Abstract
Blue mold caused by Penicillium expansum is one of the most common apple diseases, and it is becoming a serious threat in apple production. The strain Bacillus amyloliquefaciens Ba168 showed high levels of antimicrobial activity in our previous study. To analyze the antimicrobial protein of Ba168, a high-resolution LC-MS/MS proteomic analysis was performed. A total of 1155 proteins were identified from 5233 unique peptides. A total of 16 potential antimicrobial-activity-related proteins were identified; 10 of these proteins have direct antimicrobial effects, while 6 of these proteins are associated with the formation of antimicrobial substances. Then, an antifungal protein of Ba168 was isolated and purified by the sequential chromatography of DEAE Bio-sep FF anion exchange and Sephadex G-75. The single protein, named BP8-2, showed antifungal activity towards Penicillium expansum. The peptide mass fingerprinting of the protein band of BP8-2 had a high similarity with the amino acid sequences of flagellin protein. The results showed that BP8-2 significantly inhibited the growth of P. expansum and slowed the spread of apple blue mold. The results indicated that flagellin is one of the important antimicrobial substances from Ba168.
1. Introduction
Apple blue mold, caused by Penicillium expansum, is one of the most severe diseases, accounting for approximately 20% to 25% of economic loss during the postharvest storage and transport of fruits and vegetables [1]. Although people widely use chemical fungicides as an effective management strategy [2], sustainable and environmentally friendly strategies are required due to increasing environmental pollution, pesticide residues, and pesticide resistance [3]. Compared with the chemical synthetic fungicides of postharvest diseases, natural antagonistic microorganisms for biological control have emerged as a promising alternative [4]. Strains belonging to bacteria have been used as effective biocontrol agents (BCAs) for controlling multiple plant diseases [5,6,7], and bacillus-based productions comprise almost half of the commercially available biocontrol products all over the world [8]. In spite of much interest in the biocontrol of postharvest diseases, the biocontrol research of apple blue mold and its mechanisms is in the preliminary stage [9].
Bacillusamyloliquefaciens is an environmentally friendly Gram-positive bacterium, exhibiting rapid propagation and stress resistance [7,10]. The mechanisms of bacteriostatic inhibition involved in B. amyloliquefaciens include the production of antagonistic substances, the induction of host resistance, and competition between nutrition and growth space. The antagonistic substances produced by B. amyloliquefaciens, such as antibiotics, bacteriocin, cell-wall-degrading enzymes, and other antimicrobial proteins, are important in the prevention and inhibition of pathogens [11,12]. Lipopeptides (LPs) and polyketides are well-known antibiotics produced by Bacillus for plant protection, including surfactin, fengycin, iturin, difficidin, bacilysin, macrolactin, bacillaene, bacillomycin D, and bacillibactin [13]. However, there are still many secondary metabolites without separation and identification.
In our previous study, Bacillus amyloliquefaciens Ba168 was isolated from the Qingling Mountains of China; it has a significant control effect on a variety of plant diseases, including bacteria, fungi, and viruses [14,15]. B. amyloliquefaciens Ba168 has the characteristics of strong stress resistance, fast growth, and good stability, and it has been used in the control of tomato bacterial wilt, wheat yellow mosaic disease, wheat take-all, etc. However, the antibacterial substance and its antibacterial mechanism in the Ba168 were not studied. To rapidly characterize the unidentified active metabolites in fermentation broth, the proteins secreted by Ba168 in liquid medium were first identified using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis and identification. The antifungal protein was purified by column chromatography and identified through LC–MS/MS analysis.
2. Results
2.1. Identification of Antimicrobial Proteins in Ba168
In order to establish the protein expression profile of Ba168, proteomic analyses of Ba168 were performed using high-resolution LC-MS/MS. A total of 1155 proteins were identified with >95% confidence from 5233 unique peptides. A total of 16 potential antimicrobial-activity-related proteins were identified based on previous reports. In total, 10 of the identified proteins exhibited direct antimicrobial activity—namely, oxalate decarboxylase, a chitin-binding protein, flagellin, carboxypeptidase, aminopeptidase, an antimicrobial peptide, serine protease, subtilisin, leucine dehydrogenase, and β-glucanase. The other six proteins were associated with the formation of antimicrobial substances—namely, surfactin synthase, polyketide synthase, bacillomycin D synthetase C, bacillomycin D synthetase A, bacilysin biosynthesis protein, and antilisterial bacteriocin subtilosin biosynthesis protein (Table 1). The peptide mass spectra of eight proteins obtained by LC-MS/MS are shown in additional materials. The unique peptide sequences identified were as follows in: surfactin synthetase A, QAGCSADELSK (+MSn 583.2609 m/z); aminopeptidase, SSLPHGVASGK (+MSn 347.189 m/z); carboxypeptidase, HGYLAGEDR (+MSn 509.2411 m/z); polyketide synthase, AATDDSGNELPR (+MSn 623.289 m/z); chitin-binding protein, IASANGGSGQIDFGLDK (+MSn 825.4102 m/z); oxalate decarboxylase, TIASALVVVPGAMR (+MSn 757.4224 m/z); antimicrobial peptide, LVQSPNGNFAASFVLDGTK (+MSn 983.0076 m/z); flagellin, NAQDGISLIQTSEGALNETHSLLQR (+MSn 1348.1851 m/z).

Table 1.
The 16 potential antimicrobial proteins identified in Ba168.
2.2. Purification of the Antifungal Protein in Ba168
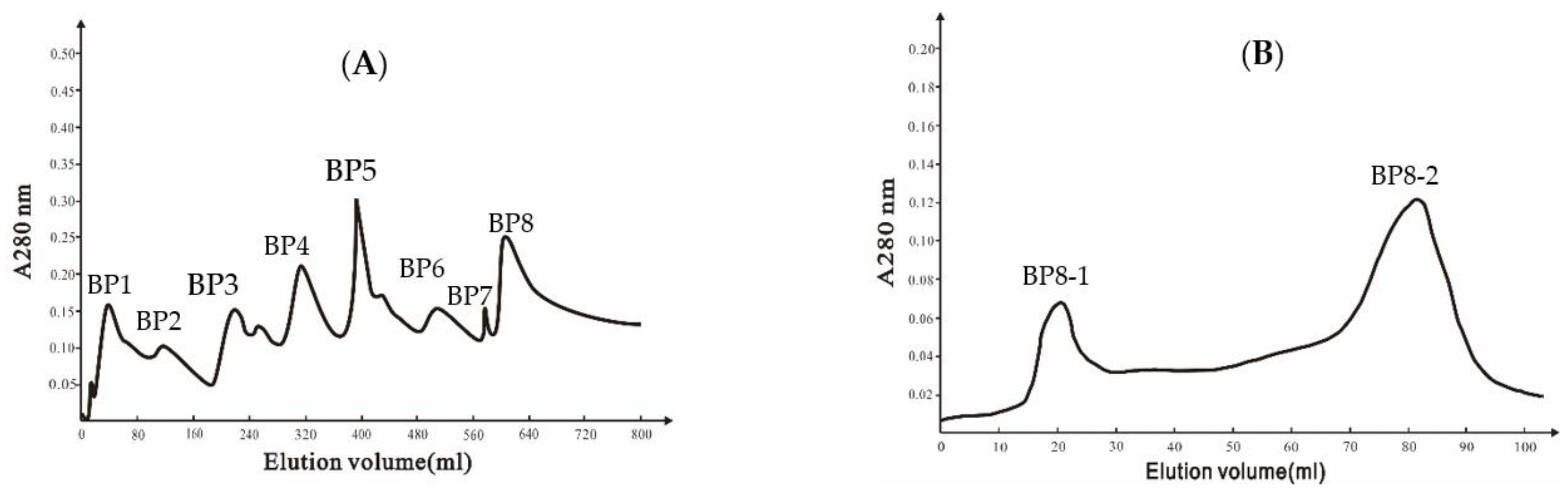
A DEAE Bio-sep FF chromatography column was used to isolate extracellular proteins from strain Ba168, and eight peaks were collected (Figure 1A). The eighth peak (BP8) showed the highest inhibitory ability against P. expansum. The eighth peak was further separated by Sephadex G-75 chromatography column, and two peaks were collected (Figure 1B), and the second peak (BP8-2) showed inhibitory ability against P. expansum.
