A Water-Soluble Epoxy-Based Green Crosslinking System for Stabilizing PVA Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
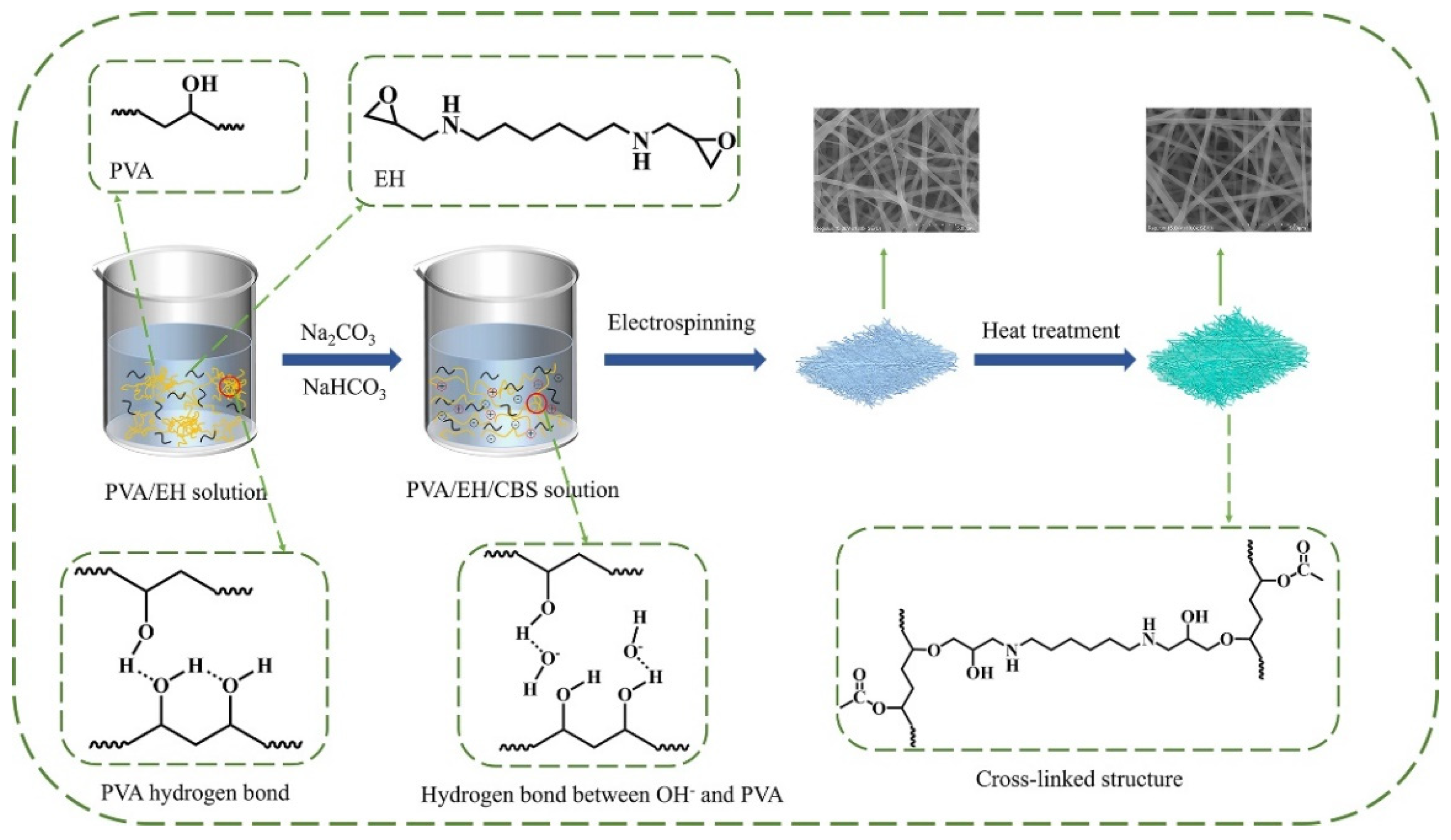
2.2. Preparation of Electrospinning Solutions
2.3. Preparation of Nanofibers
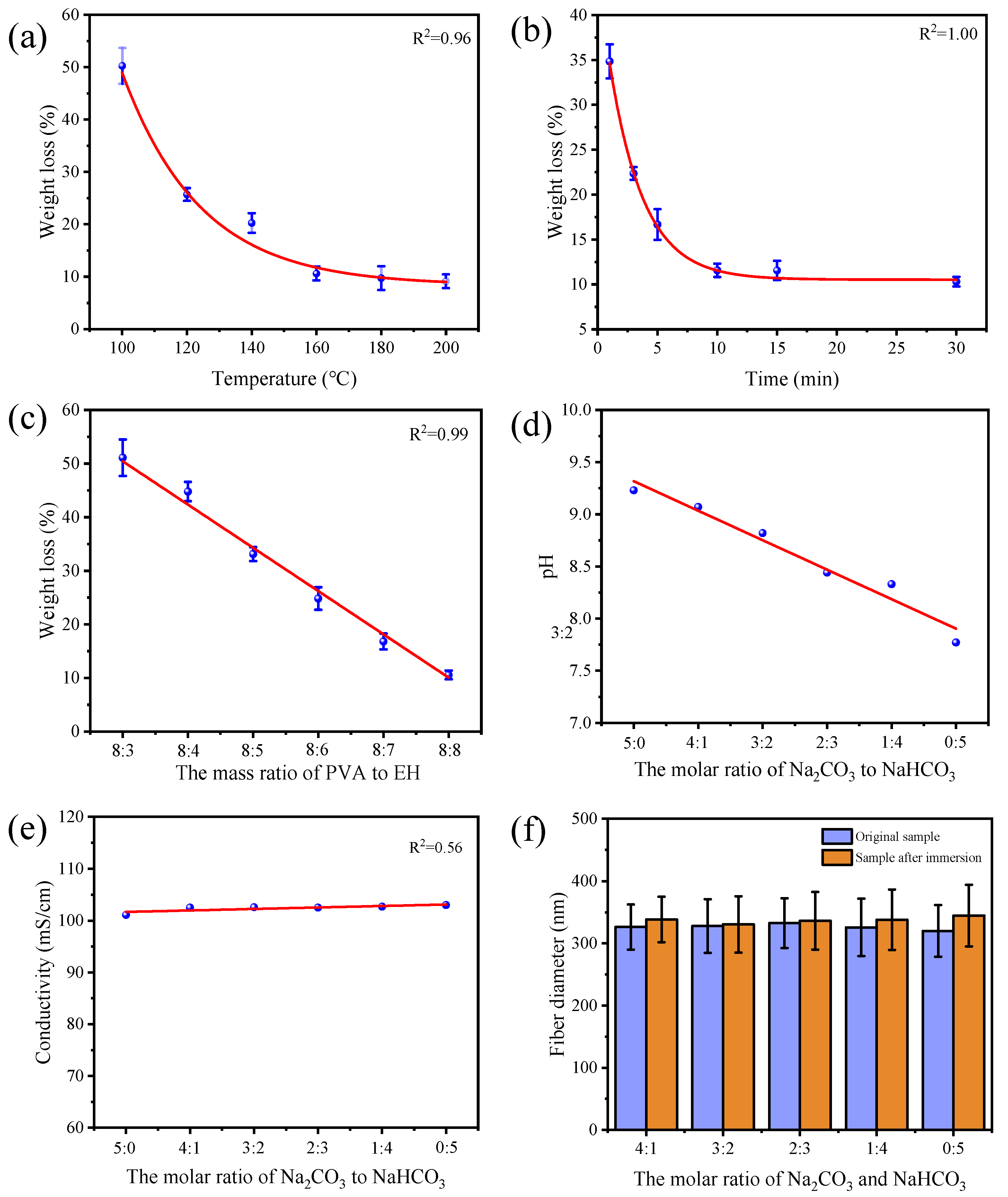
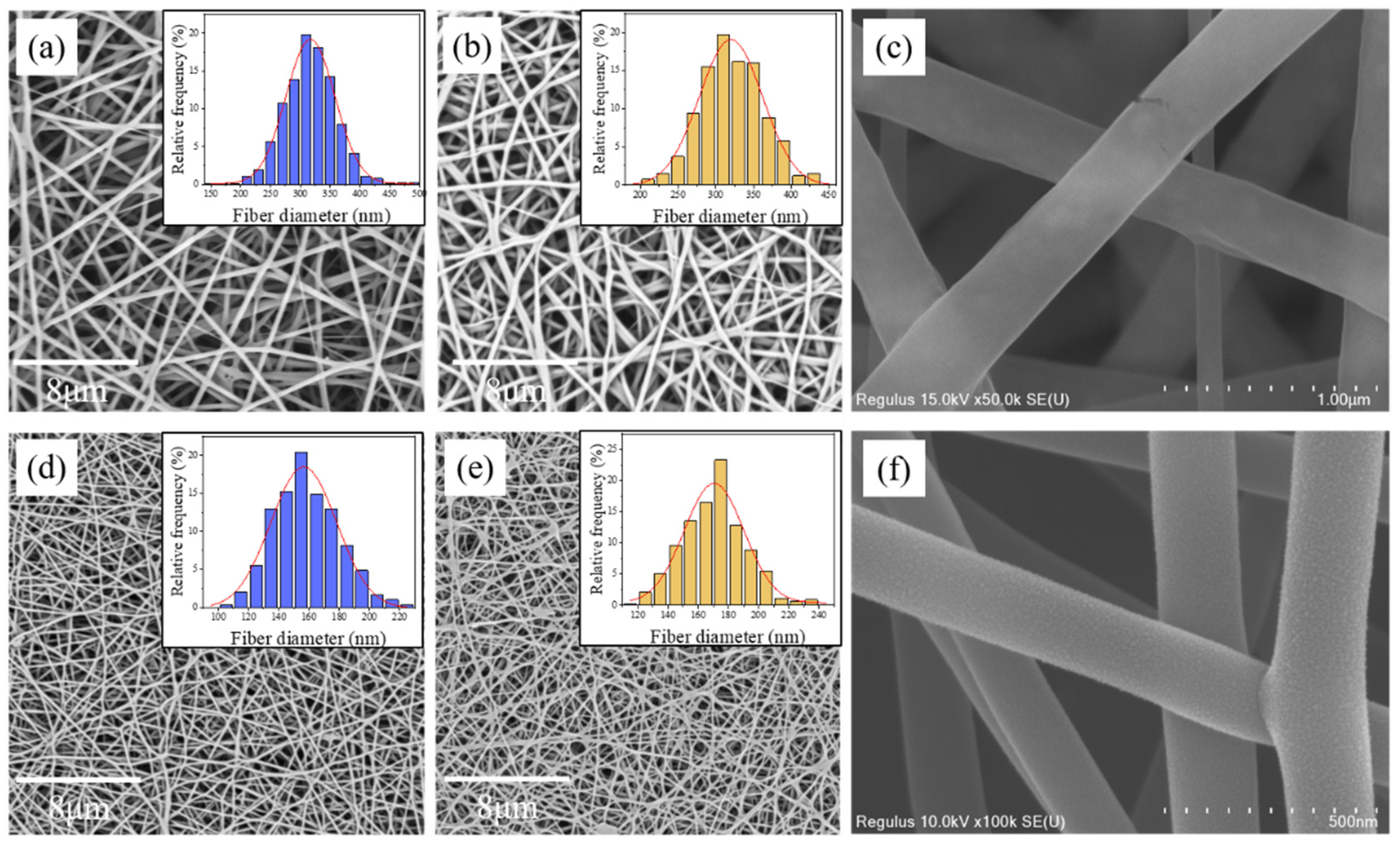
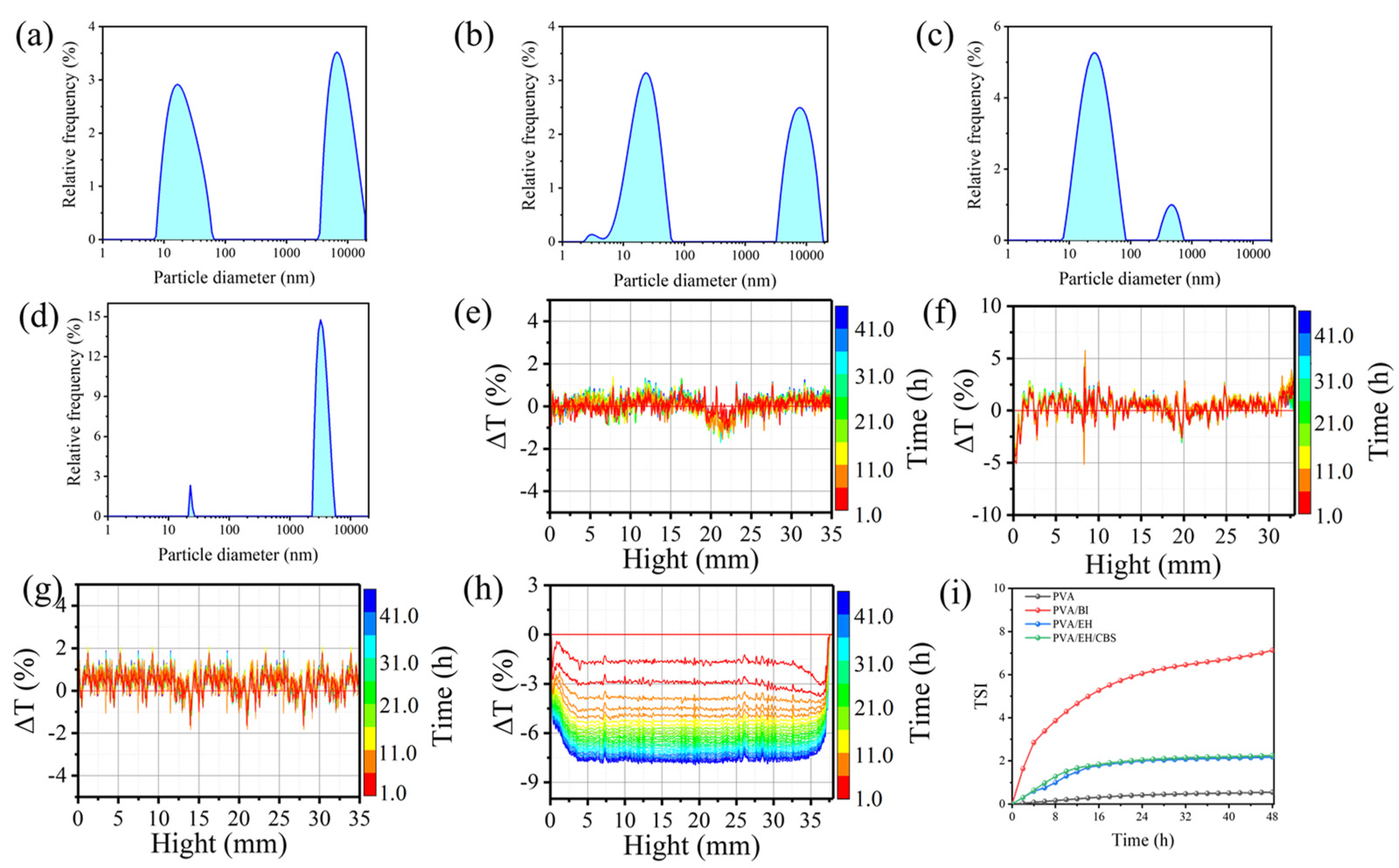
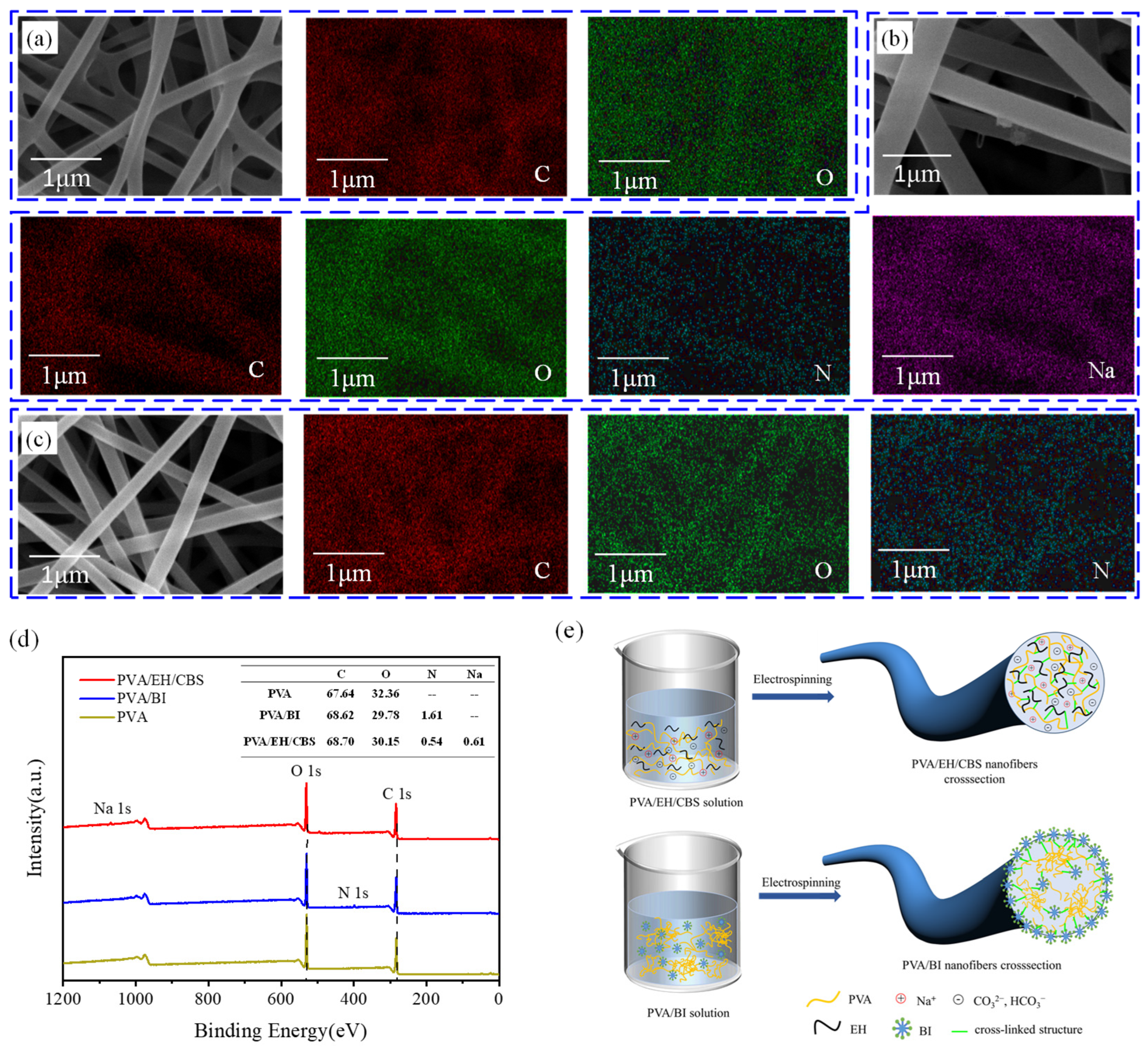
2.4. Characterizations
2.5. Water Resistance of Nanofibers
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly electrospun membranes loaded with visible-light-responding nanoparticles for multifunctional usages: Highly efficient air filtration, dye scavenging, and bactericidal activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Chen, L.-F.; Lu, Y.; Yu, L.; Lou, X.W. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2018, 37, 1917–1928. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Han, S.-J.; Shin, Y.-S.; Kang, I.-K. Fabrication of Biodegradable Polyester Nanocomposites by Electrospinning for Tissue Engineering. J. Nanomater. 2011, 2011, 929378. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, M.; Guo, R.; Huang, Q.; Wang, S.; Shi, X. Fabrication of multiwalled carbon nanotube-reinforced electrospun polymer nanofibers containing zero-valent iron nanoparticles for environmental applications. J. Mater. Chem. 2010, 20, 5700–5708. [Google Scholar] [CrossRef]
