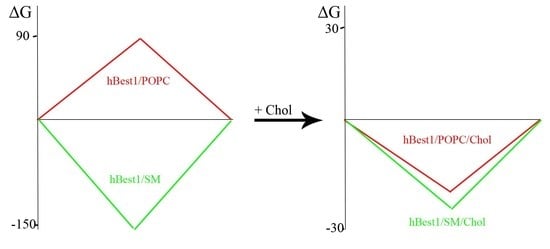
Cholesterol Alters the Phase Separation in Model Membranes Containing hBest1
Abstract
:1. Introduction
2. Results and Discussion:
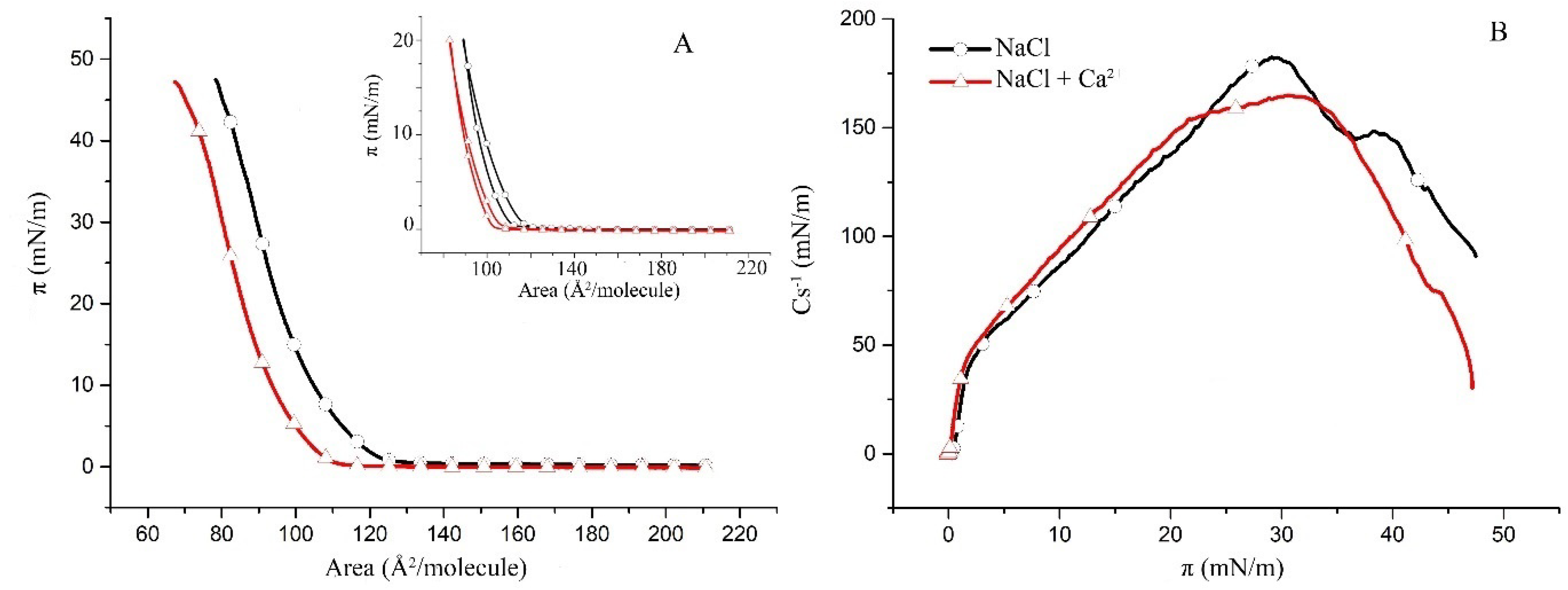
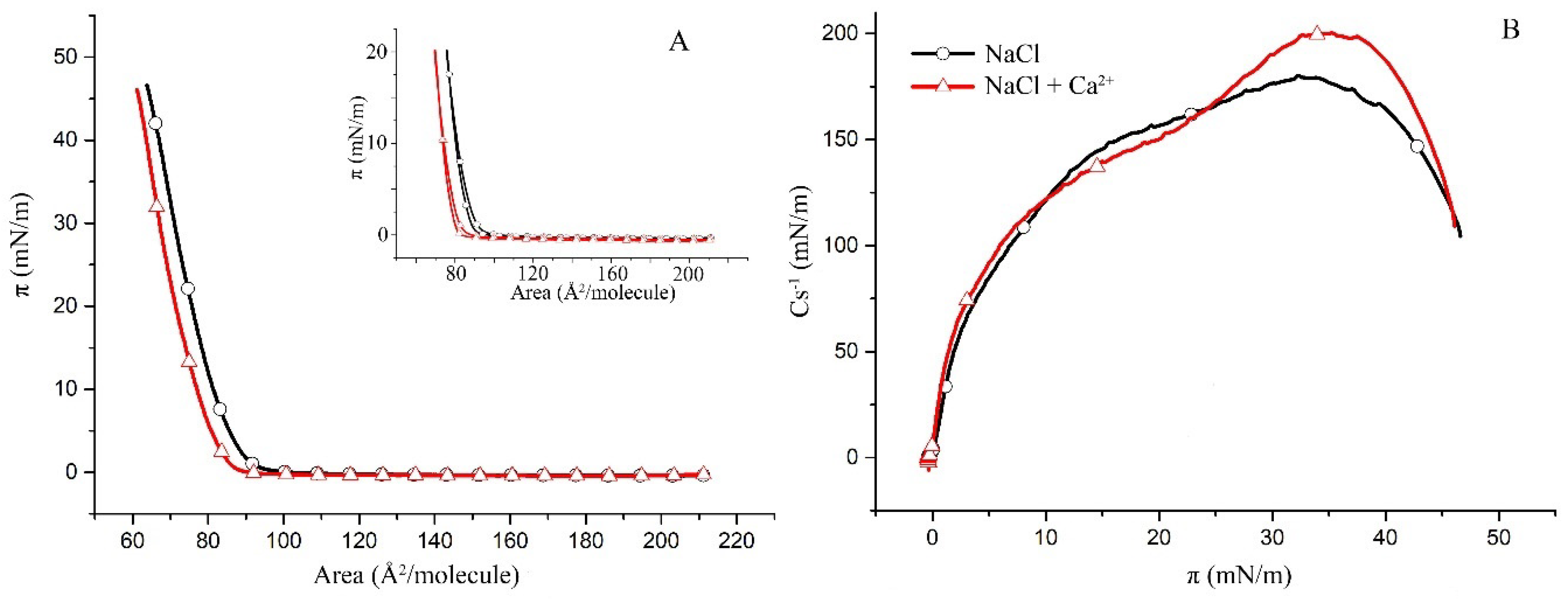
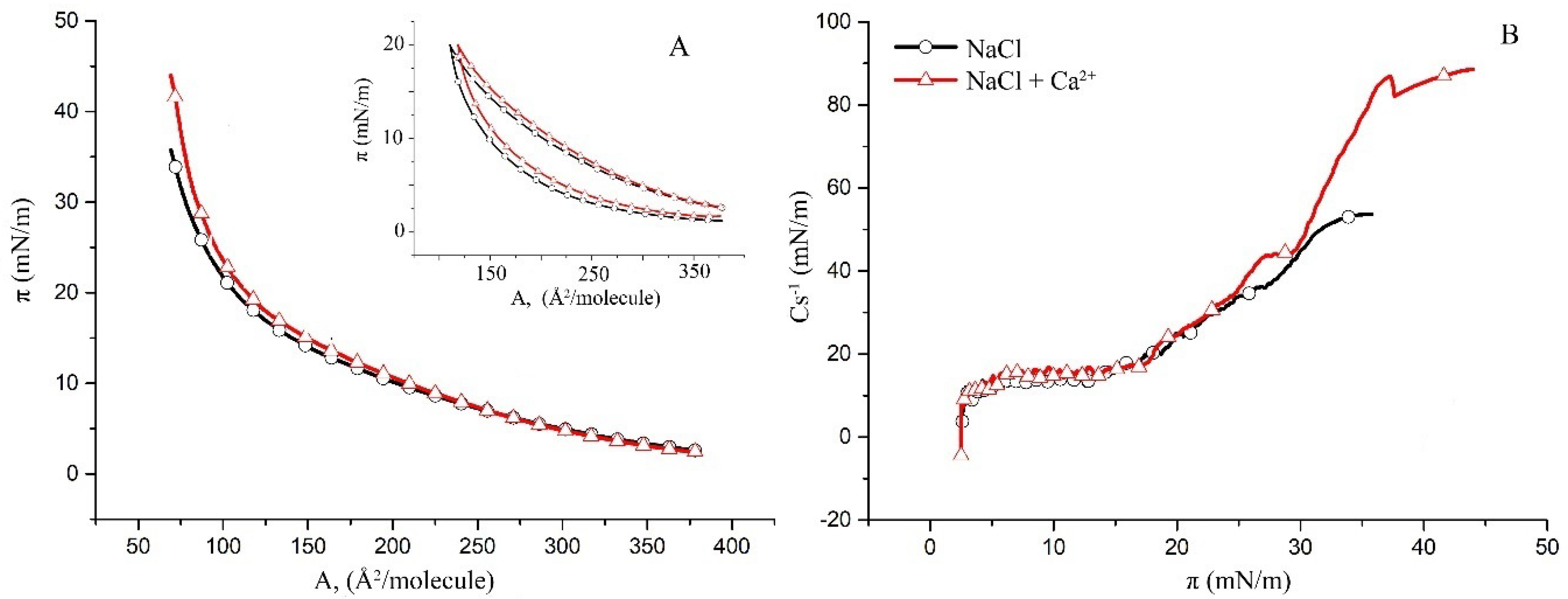
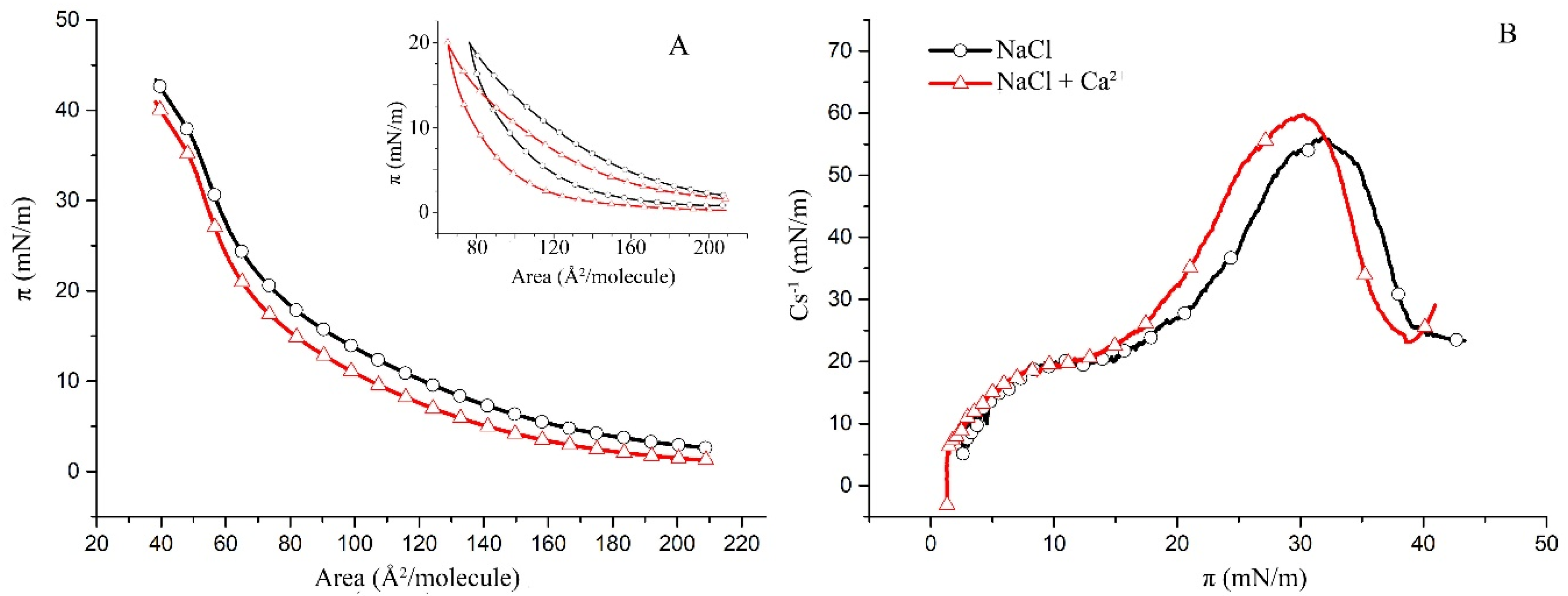
2.1. Surface Properties of Binary Monolayers Containing Cholesterol
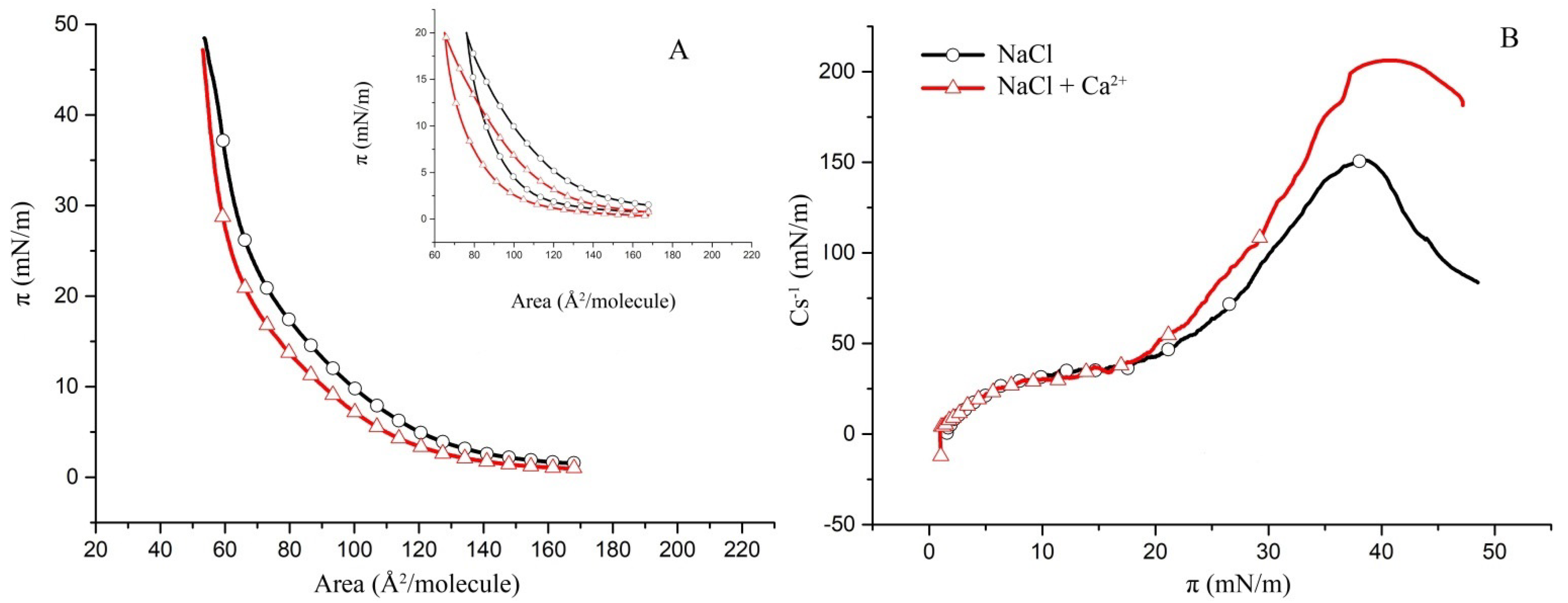
2.2. Surface Properties of hBest1/POPC/Chol and hBest1/SM/Chol Monolayers
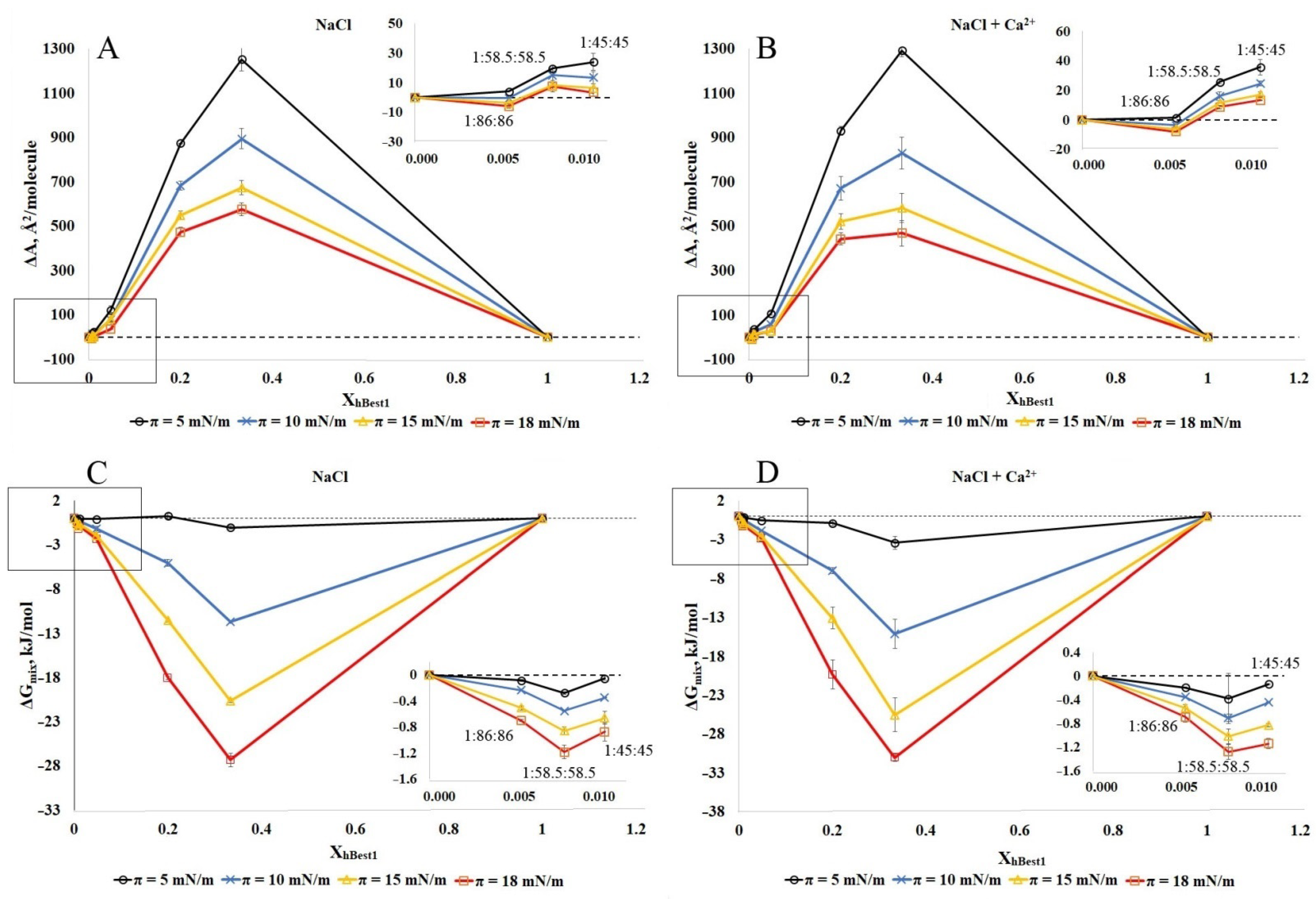
2.3. Analysis of Mixing and Phase Separation in hBest1/POPC/Chol and hBest1/SM/Chol Monolayers
3. Materials and Methods
3.1. Cell Cultures and Purification of hBest1
3.2. Monolayers Experiments
3.3. Analysis of the Miscibility and Stability in Insoluble Two-Component Monolayers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Petrukhin, K.; Koisti, M.J.; Bakall, B.; Li, W.; Xie, G.; Marknell, T.; Sandgren, O.; Forsman, K.; Holmgren, G.; Andreasson, S.; et al. Identification of the gene responsible for Best macular dystrophy. Nat. Genet. 1998, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, A.; Stohr, H.; Passmore, L.A.; Kramer, F.; Rivera, A.; Weber, B.H. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best’s disease). Hum. Mol. Genet. 