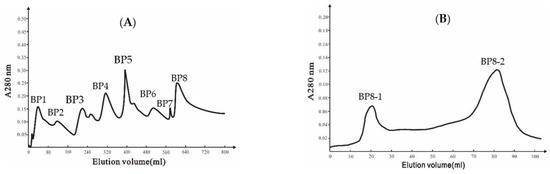
Figure 1.
(A) Ion-exchange chromatography of DEAE Bio-sep FF resin of Ba168 proteins; (B) gel chromatography of SephadexG-75 column of active fraction (peak BP8 in Figure 1A).
2.3. Inhibitory Effects of the Antifungal Protein BP8-2 on P. expansum In Vitro
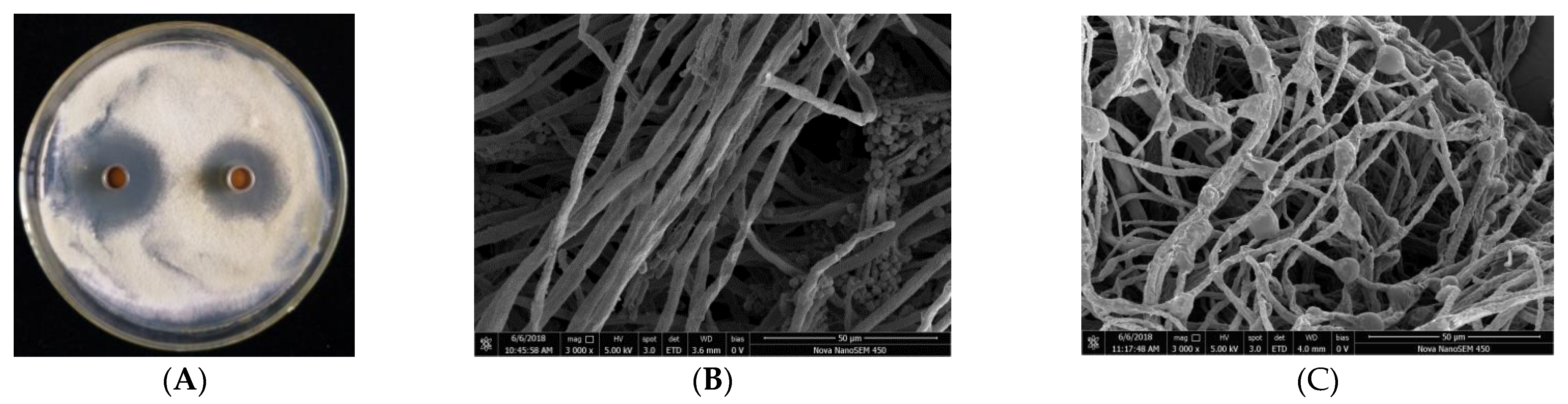
The antifungal capacity of the protein BP8-2 was tested in vitro and in vivo, with apple blue mold (caused by P. expansum) as the test pathogen. The protein BP8-2 could significantly inhibit the growth of P. expansum in vitro (Figure 2A). As observed by scanning electron microscopy (SEM), the untreated hyphae of P. expansum were smooth and uniform, and distinct sporangia could be observed (Figure 2B), while P. expansum treated with protein BP8-2 showed enlarged hyphae, vesicle formation, distortion, or empty cells devoid of cytoplasm (Figure 2C), and no conidia or spores were found in the treated P. expansum. The MIC of the BP8-2 against P. expansum was 45 µg/mL.

Figure 2.
(A) Inhibition of P. expansum by protein BP8-2. (B) Untreated hyphae of P. expansum under SEM. (C) Treated hyphae of P. expansum under SEM.
2.4. Control Effect of the Antifungal Protein BP8-2 on P. expansum In Vivo
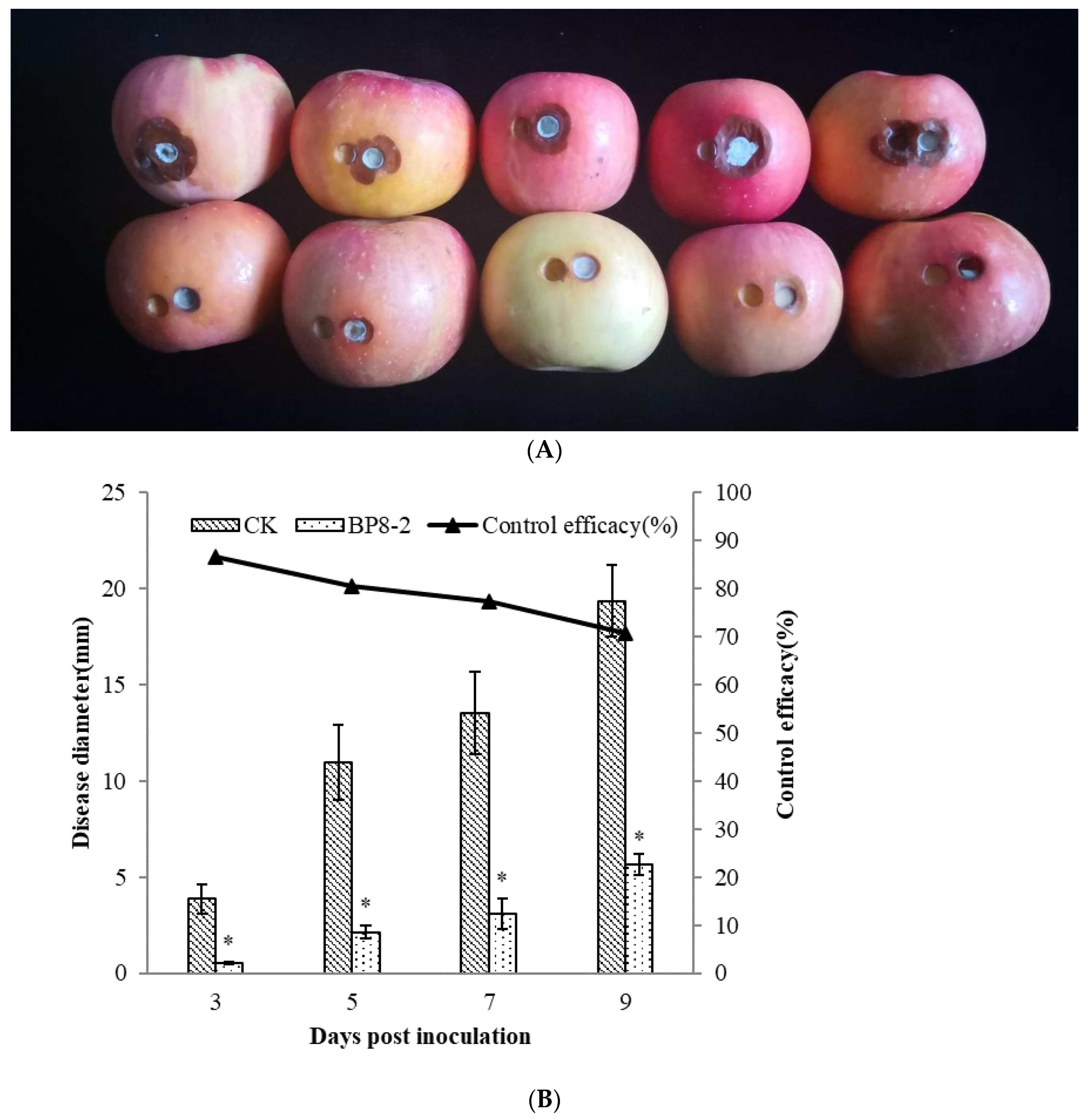
In order to test the antifungal capacity of the protein BP8-2 in vivo, the control effect of protein BP8-2 against P. expansum in red Fuji apples was tested. Protein BP8-2 could significantly inhibit the spread of P. expansum on red Fuji apples (Figure 3A), and its control effect against P. expansum on red Fuji apples was 86.56%, 80.57%, 77.22%, and 70.85% within 3, 5, 7, and 9 days post inoculation, respectively (Figure 3B).
