2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study
Abstract
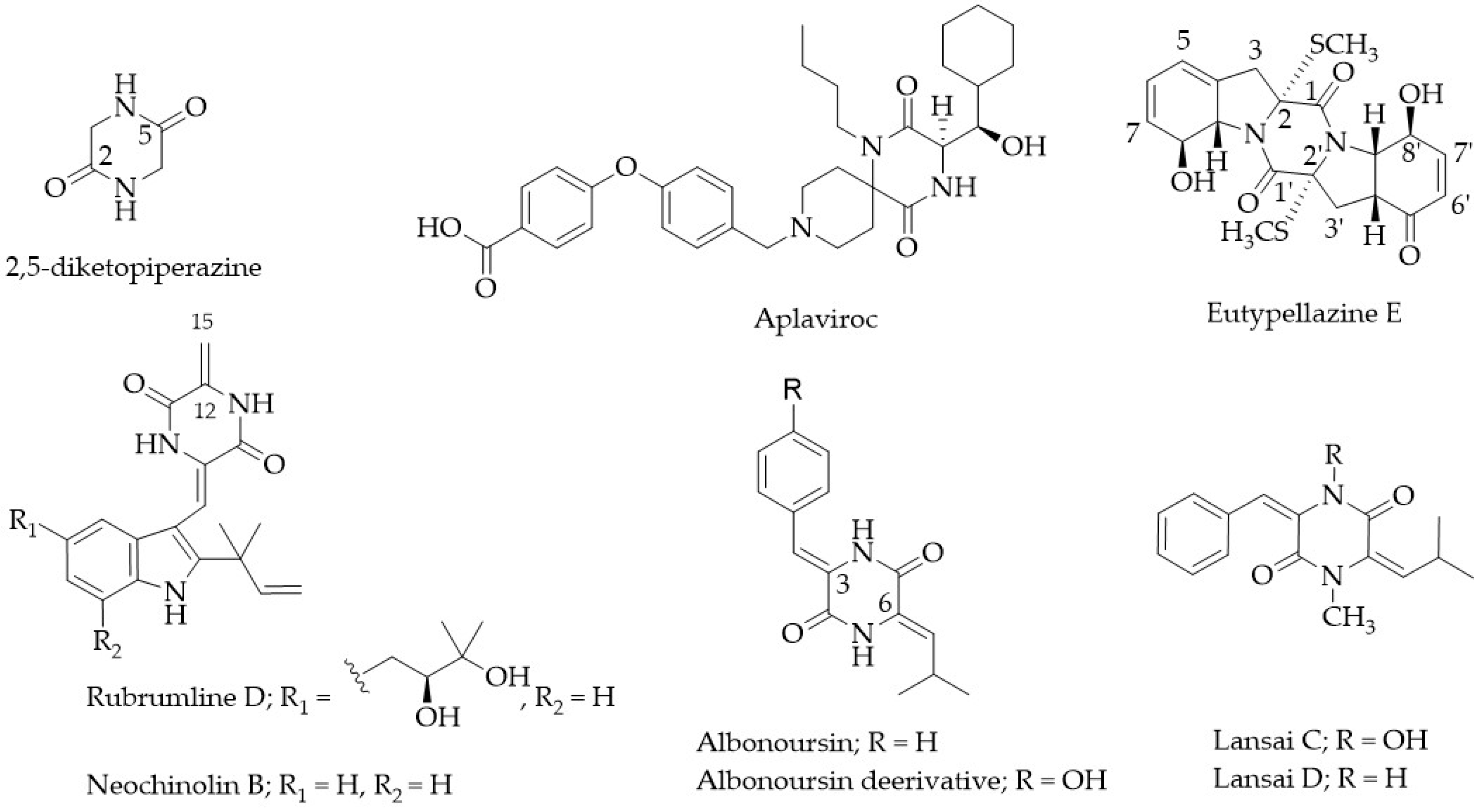
:1. Introduction
2. Results and Discussion
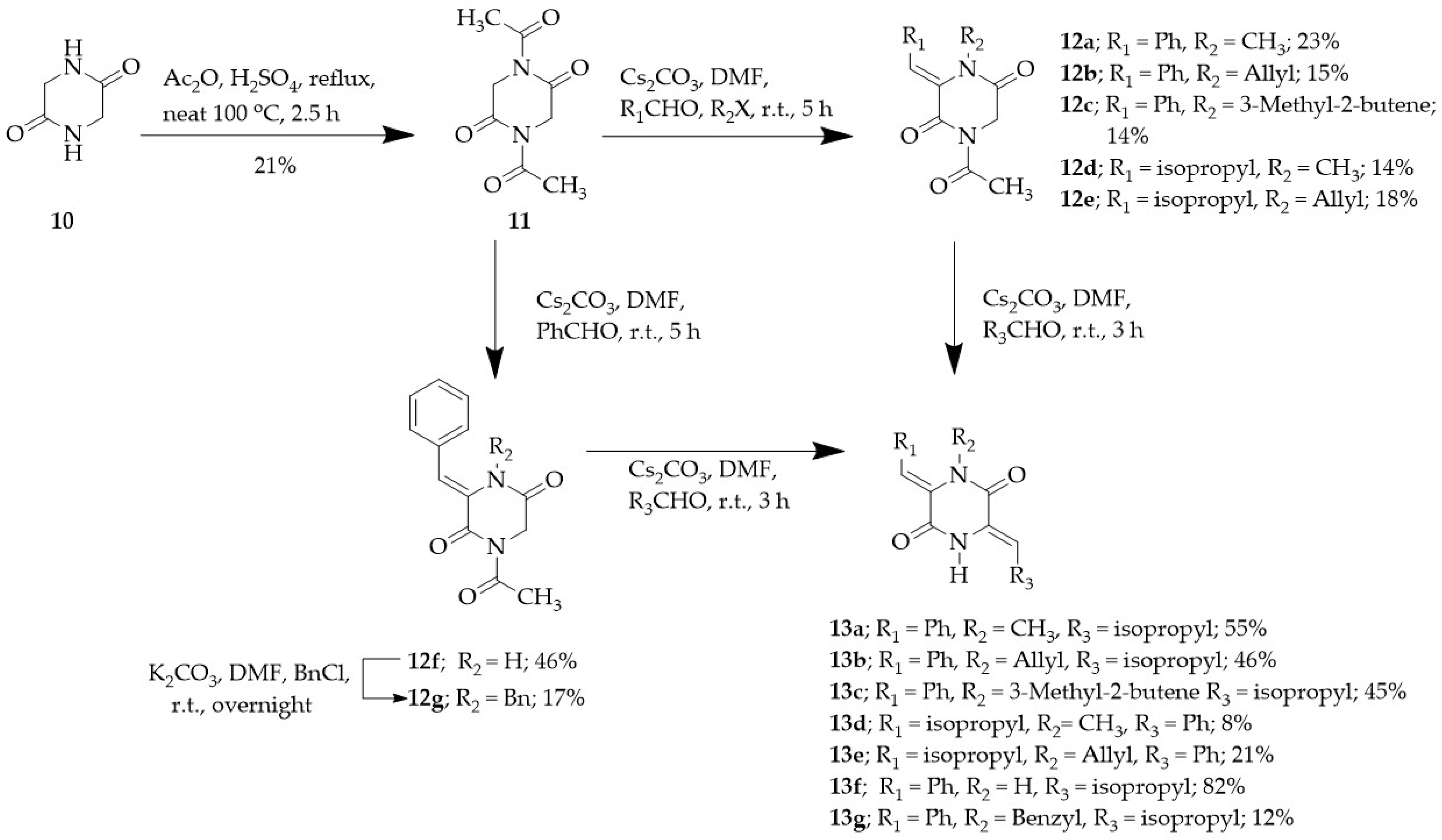
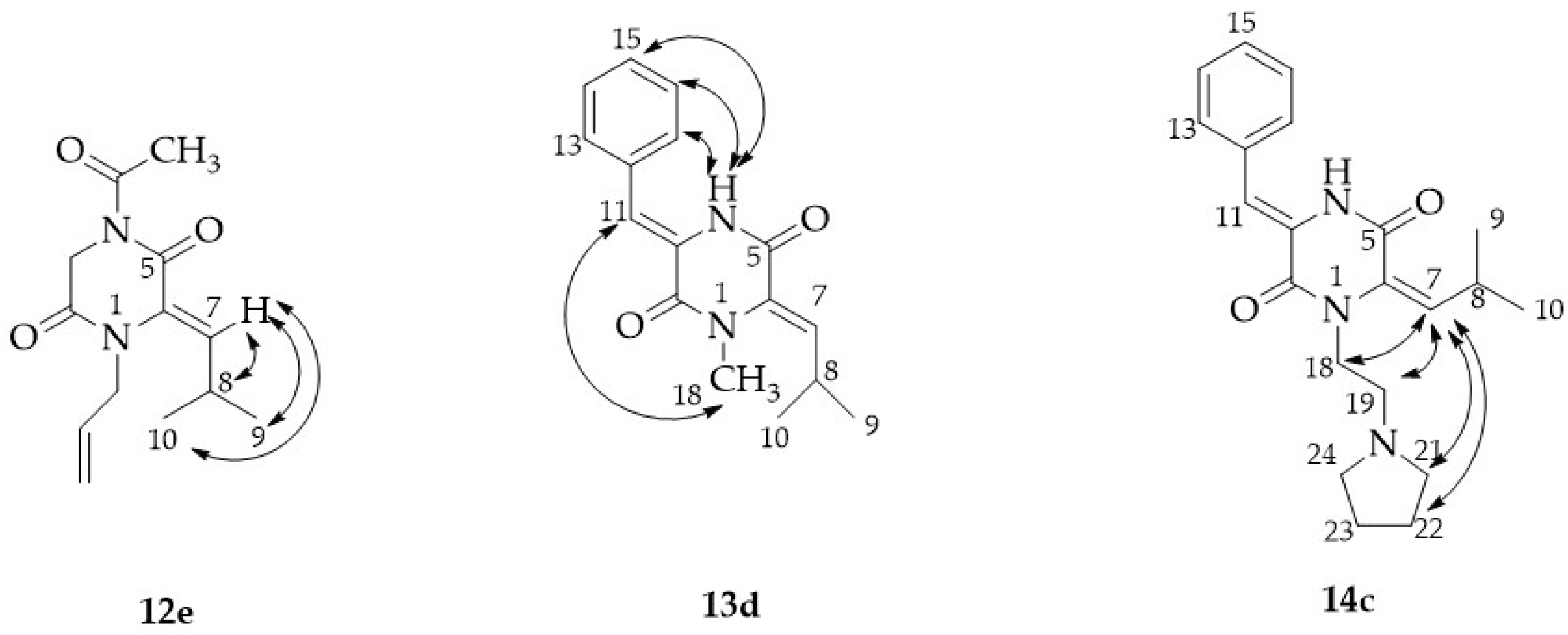
2.1. Synthesis
2.2. Virus Propagation Inhibition Assay
2.3. Molecular Docking Study
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis of 2,5-DKP Derivatives
3.2.1. Methyl (N-tert-butoxycarbonyl)-l-leucyl-l-phenylalaninate (3)
3.2.2. Methyl-l-leucyl-l-phenylalaninate (4)
3.2.3. (3S,6S)-3-Benzyl-6-isobutyl-2,5-diketopiperazine (5)
3.2.4. (3S,6S)-3-Benzyl-6-isobutyl-1,4-dimethyl-2,5-diketopiperazine (6)
3.2.5. General Procedure for the Synthesis of (3S,6S)3-Benzyl-1,4-disubstituted-6-isobutyl-2,5-diketopiperazine (7–9)
(3S,6S)-1,3,4-Tribenzyl-6-isobutyl-2,5-diketopiperazine (7)
(3S,6S)-3-Benzyl-6-isobutyl-1,4-bis(3-methylbut-2-en-1-yl)-2,5-diketopiperazine (8)
(3S,6S)-1,4-Diallyl-3-benzyl-6-isobutyl-2,5-diketopiperazine (9)
3.2.6. 2,5-Diketopiperazine (10)
3.2.7. 1,4-Diacetyl-2,5-diketopiperazine (11)
3.2.8. General Procedure for the Synthesis of (3Z)-4-Acetyl-3-benzylidene-1-substituted-2,5-diketopiperazine (12a–e)
(3Z)-1-Acetyl-3-benzylidene-4-methyl-2,5-diketopiperazine (12a)
(3Z)-1-Acetyl-4-allyl-3-benzylidene-2,5-diketopiperazine (12b)
(3Z)-1-Acetyl-3-benzylidene-4-(3-methylbut-2-ene-1-yl)-2,5-diketopiperazine (12c)
(6Z)-4-Acetyl-6-(2-methylpropylidene)-1-methyl-2,5-diketopiperazine (12d)
(6Z)-4-Acetyl-1-allyl-6-(2-methylpropylidene)-2,5-diketopiperazine (12e)