1998, 7, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Tsunenari, T.; Yau, K.W.; Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA 2002, 99, 4008–4013. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Yoon, B.E.; Berglund, K.; Oh, S.J.; Park, H.; Shin, H.S.; Augustine, G.J.; Lee, C.J. Channel-mediated tonic GABA release from glia. Science 2010, 330, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Woo, D.H.; Han, K.S.; Shim, J.W.; Yoon, B.E.; Kim, E.; Bae, J.Y.; Oh, S.J.; Hwang, E.M.; Marmorstein, A.D.; Bae, Y.C.; et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 2012, 151, 25–40. [Google Scholar] [CrossRef] [Green Version]
- Allikmets, R.; Seddon, J.M.; Bernstein, P.S.; Hutchinson, A.; Atkinson, A.; Sharma, S.; Gerrard, B.; Li, W.; Metzker, M.L.; Wadelius, C.; et al. Evaluation of the Best disease gene in patients with age-related macular degeneration and other maculopathies. Hum. Genet. 1999, 104, 449–453. [Google Scholar] [CrossRef]
- Lotery, A.J.; Munier, F.L.; Fishman, G.A.; Weleber, R.G.; Jacobson, S.G.; Affatigato, L.M.; Nichols, B.E.; Schorderet, D.F.; Sheffield, V.C.; Stone, E.M. Allelic variation in the VMD2 gene in best disease and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1291–1296. [Google Scholar]