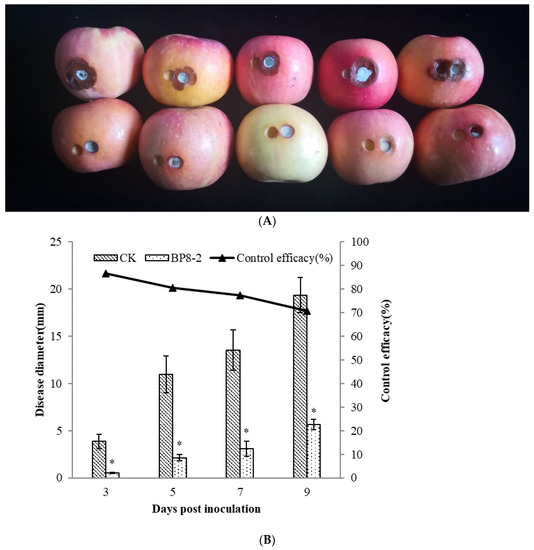
Figure 3.
The control effect of protein BP8-2 on P. expansum in red Fuji apples (A) and its data analysis (B). Statistical analysis was performed using Dunnet’s comparative test. * p <0.05, compared to control.
2.5. Identification of the Antifungal Protein BP8-2 and Amplification of Its Genomes
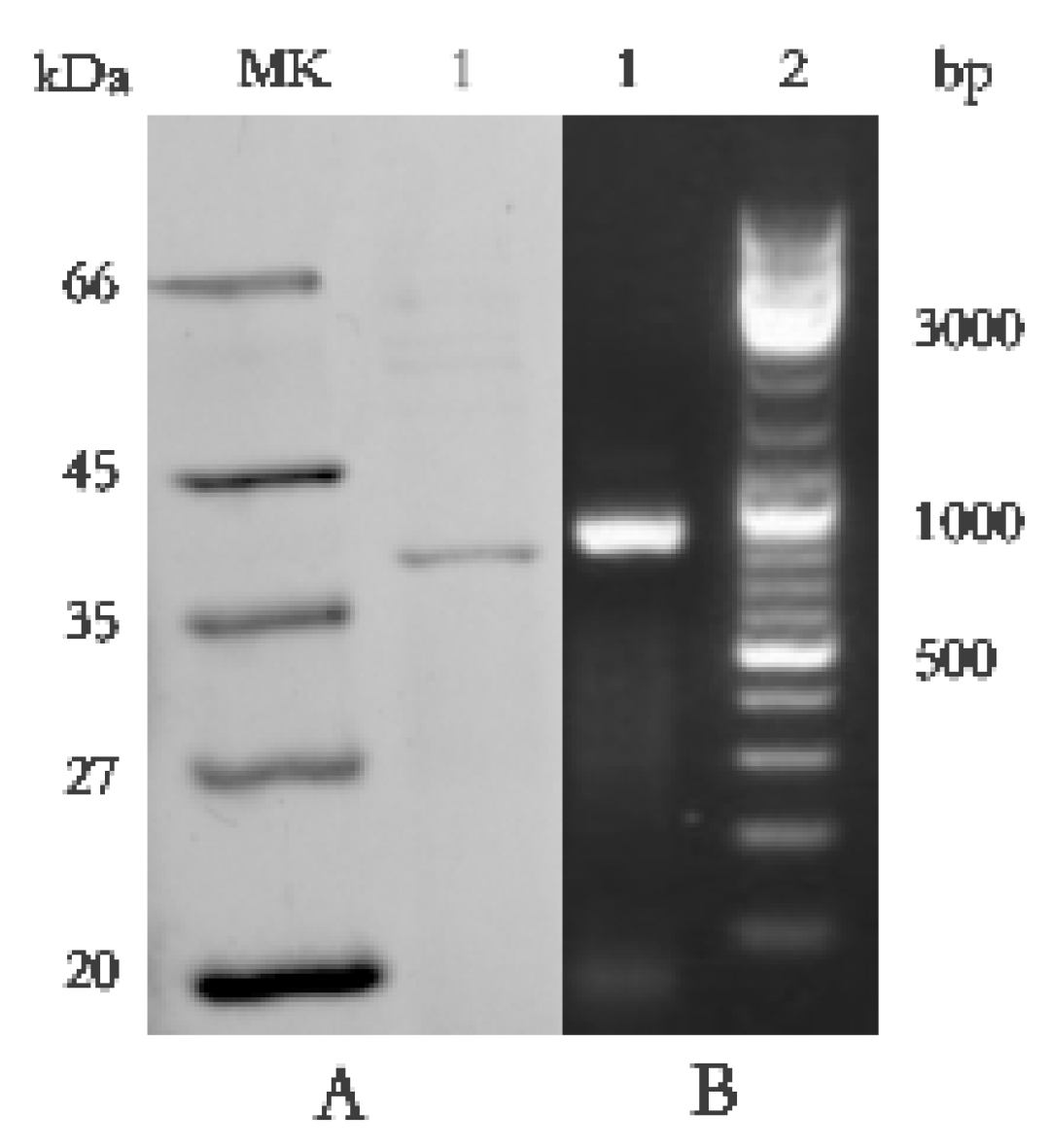
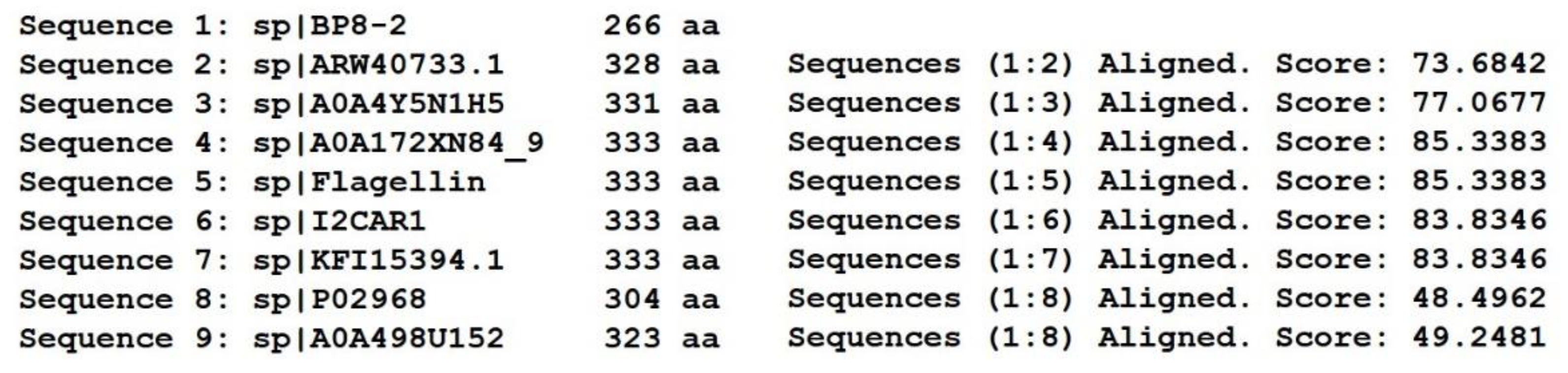
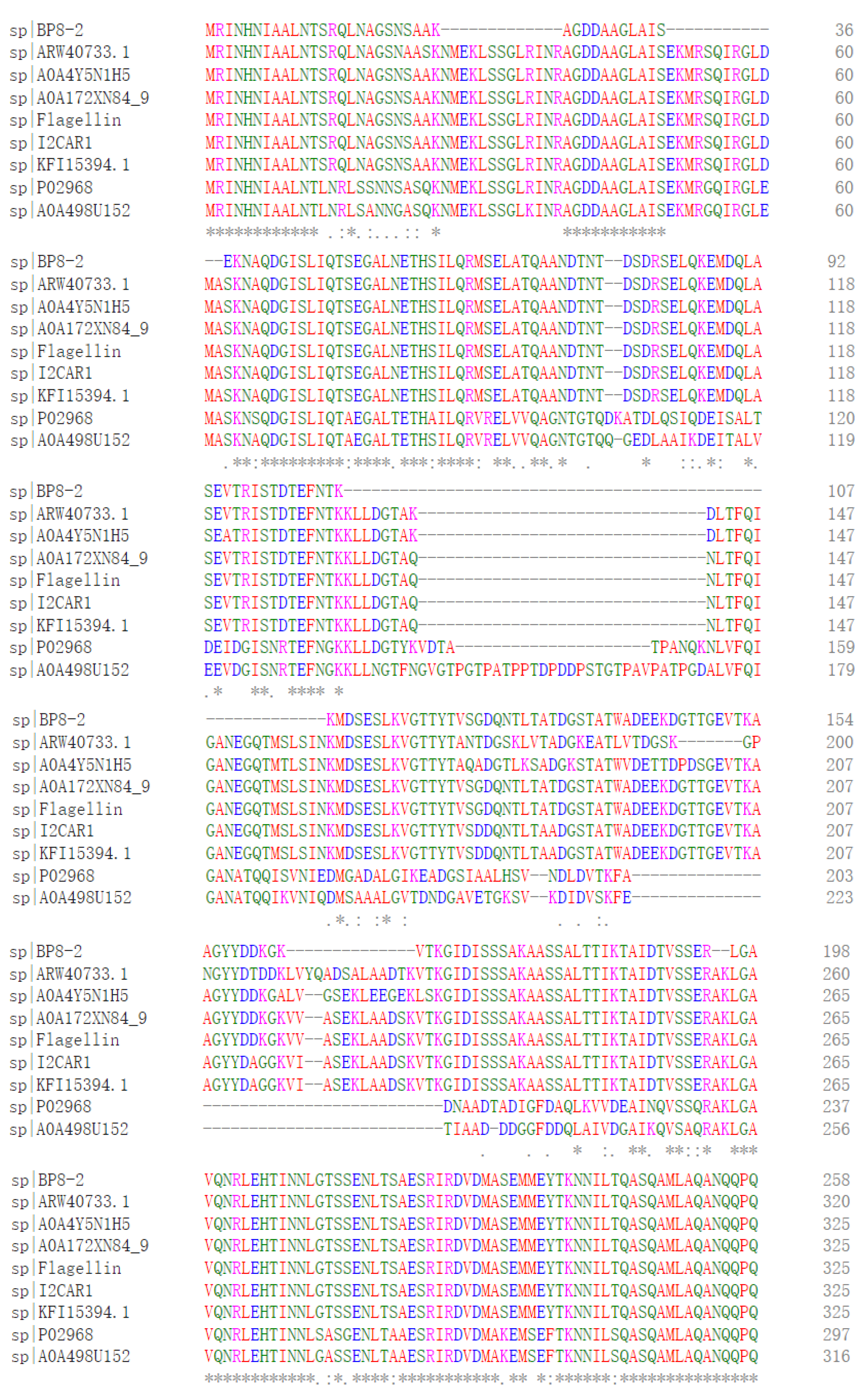
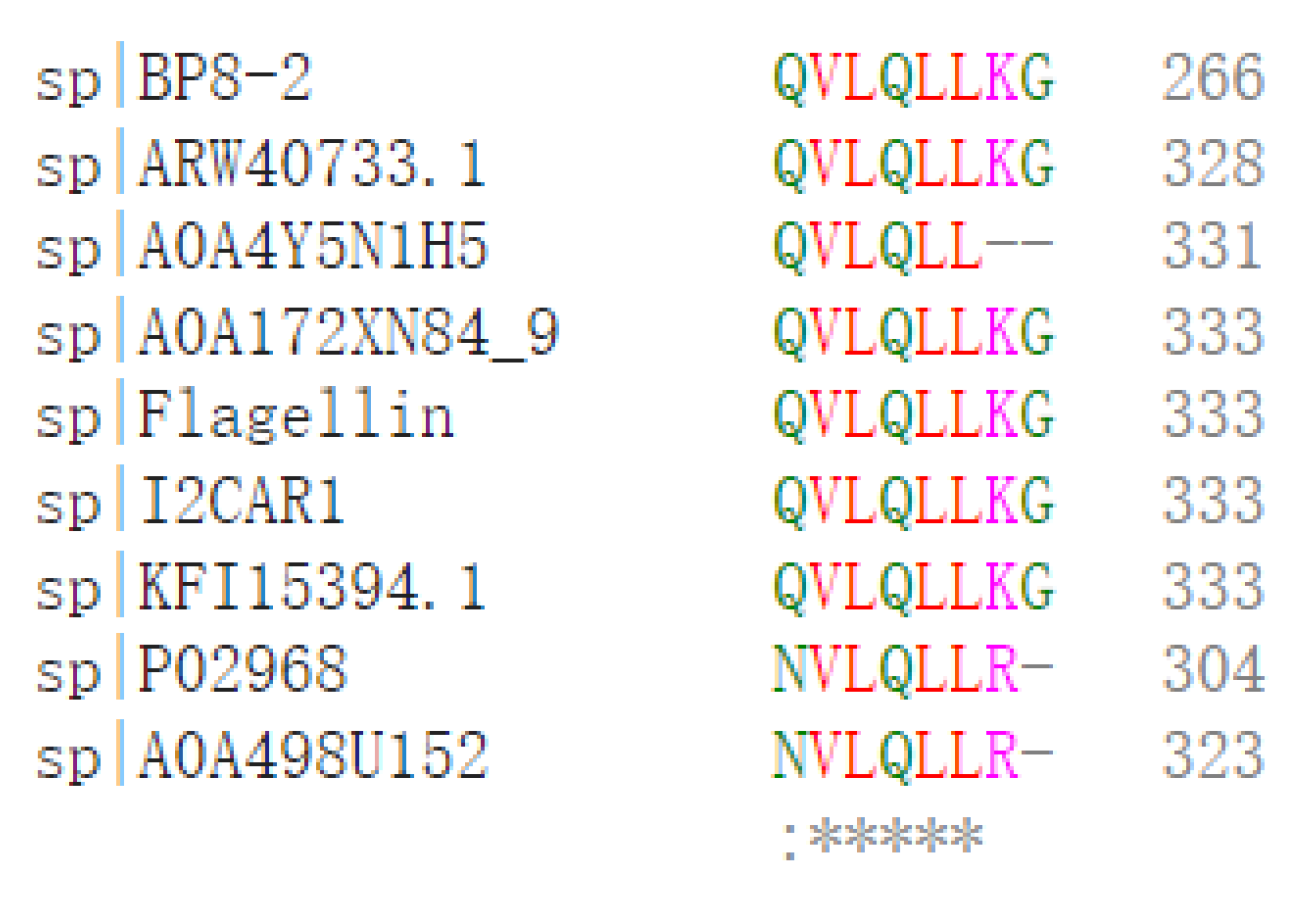
SDS-PAGE detection indicated that BP8-2 was a single protein with a molecular weight of 35 kDa (Figure 4A). The band of BP8-2 protein was cut off, enzymolysized, and then analyzed by LC-MS/MS. Having compared it with the MASCOT database, the BP8-2 showed the highest matching rate to the flagellin protein of Bacillus velezensis (NCBI: A0A172XN84_9BACI, p < 0.05), with a molecular weight of 35 532 Da and containing 333 amino acid sequences. There were 28 peptide fragments that matched, including 266 amino acid residues, with a sequence coverage of 79.9%. The protein BP8-2 peptide mapping finger sequences of Ba168 was compared with other flagellin proteins by the multiple sequence alignment analysis in the ClustalW online program (Figure 5). The sequence of BP8-2 is the most similar to the flagellin of B. velezensis and B. amyloliquefaciens. The similarity between BP8-2 and B. velezensis was the highest, 85.3383% and 83.8346%, respectively, followed by B. amyloliquefaciens, at 83.8346%, 77.6842%, and 73.6842%, respectively, while the similarity with other species of bacillus was only 48.4962% and 49.2481%. These results indicated that the antifungal protein BP8-2 identified in the current study was likely to be flagellin.