3.2.9. (3Z)-1-Acetyl-3-benzylidene-2,5-diketopiperazine (12f)
3.2.10. (3Z)-1-Acetyl-4-benzyl-3-benzylidene-2,5-diketopiperazine (12g)
3.2.11. General Procedure for the Synthesis of (3Z,6Z)-3-Benzylidene-6-(2-methyl propylidene)-4-substituted-2,5-diketopiperazine (13a–f)
(3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-4-methyl-2,5-diketopiperazine (13a)
(3Z,6Z)-4-Allyl-3-benzylidene-6-(2-methylpropylidene)-2,5-diketopiperazine (13b)
(3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-4-(3-methyl but-2-en-1-yl)-2,5-diketopiperazine (13c)
(3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-1-methyl-2,5-diketopiperazine (13d)
(3Z,6Z)-1-Allyl-3-benzylidene-6-(2-methylpropylidene)-2,5-diketopiperazine (13e)
(3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-2,5-diketopiperazine (13f)
(3Z,6Z)-4-Benzyl-3-benzylidene-6-(2-methylpropylidene)-2,5-diketopiperazine (13g)
3.2.12. General Procedure for the Synthesis of (3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-1,4-disubstituted-2,5-diketopiperazine (14a–b)
(3Z,6Z)-3-Benzylidene-6-(2-methylpropylidene)-1,4-bis(-3-methylbut-2-en-1yl)-2,5-diketopiperazine (14a)
(3Z,6Z)-1,4-Dibenzyl-3-benzylidene-6-(2-methylpropylidene)-2,5-diketo piperazine (14b)
(3Z,6E)-3-Benzylidene-6-(2-methylpropylidene)-1-(1-ethyl pyrrolidine)-2,5-diketopiperazine (14c)
3.3. Virus Propagation Inhibition Assay
3.4. Hemagglutination Assay (HA)
4. Cytotoxicity