- Davidson, A.E.; Millar, I.D.; Urquhart, J.E.; Burgess-Mullan, R.; Shweikh, Y.; Parry, N.; O’Sullivan, J.; Maher, G.J.; McKibbin, M.; Downes, S.M.; et al. Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. Am. J. Hum. Genet. 2009, 85, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.A.; Guziewicz, K.E.; Lee, C.J.; Kalathur, R.C.; Pulido, J.S.; Marmorstein, L.Y.; Marmorstein, A.D. Bestrophin 1 and retinal disease. Prog. Retin. Eye Res. 2017, 58, 45–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef] [Green Version]
- Sezgin, E.; Schwille, P. Model membrane platforms to study protein-membrane interactions. Mol. Membr. Biol. 2012, 29, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef]
- Sych, T.; Gurdap, C.O.; Wedemann, L.; Sezgin, E. How Does Liquid-Liquid Phase Separation in Model Membranes Reflect Cell Membrane Heterogeneity? Membranes 2021, 11, 323. [Google Scholar] [CrossRef]
- Heberle, F.A.; Feigenson, G.W. Phase separation in lipid membranes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004630. [Google Scholar] [CrossRef] [Green Version]
- Mladenova, K.; Petrova, S.D.; Andreeva, T.D.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Kalvachev, Y.; Balashev, K.; Bhattacharya, S.S.; Chakarova, C.; Lalchev, Z.; et al. Effects of Ca(2+) ions on bestrophin-1 surface films. Colloids Surf. B Biointerfaces 2017, 149, 226–232. [Google Scholar] [CrossRef]