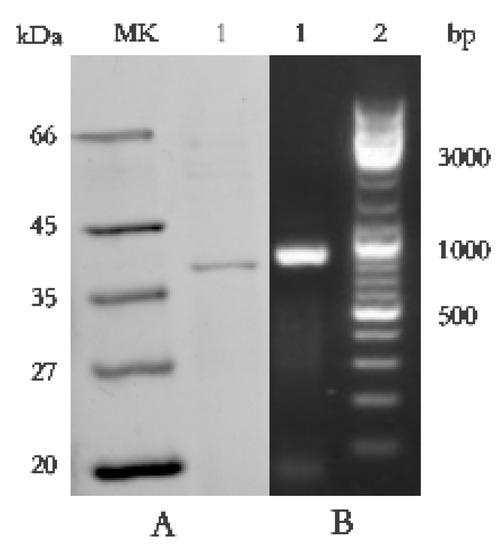
Figure 4.
(A) Molecular weight and SDS-PAGE of the purified protein BP8-2. Lane MK, molecular mass stand; lane 1, purified protein BP8-2. (B) PCR amplification of hag gene corresponding to flagellin (line1).

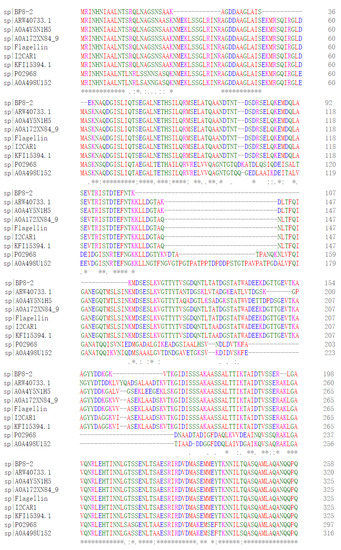
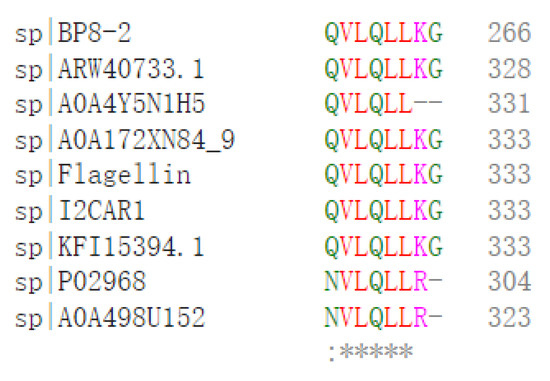
Figure 5.
The protein BP8-2 peptide mapping finger sequences of Ba168 was compared with other flagellin proteins by the multiple sequence alignment analysis. Sequence 1~9: 1~BP8-2; 2~ARW40733.1 Flagellin (B. amyloliquefaciens); 3~A0A4Y5N1H5 Flagellin (B. amyloliquefaciens); 4~A0A172XN84_9 Flagellin (B. velezensis); 5~Flagellin (B. amyloliquefaciens Ba168); 6~I2CAR1 Flagellin (B. amyloliquefaciens Y2); 7~KFI15394.1 flagellin (B. velezensis); 8~P02968 Flagellin (B. subtilis strain 168); 9~A0A498U152 Flagellin (B. safensis). (* means the amino acid sequence is the same.)
In order to further verify the ability of flagellin production in Ba168, the hag gene, coding for flagellin was amplified. A fragment of 1002 bp was obtained from Ba168, and it showed 98% similar identity to gene hag (Figure 4B). The amplified gene sequence was translated into amino acid sequence, and the multiple protein sequence alignment analysis was carried out (Figure 5). It was found that the sequences of BP8-2 and the translated flagellin were basically the same, except for the inconsistency of individual amino acid sites. This result indicated that the protein BP8-2 isolated in our study was flagellin.
3. Discussion
In this study, a complete profile of secreted proteins from B. amyloliquefaciens Ba168 was constructed by LC-MS/MS. A total of 1155 proteins were identified, and 16 of them were identified as antimicrobial peptides or antimicrobial-activity-related enzymes based on literature reports. One antagonistic protein BP8-2 showed considerable inhibitory activity against apple blue mold and it was identified as flagellin. These results provide a new theoretical basis for the mechanisms underlying the antagonistic activity of biocontrol bacteria B. amyloliquefaciens Ba168.
LC-MS/MS combined with the MASCOT database was used to rapidly identify the active proteins in Ba168. The considerable antimicrobial proteins or peptides, with effective biocontrol activities, were secreted from B. amyloliquefaciens [29,31,33,34]. However, the conventional antimicrobial peptide research requires several complete separation procedures, including the separation, purification, and identification of isolate substances, following functional research. The mass spectrometry technology offers the possibility of the large-scale rapid identification of proteins without the need for the complete separation of active substances for screening and optimizing the antimicrobial substances production. Caldeira et al. rapidly identified an active lipopeptide in B. amyloliquefaciens CCMI 105, by using the LC-ESI-MS methods combined with antifungal tests [35]. Comparing with LC-ESI-MS and other isolation and identification methods, LC-MS/MS can isolate more novel resistant proteins [36]; however, no research has been reported on B. amyloliquefaciens by LC-MS/MS. In this study, we adopted proteins that were identified by LC-MS/MS in B. amyloliquefaciens Ba168, and new antimicrobial proteins were isolated.
The genome analysis of B. amyloliquefaciens FZB42 showed several genes encoding direct synthesis of the polyketides macrolactin, bacillaene, and difficidin [29]. Likewise, our study indicated that the biocontrol effect of Ba168 is the result of a combination of various antimicrobial substances and growth-promoting factors rather than the action of a single substance. In this study, a total of 16 potential antimicrobial-activity-related proteins were identified in Ba168, 10 of which exhibit directed antimicrobial activity and 6 of which are associated with the formation of antimicrobial substances. Among these proteins, surfactin synthase and polyketide synthase are key synthetic enzymes of lipopeptides and polyketides, which are important antimicrobial substances produced by B. amyloliquefaciens and exhibit good inhibitory effects against many plant pathogens [37]. Glucanase inhibits the growth of pathogens by hydrolyzing glucan polymers, which are the main component of the cell wall for most pathogenic fungi [38]. Chitin-binding proteins exhibit antifungal activity by binding to monosaccharides and polysaccharides [39]. In addition, the chitin can induce disease resistance in plants and promote plant growth [40]. It is noticed that the synthetic enzymes of fengycin, the important lipopetides, were not identified in Ba168. This result is consistent with previous reports that not all B. amyloliquefaciens strains produce all the lipopeptides. For example, B. amyloliquefaciens subsp. plantarum B9601-Y2 can produce polyketides and fengycin, but no surfactin has been detected. The underlying reason for this may be that some peptide synthetases involved in the nonribosomal synthesis of surfactin were partially deleted [41]. The findings of these studies indicate that Ba168 can produce a variety of antagonistic proteins for disease prevention and control, which provides a basis for the further application of Ba168.