5. Molecular Docking Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Borthwick, A.D. 2,5-Diketopiperazine: Synthesis, reactions, medicinal chemistry and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Timothé, M.; Nicolas, G.; Ganesan, A.; Mihaela, G.; Dominique, B. Three cheers for nitrogen: Aza-DKPs, the aza analogues of 2, 5-Diketopiperazines. RSC Adv. 2020, 10, 43358–43370. [Google Scholar] [CrossRef]
- Gong, G.; Qi, J.; Lv, Y.; Dong, S.; Cao, C.; Li, D.; Zhao, L.; Li, Z.; Chen, X. Discovery of 1,3-Disubstituted 2, 5-Diketopiperazine Derivatives as Potent Class I HDACs Inhibitors. Chem. Pharm. Bull. 2020, 68, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Liu, X.H.; Zheng, Y.Y.; Ning, X.Y.; Zhang, Y.; Fu, X.M.; Li, X.; Shao, C.L.; Wang, C. 2,5-Diketopiperazines from a Sponge-Derived Fungus Aspergillus sclerotiorum. Front. Microbiol. 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and Biological Activities of Diketopiperazines from Marine Organisms: A Review. Mar. Drugs. 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.M.; Yi, X.X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C.H. An update on 2,5-diketopiperazines from marine organisms. Mar. Drugs. 2014, 12, 6213–6235. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A–M, thiodoketopiperazine-type alkaloids from deep sea derived fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and its analogues as potential entry inhibitors of influenza viruses, targeting viral hemagglutinin. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef]