- Andreeva, T.D.; Petrova, S.D.; Mladenova, K.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Petseva, Y.; Mladenov, N.; Balashev, K.; Lalchev, Z.; Doumanov, J.A. Effects of Ca(2+), Glu and GABA on hBest1 and composite hBest1/POPC surface films. Colloids Surf. B Biointerfaces 2018, 161, 192–199. [Google Scholar] [CrossRef]
- Doumanov, J.A.; Mladenova, K.; Moskova-Doumanova, V.; Andreeva, T.D.; Petrova, S.D. Self-organization and surface properties of hBest1 in models of biological membranes. Adv. Colloid Interface Sci. 2022, 302, 102619. [Google Scholar] [CrossRef] [PubMed]
- Videv, P.; Mladenov, N.; Andreeva, T.; Mladenova, K.; Moskova-Doumanova, V.; Nikolaev, G.; Petrova, S.D.; Doumanov, J.A. Condensing Effect of Cholesterol on hBest1/POPC and hBest1/SM Langmuir Monolayers. Membranes 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Rujoi, M.; Borchman, D.; DuPre, D.B.; Yappert, M.C. Interactions of Ca(2+) with sphingomyelin and dihydrosphingomyelin. Biophys. J. 2002, 82, 3096–3104. [Google Scholar] [CrossRef] [Green Version]
- Feng, R.-J.; Lin, L.; Li, Y.-Y.; Liu, M.-H.; Guo, Y.; Zhang, Z. Effect of Ca(2+) to Sphingomyelin Investigated by Sum Frequency Generation Vibrational Spectroscopy. Biophys. J. 2017, 112, 2173–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Faraudo, J.; Martín-Molina, A. Specific Ion Effects in Cholesterol Monolayers. Materials 2016, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar] [CrossRef]