Flagellin is a structural protein that assembles into the lash-like filament of a bacterial flagellum, which is exclusively found in bacteria [42]. In vertebrates, flagellin is a main component of the flagellar filament. Flagellin is also a specific ligand that stimulates innate immunity through direct interaction with host Toll-like receptor 5 (TLR5). Because flagellin activates the immune response, the development of flagellin as a vaccine adjuvant in subunit vaccines or antigen fusion vaccines has attracted interest [43]. Similarly, flagellin can induce defensive responses in plant cells and has a significant inhibitory effect on a variety of pathogenic fungi [44]. Ren et al. reported that a flagellin-like protein isolated from Bacillus subtilis showed antagonistic activity against Botrytis cinerea [45]. Fang et al. isolated an antifungal flagellin protein from B. amyloliquefaciens using the SDS-PAGE-MS methods [46]. In our work, an antifungal protein named BP8-2 was isolated from B. amyloliquefaciens Ba168, and it was identified as a flagellin protein using LC-MS/MS. The gene hag coding for flagellin was amplified from strain Ba168. Protein BP8-2 showed significant inhibitory activity against apple blue mold, inhibited the mycelia growth and spore production in the fungi. Our study provides further evidence that flagellin produced by Ba168 has antimicrobial activity.
Biocontrol bacteria exhibit their biocontrol capacity predominantly by occupying the infection sites of pathogens on plants, competing with pathogens for water and nutrition, inducing plant systemic resistance, producing metabolites to inhibit the growth of pathogens, as well as competing with pathogens for ecological niches [47]. The BP8-2 protein could lead to abnormal mycelium growth and branching, increased branches, and local swellings. It is assumed that flagellin may have the ability to degrade cell walls. More in-depth mechanisms of the antagonistic activity of flagellin against fungal pathogens need to be studied. More antimicrobial proteins in B. amyloliquefaciens Ba168 will be purified in future studies.
4. Materials and Methods
4.1. Strain and Cultivation Conditions
Strain Bacillus amyloliquefaciens Ba168 was submitted to the China General Microbiological Culture Collection Center on 21 August 2012. The preservation number is CGMCC 6462. The strain was stored under freezing conditions at −20 °C and revived 24 h before inoculation in pure inorganic nitrogen medium (20 g/L glucose, 20 g/L (NH)4SO4, 1 g/L K2HPO4, 0.5 g/L MgSO4, 0.5 g/L NaCl; pH 7.0).
Penicillium expansum was isolated from apples and preserved in the Key Laboratory of Molecular Biology for Agriculture, Northwest A&F University. Penicillium expansum was cultured on PDA medium at 25 °C for 5~7 days.
4.2. Protein Extraction
Ba168 was cultivated at 32 °C and 150 rpm for 72 h, and centrifuged at 12,000× g at 4 °C for 5 min. The supernatants were collected, and 313 g ammonium sulfate was added to every 1 L of the supernatants. The mixture was stored at 4 °C for 24 h, and then centrifuged at 12,000× g, 4 °C for 10 min to collect proteins. The collected proteins were diluted in 10 mmol Tris-HCl (pH 7.4) buffer, centrifuged at 12,000× g for 5 min. The supernatants, which are the protein solutions of Ba168, were transferred to a clean tube and stored at 4 °C for further detection.
4.3. Preparation of Proteins for LC-MS/MS
The sample containing 30 μg protein was added into 30 μL SDT buffer (4% SDS, 100 mM DTT, 150 mM Tris-HCl; pH 8.0). Then, the low-molecular-weight components, such as detergent and DTT, were removed by repeated ultrafiltration (Microcon units, 10 kD) using UA buffer (8 M urea, 150 mM Tris-HCl; pH 8.0). It was washed 3 times with 100 μL UA buffer and 2 times with 100 μL 25 mM NH4HCO3. Then, 100 μL iodoacetamide was added to the filtrate (IAA was 100 mM in UA buffer) and incubated in the dark for 30 min to block the reduction of cysteine residues. Finally, the protein suspension was digested overnight with 4 μg trypsin (Promega, Madison, WI, USA) in 40 μL 25 mM NH4HCO3 buffer at 37 °C to obtain polypeptides. Sample peptides were desalted on C18 containers (EmporeTM SPE Cartridges C18 (standard density), bed I.D. 7 mm, volume 3 mL, Sigma, Saint Louis, MO, USA), centrifuged, and concentrated under vacuum condition, redissolved with 40 μL 0.1% (v/v) formic acid. The UV spectral density at 280 nm was measured to estimate the peptide content.
4.4. LC-MS/MS Analysis
Each protein sample was injected for nano LC-MS/MS analysis. Loading onto the peptide mixture with a reversed-phase trap column (Thermo Scientific Acclaim PepMap100, 100 μm × 2 cm, nanoViperC18, Waltham, MA, USA) connected to a C18 reversed-phase analytical column (Thermo Scientific Easy column, 10 cm length, 75 μm inner diameter, 3 μm resin, Waltham, MA, USA) in buffer A (0.1% formic acid) and separated by buffer B (84% acetonitrile and 0.1% formic acid) in a linear gradient flow rate of 300 nL/min controlled by Intelli Flow technology. Linear gradients of 0–35% buffer B for 50 min and 35–100% buffer B for 5 min were used in 100% buffer B for 5 min.
A Q Exactive mass spectrometer (Thermo Scientific, Waltham, MA, USA) was used to perform LC-MS/MS. This mass spectrometer was coupled to an Easy nLC (Proxeon Biosystems, now Thermo Fisher Scientific, Waltham, MA, USA) for 60 min. The mass spectrometer was operated in positive ion mode. MS data were scanned for the most abundant precursor HCD fragments in a dynamic selection survey (300–1800 m/z) using a data-dependent top10 method. The automatic gain control (AGC) target was set to 3e6 and the maximum injection time was set to 10 ms. The resolution of the measurement scan was 70,000 at m/z 200, and the resolution of the HCD spectrum was 17,500 at m/z 200. The isolation width was 2 m/z. The normalized collision energy was 30 eV, and the underfill ratio, which specifies the minimum percentage of the target value that could be achieved at maximum fill time, was defined as 0.1%. The instrument was operated in peptide recognition mode.
4.5. Data Analysis
Using the MASCOT search engine for searching MS/MS spectra (Matrix Science, London, UK; version 2.2), the search parameters were as follows: database: UniProt; taxonomy: B. amyloliquefaciens (32,598). Antimicrobial proteins were analyzed based on current reports. For protein identification, the following options were used: peptide mass tolerance = 20 ppm, MS/MS tolerance = 0.1 Da, enzyme = trypsin, missed cleavages = 2, fixed modification = carbamidomethyl (C), variable modification = oxidation (M). The mass spectrometry proteomics data have been deposited into the Proteome X change Consortium (http://proteomecentral.proteomexchange.org, accessed on 26 June 2022) via the PRIDE partner repository [48] with the dataset identifier PXD014057.
Multiple sequence alignment analysis of proteins was undertaken in the ClustalW online program (https://www.genome.jp/tools-bin/clustalw, https://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 26 June 2022).
4.6. Purification of the Antifungal Protein
The collected proteins of Ba168 were dissolved in buffer A (10 mmol Tris-HCl) of pH 7.4, then purified using DEAE Bio-sep FF chromatography column using a linear buffer B (0.1–0.7 mol/L NaCl gradient solution). The protein from each peak was collected in a tube. Using Penicillium expansum as the test fungus to measure the activity of each peak, it was found that the activity of the 8th peak BP8 was the highest, so the 8th peak was collected and then further purified by gel filtration chromatography using a 2.5 cm × 75 cm Sephadex G-75 column eluted with buffer A. Two peaks were collected and the activity of each peak was measured again. The antifungal activity peak was freeze-dried and suspended in buffer A.
4.7. Inhibitory Effect of the Antifungal Protein BP8-2 on P. expansum In Vitro
The inhibitory effect of protein BP8-2 on P. expansum in vitro was determined as Wiggins and Kinkel [49]. Specifically, 10 mL sterile water was added and P. expansum was cultured on PDA for 7-days, spores and mycelia were collected by scraping with a sterile loop. Next, 200 μL of the resulting pathogen suspension was sprayed on each PDA plate. Two Oxford cups were then placed symmetrically at the edge of plates, 100–200 μL of BP8-2 protein solution (the protein concentration was 200 µg/mL) was added to each cup. The plates were incubated for 2 days at 25 °C, and the diameter of the fungistatic circle was measured and recorded.