- Wang, P.; Xi, L.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W. Diketopiperazine derivatives from the marine-derived actinomycete Streptomyces sp. FXJ7.328. Mar. Drugs. 2013, 11, 1035–1049. [Google Scholar] [CrossRef] [Green Version]
- Tuntiwachwuttikul, P.; Taechowisan, T.; Wanbanjob, A.; Thadaniti, S.; Taylor, W.C. Lansai A–D, secondary metabolites from Streptomyces sp. SUC1. Tetrahedron 2008, 64, 7583–7586. [Google Scholar] [CrossRef]
- Taechowisan, T.; Wanbanjob, A.; Tuntiwachwuttikul, P.; Thadaniti, S.; Taylor, W.C. Phytopharmacology and Therapeutic Value V. In Recent Progress in Medicinal Plants; Singh, V.K., Govil, J.N., Bhardwaj, R., Eds.; Studium Press: Houston, TX, USA, 2007; Volume 23, pp. 337–343. [Google Scholar]
- Taechowisan, T.; Wanbanjob, A.; Tuntiwachwuttikul, P.; Liu, J. Anti-inflammatory activity of lansais from endophytic Streptomyces sp. SUC1 in LPS-induced RAW 256.7 cells. Food. Agric. Immunol. 2009, 20, 67–77. [Google Scholar] [CrossRef]
- Taechowisan, T.; Wanbanjob, A.; Tuntiwachwuttikul, P.; Liu, J. Anti-inflammatory effects of lansai C and D cause inhibition of STAT-1 and NF-κB activations in LPS-induced RAW 256.7 cells. Food. Agric. Immunol. 2010, 20, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, C.J.; Cuevas, F.; Romero-Estudillo, I.; Boto, A. Synthesis of diketopiperazine scaffolds with tailored N- and α- chains by selective modification of customizable units. Adv. Synth. Catal. 2020, 362, 3158–3169. [Google Scholar] [CrossRef]