- Levitt, A.S.; Alhabeb, M.; Hatter, C.B.; Sarycheva, A.; Dion, G.; Gogotsi, Y. Electrospun MXene/carbon nanofibers as supercapacitor electrodes. J. Mater. Chem. A 2019, 7, 269–277. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- De Schoenmaker, B.; Van der Schueren, L.; Zugle, R.; Goethals, A.; Westbroek, P.; Kiekens, P.; Nyokong, T.; De Clerck, K. Effect of the relative humidity on the fibre morphology of polyamide 4.6 and polyamide 6.9 nanofibres. J. Mater. Sci. 2012, 48, 1746–1754. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Manivasakan, P.; Rajendran, V. Hydrophobic and thermal behaviour of nylon 6 nanofibre web deposited on cotton fabric through electrospinning. Micro Nano Lett. 2014, 9, 519–522. [Google Scholar] [CrossRef]
- Zhang, M.; Sheng, J.; Yin, X.; Yu, J.; Ding, B. Polyvinyl Butyral Modified Polyvinylidene Fluoride Breathable-Waterproof Nanofibrous Membranes with Enhanced Mechanical Performance. Macromol. Mater. Eng. 2017, 302, 1600272. [Google Scholar] [CrossRef]
- Zhan, F.; Yan, X.; Li, J.; Sheng, F.; Li, B. Encapsulation of tangeretin in PVA/PAA crosslinking electrospun fibers by emulsion-electrospinning: Morphology characterization, slow-release, and antioxidant activity assessment. Food Chem. 2021, 337, 127763. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Yin, R.; Ma, G.; Yang, D.; Nie, J. Electrospinning of methoxy poly(ethylene glycol)-grafted chitosan and poly(ethylene oxide) blend aqueous solution. Carbohydr. Polym. 2011, 83, 270–276. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Petrova, V.A.; Golovkin, A.S.; Mishanin, A.I.; Romanov, D.P.; Chernyakov, D.D.; Poshina, D.N.; Skorik, Y.A. Cytocompatibility of Bilayer Scaffolds Electrospun from Chitosan/Alginate-Chitin Nanowhiskers. Biomedicines 2020, 8, 305. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Z.; Zhang, Q.; Ravi, M.; Yu, M.; Tan, Y.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Polymer/block copolymer blending system as the compatible precursor system for fabrication of mesoporous carbon nanofibers for supercapacitors. J. Power Sources 2019, 419, 137–147. [Google Scholar] [CrossRef]
- Peng, Y.; Ding, S.; Wen, Z.; Xu, S.; Lv, W.; Xu, Z.; Yang, Y.; Wang, Y.; Wei, Y.; Tang, Y. Thin-film encapsulation of organic electronic devices based on vacuum evaporated lithium fluoride as protective buffer layer. Appl. Phys. A 2017, 123, 108. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Li, C.; Shen, Y.; Wang, D. Cross-linked polyvinyl alcohol modified by aziridine cross-linker for effective paper sizing. Prog. Org. Coat. 2021, 161, 106482. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Bhardwaj, N.K. Mixing of oxidized starch and polyvinyl alcohol for surface sizing of paper. Nord. Pulp Pap. Res. J. 2019, 34, 343–353. [Google Scholar] [CrossRef]
- Zuber, M.; Zia, K.M.; Bhatti, I.A.; Jamil, T.; Fazal-ur-Rehman; Rizwan, A. Modification of cellulosic fabric using polyvinyl alcohol, part-II: Colorfastness properties. Carbohydr. Polym. 2012, 87, 2439–2446. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, C.; Li, X.; Yao, Y.; Lan, L.; Zhao, F.; Ye, Z.; Ying, Y.; Ping, J. All-electrospun flexible triboelectric nanogenerator based on metallic MXene nanosheets. Nano Energy 2019, 59, 268–276. [Google Scholar] [CrossRef]
- Moritani, T.O. T Functional modification of poly(vinyl alcohol) by copolymerization: IV. Self-crosslinkable poly(vinyl alcohol)s. Polymer 1997, 39, 923–931. [Google Scholar] [CrossRef]
- Han, B.; Li, J.; Chen, C.; Xu, C.; Wickramasinghe, S.R. Effects of Degree of Formaldehyde Acetal Treatment and Maleic Acid Crosslinking on Solubility and Diffusivity of Water in PVA Membranes. Chem. Eng. Res. Des. 2003, 81, 1385–1392. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Jiang, Z.; Sakai, E.; Qiu, J.; Zhu, P. Highly temperature resistant cellulose nanofiber/polyvinyl alcohol hydrogel using aldehyde cellulose nanofiber as cross-linker. Cellulose 2019, 26, 5291–5303. [Google Scholar] [CrossRef]
- Shaikh, R.P.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Crosslinked electrospun PVA nanofibrous membranes: Elucidation of their physicochemical, physicomechanical and molecular disposition. Biofabrication 2012, 4, 025002. [Google Scholar] [CrossRef]
- Tang, C.; Saquing, C.D.; Harding, J.R.; Khan, S.A. In Situ Cross-Linking of Electrospun Poly(vinyl alcohol) Nanofibers. Macromolecules 2009, 43, 630–637. [Google Scholar] [CrossRef]
- Bo, J. Study on PVA hydrogel crosslinked by epichlorohydrin. J. Appl. Polym. Sci. 1992, 46, 783–786. [Google Scholar] [CrossRef]
- Reis, A.V.; Fajardo, A.R.; Schuquel, I.T.A.; Guilherme, M.R.; Vidotti, G.J.; Rubira, A.F.; Muniz, E.C. Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Groups of Poly(vinyl alcohol) and Poly(acrylic acid): Is This Reaction Mechanism Still Unclear? J. Org. Chem. 2009, 74, 3750–3757. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Zhu, C.; Zhang, Q.; Luo, Z. Small-Angle X-ray Scattering Study on Covalently Cross-linked Polyvinyl Alcohol/Epoxy Resin Membrane in Polyphosphoric Acid. Adv. Compos. Lett. 2016, 25, 694–702. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- Czibulya, Z.; Csik, A.; Toth, F.; Pal, P.; Csarnovics, I.; Zelko, R.; Hegedus, C. The Effect of the PVA/Chitosan/Citric Acid Ratio on the Hydrophilicity of Electrospun Nanofiber Meshes. Polymers 2021, 13, 3557. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Yang, E.L. Green Electrospinning and Crosslinking of Polyvinyl Alcohol/Citric Acid. J. Nano Res. 2015, 32, 32–42. [Google Scholar] [CrossRef]
- Sonker, A.K.; Verma, V. Influence of crosslinking methods toward poly(vinyl alcohol) properties: Microwave irradiation and conventional heating. J. Appl. Polym. Sci. 2018, 135, 46125. [Google Scholar] [CrossRef]
- Arik, N.; Inan, A.; Ibis, F.; Demirci, E.A.; Karaman, O.; Ercan, U.K.; Horzum, N. Modification of electrospun PVA/PAA scaffolds by cold atmospheric plasma: Alignment, antibacterial activity, and biocompatibility. Polym. Bull. 2018, 76, 797–812. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ooi, C.W.; Song, C.P.; Wang, C.Y.; Liu, B.L.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antibacterial efficacy of quaternized chitosan/poly (vinyl alcohol) nanofiber membrane crosslinked with blocked diisocyanate. Carbohydr. Polym. 2021, 262, 117910. [Google Scholar] [CrossRef]