4.8. Determination of Minimum Inhibitory Concentration (MIC) of the Antifungal Protein BP8-2 on P. expansum
The MIC for P. expansum was tested via the microtiterplate dilution method using 96 well plate method. Specifically, 90 µL P. expansum spore suspension was added into 96-well microtitration plate hole for each, then 10 µL of the purified protein BP8-2 with different concentrations in the range of 10–200 µg/mL were added. Sterile water without P. expansum was used as a negative control. Sterile water without protein BP8-2 was used as a positive control. After being incubated at 30 °C for 24 h, microbial growth was assessed by measuring the optical density at 600 nm. The MIC was defined as the lowest concentration of flagellin that completely inhibits P. expansum growth.
4.9. Control Effect of the Antifungal Protein BP8-2 on P. expansum In Vivo
The control effect of the antifungal protein BP8-2 against P. expansum was determined in vivo [50]. After surface disinfection with 0.2% sodium hypochlorite, red Fuji apples were wounded to a depth of 7 mm × 3 mm diameter with a sterile hole punch. 100 μL of protein BP8-2 solution (200 μg/mL) were injected into each hole, 100 μL sterile water injected in each wound was treated as the control. Two hours later, 6 mm disks of P. expansum were placed on the wounds, and then sealed with sealing film. All the treated apples were transferred to a biochemical incubator and maintained at a relative humidity of 95% and temperature of 25 °C. Each treatment contained 12 fruits, and the experiment was repeated 3 times. The disease diameter was monitored at 3, 5, 7 and 9 days after inoculation. The prevention and cure effects were calculated as follows: control effect (%) = (1-treatment spot diameter/control spot diameter) × 100.
4.10. Identification of the Antifungal Protein BP8-2 and Cloning of Hag GENE in Ba168
The molecular weight of protein BP8-2 was detected by SDS-PAGE, according to the method of Laemmli [51]. Electrophoresis was carried out with 5% stacked gel and 12% running gel. After electrophoresis, the blue band was cutoff and enzymolysized with trypsin. The peptide segment was identified by LC-MS/MS analysis. MS/MS spectra were searched using the MASCOT search engine.
DNA obtained from Ba168 was subjected to PCR for the detection of the hag gene (coding for flagellin). Specific primer pairs (F: 5’-ATGAGAATCAACCACAATATCGC-3’, R: 5’-TTAACCTTTAAGCAATTGAAGAAC-3’) were designed based on the functional gene hag [52]. The amplification system was 20 µL, including 2 µL 10× dream Taq buffer, 0.5 µL dNTPs mixture (10 mM), 1 µL template DNA, 0.2 µL Taq enzyme (0.5 U/mL), 1 µL forward primer (10 µM), 1 µL reverse primer (10 µM) and 14.3 µL ddH2O. Amplification factor: 94 °C, 5 min; (94 °C, 30 s, 55 °C, 30 s, 72 °C, 40 s) for 30 cycle, 72 °C, 7 min, 4 °C termination reaction. PCR products were run according to the method of Kwok et al. [53].
Author Contributions
D.A., P.S. and M.L. designed the experiments. M.L., Y.C., L.L. and Y.M. purification and identification of the antifungal protein in Ba168; M.L. and D.G. analyzed data of LC/MS; L.L. and Z.T. cloned the hag Gene in Ba168; M.L., Y.C. and Y.M. tested the inhibitory effect of the antifungal protein BP8-2 on P. expansum. D.A., P.S. and M.L. supervised the study. P.S., M.L. and Y.C. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by SCYC202009, Key Research and Development Program of Shaanxi Province (2020NY-046) and Science and Technology Program of Shaanxi Academy of Sciences (2022K-02-05), Universities Innovation Foundation of Gansu Province (2021B-117), Natural Science Foundation of Gansu Province (21JR7RA820).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Instrument Shared Platform of Northwest A&F University, the People’s Republic of China, for the LC-MS.
Conflicts of Interest
The authors have no conflict of interest to declare.
Sample Availability
Samples of the proteins are available from the authors.
References
- Droby, S. Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Hortic. 2006, 709, 45–51. [Google Scholar] [CrossRef]
- El-Ghaouth, A.; Smilanick, J.L.; Brown, G.E.; Ippolito, A.; Wilson, C.L. Control of decay of apple and citrus fruits in semicommercial tests with Candida saitoana and 2-Deoxy-d-glucose. Biol. Control. 2001, 20, 96–101. [Google Scholar] [CrossRef]
- Kulkarni, S. Commercialisation of Microbial Biopesticides for the Management of Pests and Diseases, Recent Advances in the Diagnosis and Management of Plant Diseases; Springer: New Delhi, India, 2015. [Google Scholar]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Alvindia, D.G.; Natsuaki, K.T. Biocontrol activities of Bacillus amyloliquefaciens DGA14 isolated from banana fruit surface against banana crown rot-causing pathogens. Crop Prot. 2009, 28, 236–242. [Google Scholar] [CrossRef]
- Arguelles-Arias, A.; Ongena, M.; Halimi, B.; Lara, Y.; Brans, A.; Joris, B.; Fickers, P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Factories 2009, 8, 63. [Google Scholar] [CrossRef]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Nabila, E.A.; Soufiyan, E.A. Microbial biocontrol of post-harvest fungal rot in apples: Current state of the science. Bull. Bot. Res. 2020, 2, 28. [Google Scholar]
- Cai, W.; Xia, B.; Xia, Y.; Yi, Y.; Wang, Y. Effect of Bacillus amyloliquefaciens fermentation liquid on different types of peppers for biological control of postharvest A. alternate. Trans. Chin. Soc. Agric. Eng. 2013, 29, 253–261. [Google Scholar]
- Tan, S.; Dong, Y.; Liao, H.; Huang, J.; Song, S.; Xu, Y.; Shen, Q. Antagonistic bacterium Bacillus amyloliquefaciens induces resistance and controls the bacterial wilt of tomato. Pest Manag. Sci. 2013, 69, 1245–1252. [Google Scholar] [CrossRef]
- Ye, F.; Yang, R.; Hua, X.; Shen, Q.; Zhao, W.; Zhang, W. Modification of stevioside using transglucosylation activity of Bacillus amyloliquefaciens alpha-amylase to reduce its bitter aftertaste. LWT Food Sci. Technol. 2013, 51, 524–530. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, B.Q.; Wang, Y.; Guo, Q.G.; Lu, X.Y.; Li, S.Z.; Ma, P. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 2013, 97, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, X.; Huang, H.; Zhai, F.; An, T.C.; An, D.R. Control effect and mechanism of Bacillus amyloliquefaciens Ba-168 on wheat take-all. J. Plant Prot. 2013, 40, 475–476. [Google Scholar]
- Chao, J.J.; Yang, H.Y.; Xie, X.S.; Tian, J.; Wu, Y.F.; An, D.R. Control effect of Bacillus amyloliquefaciens on wheat yellow mosaic disease. Acta Phytophylacica Sin. 2016, 43, 588–593. [Google Scholar]
- Kesarwani, M.; Azam, M.; Natarajan, K.; Mehta, A.; Datta, A. Oxalate decarboxylase from collybia velutipes. J. Biol. Chem. 2000, 275, 7230–7238. [Google Scholar] [CrossRef]
- De, D.L.A. Plant-derived antifungal proteins and peptides. Can. J. Microbiol. 2005, 51, 1001–1014. [Google Scholar]
- Zhao, X.; Zhao, X.; Wei, Y.; Shang, Q.; Liu, Z. Isolation and identification of a novel antifungal protein from a rhizobacterium Bacillus subtilisstrain F3. J. Phytopathol. 2012, 161, 43–48. [Google Scholar] [CrossRef]