- Rajput, S.; Mclean, K.J.; Poddar, H.; Selvam, I.R.; Nagalingam, G.; Triccass, J.A.; Levy, C.W.; Munro, A.W.; Hutton, C.A. Structure-acitivity relationships of cyclo(L-tyrosyl-L-tyrpsine) derivatives binding to mycobacterium tuberculosis CYP121: Iodinated analogues promote shift to high-spin adduct. J. Med. Chem. 2019, 62, 9792–9805. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Qin, X.; Li, D.; Tu, Z.; Zhou, X.; Wang, J.; Yang, B.; Lin, X.; Liu, J.; Yang, X.; et al. Design and synthesis of novel soluble 2,5-diketopiperazine derivatives as potential anticancer agents. Eur. J. Med. Chem. 2014, 83, 236–244. [Google Scholar] [CrossRef]
- Balducci, D.; Conway, P.A.; Sapuppo, G.; Müller-Bunz, H.; Paradisi, F. Novel approach to the synthesis of aliphatic and aromatic α-keto acids. Tetrahedron 2012, 68, 7374–7379. [Google Scholar] [CrossRef]
- Ando, S.; Grote, A.L.; Koide, K. Diastereoselective synthesis of diketopiperazine Bis-α,β-epoxide. J. Org. Chem. 2011, 76, 1155–1158. [Google Scholar] [CrossRef] [Green Version]
- Lautru, S.; Gondry, M.; Genet, R.; Pernodet, J.-L. The albonoursin gene cluster of S. noursei: Biosynthesis of diketopiperazine metabolites independent of nonribosomal peptide synthetases. Chem. Biol. 2002, 9, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Fairhurst, M.E.; Zeeshan, M.; Haug, B.E.; Bayer, A. Aldol condensation on a 3-alkylidene-2,5-diketopiperazine: Synthesis of two marine natural products. Synlett 2018, 29, 1303–1306. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio—BIOVIA—Dassault Systèmes®. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/biovia-discovery-studio (accessed on 31 May 2022).