- Davies, E.K.R.J.T. Interfacial Phenomena, 1st ed.; Academic Press: New York, NY, USA, 1961. [Google Scholar] [CrossRef]
- Dynarowicz-Łątka, P.; Wnętrzak, A.; Makyła-Juzak, K. Cyclosporin A in Membrane Lipids Environment: Implications for Antimalarial Activity of the Drug--The Langmuir Monolayer Studies. J. Membr. Biol. 2015, 248, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Mladenov, N.; Petrova, S.D.; Mladenova, K.; Bozhinova, D.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Videv, P.; Veleva, R.; Kostadinova, A.; Staneva, G.; et al. Miscibility of hBest1 and sphingomyelin in surface films—A prerequisite for interaction with membrane domains. Colloids Surf. B Biointerfaces 2020, 189, 110893. [Google Scholar] [CrossRef]
- Baglioni, P.; Cestelli, O.; Dei, L.; Gabrielli, G. Monolayers of cholesterol at water-air interface: Mechanism of collapse. J. Colloid Interface Sci. 1985, 104, 143–150. [Google Scholar] [CrossRef]
- Fidalgo Rodriguez, J.L.; Caseli, L.; Minones Conde, J.; Dynarowicz-Latka, P. New look for an old molecule—Solid/solid phase transition in cholesterol monolayers. Chem. Phys. Lipids 2019, 225, 104819. [Google Scholar] [CrossRef]