- Garciabustos, J.F.; Pezzi, N.; Mendez, E. Structure and mode of action of microcin 7, an antibacterial peptide produced by Escherichia coli. Antimicrob. Agents Chemother. 1985, 27, 791. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kumar, S.K.H.; Dhakan, D.B.; Sharma, V.K. Prediction of peptidoglycan hydrolases- a new class of antibacterial proteins. BMC Genom. 2016, 17, 411. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Guo, Q.G.; Lu, X.Y.; Zhao, W.S.; Wang, P.P.; Li, S.Z.; Ma, P. Isolation and identification of an antifungal protein produced by Bacillus subtilis NCD-2. Acta Phytopathol. Sin. 2018, 48, 395–401. [Google Scholar]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.L.; Fang, Y.Q.; Sun, Y.; Luan, N.; Chen, X.; Chen, M.R.; Han, Y.J.; Yin, Y.Z.; Mwangi, J.; Niu, J.K.; et al. Antimicrobial peptide LL-37 forms complex with bacterial DNA to facilitate blood translocation of bacterial DNA and aggravate ulcerative colitis. Chin. Sci. Bull. 2018, 63, 1364–1375. [Google Scholar] [CrossRef]
- Muthu, S.; Gopal, V.B.; Soundararajan, S.; Nattarayan, K.; Narayan, K.S.; Lakshmikanthan, M.; Malairaj, S.; Perumal, P. Antibacterial serine protease from Wrightia tinctoria: Purification and characterization. Plant Physiol. Biochem. 2017, 112, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Park, S.M.; Ahn, B.Y. Isolation and characterization of an antimicrobial substance from Bacillus subtilis BY08 antagonistic to Bacillus cereus and Listeria monocytogenes. Food Sci. Biotechnol. 2013, 22, 433–440. [Google Scholar] [CrossRef]
- Li, B.L.; Wang, H.B.; Kong, F.Y.; Zhang, C.S.; Wang, J.; Li, X.K. Purification and partial characterization of antagonistic proteins from Bacillus Subtilis Tpb55. Chin. Tob. Sci. 2011, 11, 565. [Google Scholar]
- Antonio, G.; Maqueda, M.; Manuel, M.-B.; Lebbadi, M.; Vaidivia, E. Isolation and physico-chemical characterization of an antifungal and antibacterial peptide produced by Bacillus licheniformis A12. Appl. Microbiol. Biotechnol. 1993, 39, 438–442. [Google Scholar]
- Sandrin, C.; Peypoux, F.; Michel, G. Coproduction of surfactin and iturin A, lipopeptides with surfactant and antifungal properties, by Bacillus subtilis. Biotechnol. Appl. Biochem. 1990, 12, 370–375. [Google Scholar]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Piel, J.; Borriss, R.; Pühler, A.; Selbitschka, W. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2008, 140, 27–37. [Google Scholar] [CrossRef]
- Chen, X.H.; Scholza, R.; Borrissb, M.; Jungeb, H.; Mögel, G.; Kunz, S.; Borrissa, R. Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J. Biotechnol. 2009, 140, 38–44. [Google Scholar] [CrossRef]
- Wu, L.M.; Wu, H.J.; Chen, L.N.; Yu, X.F.; Borriss, R.; Gao, X.W. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015, 5, 12975. [Google Scholar] [CrossRef]
- Zheng, G.; Yan, L.Z.; Vederas, J.C.; Zuber, P. Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin. J. Bacteriol. 1999, 181, 7346–7355. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Zhao, J.; Wang, C.B.; Liang, H. Research advances on antibacterial mechanism of Bacillus amyloliquefaciens. Biotechnol Bull. 2015, 17, 293–296. [Google Scholar]
- Abdullah, M.T.; Ali, N.Y.; Suleman, P. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Prot. 2008, 27, 1354–1359. [Google Scholar] [CrossRef]
- Caldeira, A.T.; Arteiro, J.M.S.; Coelho, A.V.; Roseiro, J.C. Combined use of LC–ESI-MS and antifungal tests for rapid identification of bioactive lipopeptides produced by Bacillus amyloliquefaciens CCMI 1051. Process Biochem. 2011, 46, 1738–1746. [Google Scholar] [CrossRef]
- Neupane, S.; Ma, Q.; Mathew, F.M.; Varenhorst, A.J.; Andersen, E.J.; Nepal, M.P. Evolutionary divergence of TNL disease-resistant proteins in soybean (glycine max) and common bean (phaseolus vulgaris). Biochem. Genet. 2018, 56, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Seung, H.J.; Narayan, C.P.; Xin, D.J.; Young, S.K.; Bong-Sik, Y.; Seung, H.Y. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 2013, 41, 234–242. [Google Scholar]
- Bacon, J.S.; Gordon, A.H.; Jones, D.; Tayor, I.; Webley, D. The separation of beta-glucanases produced by Cytophaga johnsonii and their role in the lysis of yeast cell walls. Biochem. J. 1970, 120, 67. [Google Scholar] [CrossRef]
- Mehmood, M.; Hussain, K.; Latif, F.; Tabassum, M.; Gull, M.; Gill, S.; Anam, S.; Zahid, I. Synergistic action of the antifungal β-chitin binding protein cbp50 from Bacillus thuringiensis with bacterial chitinases. Curr. Proteom. 2014, 11, 23–26. [Google Scholar] [CrossRef]
- Kpb, V.D.B.; Rouge, P.P.; Coosemans, J.; Krouglova, T. Synergistic antifungal activity of two chitin-binding proteins from spindle tree (Euonymus europaeus L.). Planta 2004, 219, 221–232. [Google Scholar]
- He, P.; Hao, K.; Blom, J.; Rückert, C.; Vater, J.; Mao, Z.; Wu, Y.; Hou, M.; He, P.; He, Y. Genome sequence of the plant growth promoting strain Bacillus amyloliquefaciens subsp. plantarum B9601-Y2 and expression of mersacidin and other secondary metabolites. J. Biotechnol. 2013, 164, 281–291. [Google Scholar] [CrossRef]
- Eckhard, U.; Bandukwala, H.; Mansfield, M.J.; Marino, G.; Cheng, J.J.; Wallace, I.; Holyoak, T.; Charles, T.C.; Austin, J.; Overall, C.M.; et al. Discovery of a proteolytic flagellin family in diverse bacterial phyla that assembles enzymatically active flagella. Nat. Commun. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Lee, C.; Park, J.; Jeon, B.Y.; Hong, M. Structure-guided fusion-protein designs using Bacillus flagellin as a vaccine adjuvant. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, a134. [Google Scholar] [CrossRef]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 2010, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.J.; Shi, G.L.; Xiao, Q.W.; Liu, J.G.; Wang, Y.N. Identification and characterization of a novel Bacillus subtilis strain with potent antifungal activity of a flagellin-like protein. World J. Microbiol. Biotechnol. 2013, 29, 2343–2352. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Li, L.L.; Zheng, Y.L.; Yuan, H.H.; Zhang, X.H.; Cheng, S.Y.; Cheng, H. Extraction, isolation and identification of antimicrobial substances from Bacillus amyloliquefaciens CMN1308. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 308. [Google Scholar] [CrossRef][Green Version]
- Djordje, F.; Ivica, D.; Tanja, B.; Jelena, L.; Slaviša, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Wiggins, B.E.; Kinkel, L.L. Green manures and crop sequences influence potato diseases and pathogen inhibitory activity of indigenous streptomycetes. Phytopathology 2005, 95, 178–185. [Google Scholar] [CrossRef]
- Sadeghian, M.; Bonjar, G.H.S.; Sirchi, G.R.S. Post harvest biological control of apple bitter rot by soil-borne actinomycetes and molecular identification of the active antagonist. Postharvest Biol. Technol. 2016, 112, 46–54. [Google Scholar] [CrossRef]
- Laemmli, U.K. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 1973, 80, 575–599. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Z.J.; Han, Y.; Wang, Z.Z.; Fan, J.; Xiao, H.Z. Isolation and identification of antifungal peptides from Bacillus BH072, a novel bacterium isolated from honey. Microbiol. Res. 2013, 168, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.; Higuchi, R. Avoiding false positives with PCR. Nature 1989, 339, 237–238. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).