- Hsu, K.C.; Chen, Y.F.; Lin, S.R.; Yang, J.M. iGEMDOCK: A graphical environment of enhancing GEMDOCK using pharmacological interactions and post-screening analysis. BMC Bioinform. 2011, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, J.E.; Bowen, R.A. Effect of oseltamivir carboxylate consumption on emergence of drug-resistant H5N2 avian influenza virus in mallard ducks. Antimicrob. Agents Chemother. 2013, 57, 2171–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, K.; Perry, C.M. Oseltamivir. Drugs 2001, 61, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R. Oseltamivir in human avian influenza infection. J. Antimicrob. Chemother. 2010, 65 (Suppl. 2), ii25–ii33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneghan, C.J.; Onakpoya, I.; Thompson, M.; Spencer, E.A.; Jones, M.; Jefferson, T. Zanamivir for influenza in adults and children: Systematic review of clinical study reports and summary of regulatory comments. BMJ 2014, 348, g2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, M.M.; Skoglund, E.W.; Ison, M.G. Peramivir: An intravenous neuraminidase inhibitor. Expert Opin. Pharmacother. 2015, 16, 1889–1900. [Google Scholar] [CrossRef]
- Russell, R.J.; Haire, L.F.; Stevens, D.J.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.J.; Skehel, J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef]
- Collins, P.; Haire, L.; Lin, Y.; Liu, J.; Russell, R.J.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S.J. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.J.; Barrett, S.; Peat, T.S.; Newman, J.; Streltsov, V.A.; Waddington, L.; Saito, T.; Tashiro, M.; McKimm-Breschkin, J.L. Structural and Functional Basis of Resistance to Neuraminidase Inhibitors of Influenza B Viruses. J. Med. Chem. 2010, 53, 6421–6431. [Google Scholar] [CrossRef] [PubMed]
- Pozdnev, V.F.; Podgornova, N.N.; Zentsova, N.K.; Aukone, G.I.; Kalei, U.O. Preparation of tert-butoxycarbonyl derivatives of amino acids using di-tert-butyl pyrocarbonate. Chem. Nat. Compd. 1979, 15, 471–476. [Google Scholar] [CrossRef]
- Samsawat, T.; Jaramornburapong, C.; Phutdhawong, W.; Phutdhawong, W.S.; Taechowisan, T. Evaluating the effect of amine-geldanamycin hybrids on anticancer activity. J. Appl. Pharm. Sci. 2021, 11, 098–107. [Google Scholar] [CrossRef]
- Nitecki, D.E.; Halpern, B.; Westley, J.W. A simple route to sterically pure diketopiperazines. J. Org. Chem. 1968, 33, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, W.; Jia, Y. Synthetic studies of cyclic peptides stephanotic cid methyl ester, celogentin C, and moroidin. Tetrahedron 2014, 70, 7753–7762. [Google Scholar] [CrossRef]
- 2,5-diketopiperazine. Available online: http://en.wikipedia.org/wiki/2,5-Diketopiperazine (accessed on 17 November 2021).