- Stone, S.A.; Gosavi, P.; Athauda, T.J.; Ozer, R.R. In situ citric acid crosslinking of alginate/polyvinyl alcohol electrospun nanofibers. Mater. Lett. 2013, 112, 32–35. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Li, T.; Wang, W.; Wang, W.; Yu, G. Novel crosslinked chitosan for enhanced adsorption of hexavalent chromium in acidic solution. Chem. Eng. J. 2018, 347, 782–790. [Google Scholar] [CrossRef]
- Das, G.; Deka, B.K.; Lee, S.H.; Park, Y.-B.; Yoon, Y.S. Poly(vinyl alcohol)/silica nanoparticles based anion-conducting nanocomposite membrane for fuel-cell applications. Macromol. Res. 2015, 23, 256–264. [Google Scholar] [CrossRef]
- Hesheng, L.F.X. Preparation and Properties of Poly(vinyl alcohol) modified with Tri-hydroxymethyl Aminomethane. Polym. Mater. Sci. Eng. 2018, 34, 138–144. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, R.; Wu, C.; Qian, R. Viscometric investigation of intramolecular hydrogen bonding cohesional entanglement in extremely dilute aqueous solution of poly vinyl alcohol. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2421–2427. [Google Scholar] [CrossRef]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Strong adsorption properties and mechanism of action with regard to tetracycline adsorption of double-network polyvinyl alcohol-copper alginate gel beads. J. Hazard. Mater. 2022, 422, 126863. [Google Scholar] [CrossRef] [PubMed]

