M.M.; Schaefer, Z.L.; Gangoli, V.S.; Cheng, Y.; Caliman, V.; Wong, M.S.; Silva, G.G. Surface modification of carbon black nanoparticles by dodecylamine: Thermal stability and phase transfer in brine medium. Carbon 2014, 72, 287–295. [Google Scholar] [CrossRef]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mat. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Labun, P.; Grulova, D.; Salamon, I.; Šeršeň, F. Calculating the silicon in horsetail (Equisetum arvense L.) during the vegetation season. Food Nutr. Sci. 2013, 4, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Szulc, W.; Rutkowska, B.; Hoch, M.; Ptasiński, D.; Kazberuk, W. Plant available silicon in differentiated fertilizing conditions. Plant Soil Environ. 2019, 65, 233–239. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerriero, G.; Stokes, I.; Exley, C. Is callose required for silicification in plants? Biol. Lett. 2018, 14, 20180338. [Google Scholar] [CrossRef] [Green Version]
- Alyosef, H.A.; Schneider, D.; Wassersleben, S.; Roggendorf, H.; Weiß, M.; Eilert, A.; Denecke, R.; Hartmann, I.; Enke, D. Meso/macroporous silica from miscanthus, cereal remnant pellets, and wheat straw. ACS Sustain. Chem. Eng. 2015, 3, 2012–2021. [Google Scholar] [CrossRef]
- Rosiah, O.; Nor, H.A.; Khamirul, A.M.; Mohd Nizar, H.; Ismayadi, I.; Syazwan, M. Effect of temperature towards rice husk silica characterization with different preparation methods. IJBAS-IJENS 2017, 17, 15–20. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Steenari, B.M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of ash sintering during combustion of agricultural residues and the effect of additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Wang, L.; Skreiberg, Ø.; Becidan, M.; Li, H. Sintering of rye straw ash and effect of additives. Energy Procedia 2014, 61, 2008–2011. [Google Scholar] [CrossRef] [Green Version]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 16421. [Google Scholar] [CrossRef]
- Sulym, I.; Goncharuk, O.; Sternik, D.; Terpilowski, K.; Derylo-Marczewska, A.; Borysenko, M.V.; Gun’ko, V.M. Nanooxide/polymer composites with silica@PDMS and ceria-zirconia-silica@PDMS: Textural, morphological, and hydrophilic/hydrophobic features. Nanoscale Res. Lett. 2017, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef]
- Choi, K.; Lee, S.; Park, J.O.; Park, J.-A.; Cho, S.-H.; Lee, S.Y.; Lee, J.H.; Choi, J.-W. Chromium removal from aqueous solution by a PEI-silica nanocomposite. Sci. Rep. 2018, 8, 1438. [Google Scholar] [CrossRef] [Green Version]
- Dinker, M.K.; Kulkarni, P.S. Recent advances in silica-based materials for the removal of hexavalent chromium: A Review. J. Chem. Eng. Data 2015, 60, 2521–2540. [Google Scholar] [CrossRef]
- Hao, S.; Verlotta, A.; Aprea, P.; Pepe, F.; Caputo, D.; Zhu, W. Optimal synthesis of amino-functionalized mesoporous silicas for the adsorption of heavy metal ions. Micropor. Mesopor. Mat. 2016, 236, 250–259. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Francisco, W.; Canuto de Menezes, B.R.; Cividanes, L.D.; Coutinho, A.R.; Thim, G.P. Carbon nanotube functionalized with dodecylamine for the effective dispersion in solvents. Appl. Surf. Sci. 2015, 357, 2154–2159. [Google Scholar] [CrossRef]
- Council Directive 98/83/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31998L0083&from=EN (accessed on 12 April 2021).
- Dong, X.; Wu, Z.; Li, X.; Xiao, L.; Yang, M.; Li, Y.; Duan, J.; Sun, Z. The size-dependent cytotoxicity of amorphous silica nanoparticles: A systematic review of in vitro studies. Int. J. Nanomed. 2020, 15, 9089–9113. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [Green Version]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int. J. Nanomed. 2011, 6, 2821–2835. [Google Scholar]
- Nemmar, A.; Albarwani, S.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Attoub, S.; Ali, B.H. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int. J. Nanomed. 2014, 9, 2779–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Bu, D.; Zhang, N.; Tian, D.; Ma, L.; Yang, H. Cytotoxicity of mesoporous silica modified by amino and carboxyl groups on vascular endothelial cells. Environ. Toxicol. 2021, 36, 1422–1433. [Google Scholar] [CrossRef]


| Biomass | Mean Size(nm) |
|---|---|
| Horsetail (Equisetum arvense) | 166 ± 34 688 ± 49 890 ± 93 |
| Common reed (Phragmites australis) | 1867 ± 668 5521 ± 55 |
| Wheat straw (Triticum aestivum) | 422 ± 38 1395 ± 245 |
| Rye straw (Secale cereale) | 317 ± 6 1199 ± 180 |
| Silica Source | Horsetail | Common Reed | Wheat Straw | Rye Straw |
|---|---|---|---|---|
| SSA (m2/g) | 305 | 249 | 293 | 236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara-Lora, I.; Wronski, N.; Bialas, A.; Osip, H.; Czosnek, C. Efficient Adsorption of Chromium Ions from Aqueous Solutions by Plant-Derived Silica. Molecules 2022, 27, 4171. https://doi.org/10.3390/molecules27134171
Guevara-Lora I, Wronski N, Bialas A, Osip H, Czosnek C. Efficient Adsorption of Chromium Ions from Aqueous Solutions by Plant-Derived Silica. Molecules. 2022; 27(13):4171. https://doi.org/10.3390/molecules27134171
Chicago/Turabian StyleGuevara-Lora, Ibeth, Norbert Wronski, Anna Bialas, Honorata Osip, and Cezary Czosnek. 2022. "Efficient Adsorption of Chromium Ions from Aqueous Solutions by Plant-Derived Silica" Molecules 27, no. 13: 4171. https://doi.org/10.3390/molecules27134171
APA StyleGuevara-Lora, I., Wronski, N., Bialas, A., Osip, H., & Czosnek, C. (2022). Efficient Adsorption of Chromium Ions from Aqueous Solutions by Plant-Derived Silica. Molecules, 27(13), 4171. https://doi.org/10.3390/molecules27134171