- Liao, S.; Qin, C.; Yao, J.; Li, J.; Zhou, X.; Wang, J.; Huang, Z.; Liu, Y. One-pot synthesis of polysubstituted 3-amino-2-oxo-2,7-dihydro-1H-azepines. Synthesis 2014, 46, 621–628. [Google Scholar] [CrossRef]
- Katrizky, A.R.; Fan, W.-Q.; Szajda, M.; Li, Q.-L.; Caster, K.C. Conjugated systems derived from piperazine-2,5-dione. J. Heterocyclic. Chem. 1988, 25, 591–597. [Google Scholar] [CrossRef]
- Shin, C.-G.; Chigira, Y.; Masaki, M.; Ohta, M. Synthesis of albonoursin. Bull. Chem. Soc. Jpn. 1969, 42, 191–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, R.; Chen, P. Influenza virus propagation in embryonated chicken eggs. J. Vis. Exp. 2015, 97, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tim, M. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]



| Compounds | HA Titer * Concentrations (µg/mL) | IC50 (µg/mL) | |||
|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | LLC-MK2 | |
| LS-C | Negative | NT | Negative | >1:3072 | 507.84 |
| LS-D | 1:12 | NT | 1:24 | >1:3072 | 437.33 |
| 6 | 1:48 | 1:12 | 1:6 | >1:3072 | NT |
| 7 | Negative | 1:6 | 1:6 | >1:3072 | NT |
| 8 | 1:24 | 1:1.5 | 1:3 | >1:3072 | NT |
| 9 | Negative | 1:6 | 1:24 | >1:3072 | NT |
| 13a | Negative | Negative | 1:1.5 | >1:3072 | NT |
| 13b | Negative | Negative | Negative | >1:3072 | 330.28 |
| 13c | Negative | Negative | Negative | >1:3072 | 386.42 |
| 13d | Negative | Negative | Negative | >1:3072 | 353.51 |
| 13e | 1:24 | 1:1.5 | 1:6 | >1:3072 | NT |
| 13g | 1:6 | 1:1.5 | 1:384 | >1:3072 | NT |
| 14a | 1:12 | 1:786 | 1:6 | >1:3072 | NT |
| 14b | 1:24 | 1:6 | 1:1.5 | >1:3072 | NT |
| 14c | Negative | Negative | Negative | >1:3072 | 287.65 |
| 1-Adamantanamine hydrochloride | Negative | Negative | Negative | Negative | NT |
| Oseltamivir carboxylate | Negative | Negative | Negative | Negative | NT |
| PBS | >1:3072 | NT | |||
| Compounds | Binding Energy (kcal/mol) | Amino Acid Residues | H–bond Length (Å) |
|---|---|---|---|
| 7 | −85.34 | ASN249 | 1.84 |
| 9 | −77.18 | ARG371 | 2.09 |
| 13a | −85.09 | ARG371 | 2.45 |
| 13b | −77.87 | ARG371, GLN432 | 2.58, 1.87 |
| 13c | −91.93 | ARG371, ARG371, GLN432 | 2.76, 2.18, 2.84 |
| 13d | −101.86 | ARG371 | 2.24 |
| 14c | −102.25 | ARG371 | 1.84 |
| LS-C | −81.02 | ARG371 | 3.00 |
| Oseltamivir carboxylate | −93.75 | ASP151, ARG152, ARG292, ARG371, TYR406 | 2.58, 1.94, 1.95, 1.95, 2.49 |
| Zanamivir | −107.07 | ARG118, GLU119, ASP151, ASP152, TRP178, GLU277, ARG292, ARG294, ARG371 | 2.15, 3.02, 2.68, 2.39, 2.87, 2.28, 2.80, 2.05, 2.06, |
| Peramivir | −115.88 | ARG118, ASP151, ARG152, GLU277, ARG292, ARG371 | 2.25, 2.11, 1.91, 2.43, 2.49, 2.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winyakul, C.; Phutdhawong, W.; Tamdee, P.; Sirirak, J.; Taechowisan, T.; Phutdhawong, W.S. 2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study. Molecules 2022, 27, 4200. https://doi.org/10.3390/molecules27134200
Winyakul C, Phutdhawong W, Tamdee P, Sirirak J, Taechowisan T, Phutdhawong WS. 2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study. Molecules. 2022; 27(13):4200. https://doi.org/10.3390/molecules27134200
Chicago/Turabian StyleWinyakul, Chanakan, Weerachai Phutdhawong, Poomipat Tamdee, Jitnapa Sirirak, Thongchai Taechowisan, and Waya S. Phutdhawong. 2022. "2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study" Molecules 27, no. 13: 4200. https://doi.org/10.3390/molecules27134200