| Serial Number | Na2CO3 (mol/L) | NaHCO3 (mol/L) | Na2CO3/NaHCO3 Molar Ratio |
|---|---|---|---|
| 1 | 0.2 | 0 | 5/0 |
| 2 | 0.16 | 0.04 | 1/4 |
| 3 | 0.12 | 0.08 | 3/2 |
| 4 | 0.08 | 0.12 | 2/3 |
| 5 | 0.04 | 0.16 | 1/4 |
| 6 | 0 | 0.2 | 0/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Fang, K.; Wang, W.; Niu, H. A Water-Soluble Epoxy-Based Green Crosslinking System for Stabilizing PVA Nanofibers. Molecules 2022, 27, 4177. https://doi.org/10.3390/molecules27134177
Zhang Y, Fang K, Wang W, Niu H. A Water-Soluble Epoxy-Based Green Crosslinking System for Stabilizing PVA Nanofibers. Molecules. 2022; 27(13):4177. https://doi.org/10.3390/molecules27134177
Chicago/Turabian StyleZhang, Yujian, Kuanjun Fang, Wei Wang, and Haitao Niu. 2022. "A Water-Soluble Epoxy-Based Green Crosslinking System for Stabilizing PVA Nanofibers" Molecules 27, no. 13: 4177. https://doi.org/10.3390/molecules27134177
APA StyleZhang, Y., Fang, K., Wang, W., & Niu, H. (2022). A Water-Soluble Epoxy-Based Green Crosslinking System for Stabilizing PVA Nanofibers. Molecules, 27(13), 4177. https://doi.org/10.3390/molecules27134177