- Mangiarotti, A. Hopanoids Like Sterols Form Compact but Fluid Films. Langmuir 2019, 35, 9848–9857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, C.; Xu, G.; Sun, R. Effects of Concentration and Surface Pressure on MBP Interaction with Cholesterol in Langmuir Films. Scanning 2017, 2017, 1542156. [Google Scholar] [CrossRef] [PubMed]
- Torrent-Burgués, J. Lysozyme Influence on Monolayers of Individual and Mixed Lipids. Colloids Interfaces 2022, 6, 15. [Google Scholar] [CrossRef]
- Dynarowicz-Łatka, P.; Hac-Wydro, K. Interactions between phosphatidylcholines and cholesterol in monolayers at the air/water interface. Colloids Surf. B Biointerfaces 2004, 37, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Momsen, M.M.; Smaby, J.M.; Brockman, H.L.; Brown, R.E. Cholesterol decreases the interfacial elasticity and detergent solubility of sphingomyelins. Biochemistry 2001, 40, 5954–5963. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, F.C. Molecular Interaction in Mixed Monolayers. In Proceedings of the Second International Congress of Surface Activity: Gas-Liquid and Liquid-Liquid Interface, Butterworths, London, UK, 1957; p. 85. [Google Scholar]
- Mladenova, K.; Moskova-Doumanova, V.; Tabashka, I.; Petrova, S.; Lalchev, Z.; Doumanov, J. Establishment and characterization of stably transfected mdck cell line, expressing hbest1 protein. Bulg. J. Agric. Sci. 2013, 19, 159–162. [Google Scholar]
- Mladenova, K.; Petrova, S.D.; Georgiev, G.A.; Moskova-Doumanova, V.; Lalchev, Z.; Doumanov, J.A. Interaction of Bestrophin-1 with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) in surface films. Colloids Surf. B Biointerfaces 2014, 122, 432–438. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Videv, P.; Mladenova, K.; Andreeva, T.D.; Park, J.H.; Moskova-Doumanova, V.; Petrova, S.D.; Doumanov, J.A. Cholesterol Alters the Phase Separation in Model Membranes Containing hBest1. Molecules 2022, 27, 4267. https://doi.org/10.3390/molecules27134267
Videv P, Mladenova K, Andreeva TD, Park JH, Moskova-Doumanova V, Petrova SD, Doumanov JA. Cholesterol Alters the Phase Separation in Model Membranes Containing hBest1. Molecules. 2022; 27(13):4267. https://doi.org/10.3390/molecules27134267
Chicago/Turabian StyleVidev, Pavel, Kirilka Mladenova, Tonya D. Andreeva, Jong Hun Park, Veselina Moskova-Doumanova, Svetla D. Petrova, and Jordan A. Doumanov. 2022. "Cholesterol Alters the Phase Separation in Model Membranes Containing hBest1" Molecules 27, no. 13: 4267. https://doi.org/10.3390/molecules27134267
APA StyleVidev, P., Mladenova, K., Andreeva, T. D., Park, J. H., Moskova-Doumanova, V., Petrova, S. D., & Doumanov, J. A. (2022). Cholesterol Alters the Phase Separation in Model Membranes Containing hBest1. Molecules, 27(13), 4267. https://doi.org/10.3390/molecules27134267