An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring
Abstract
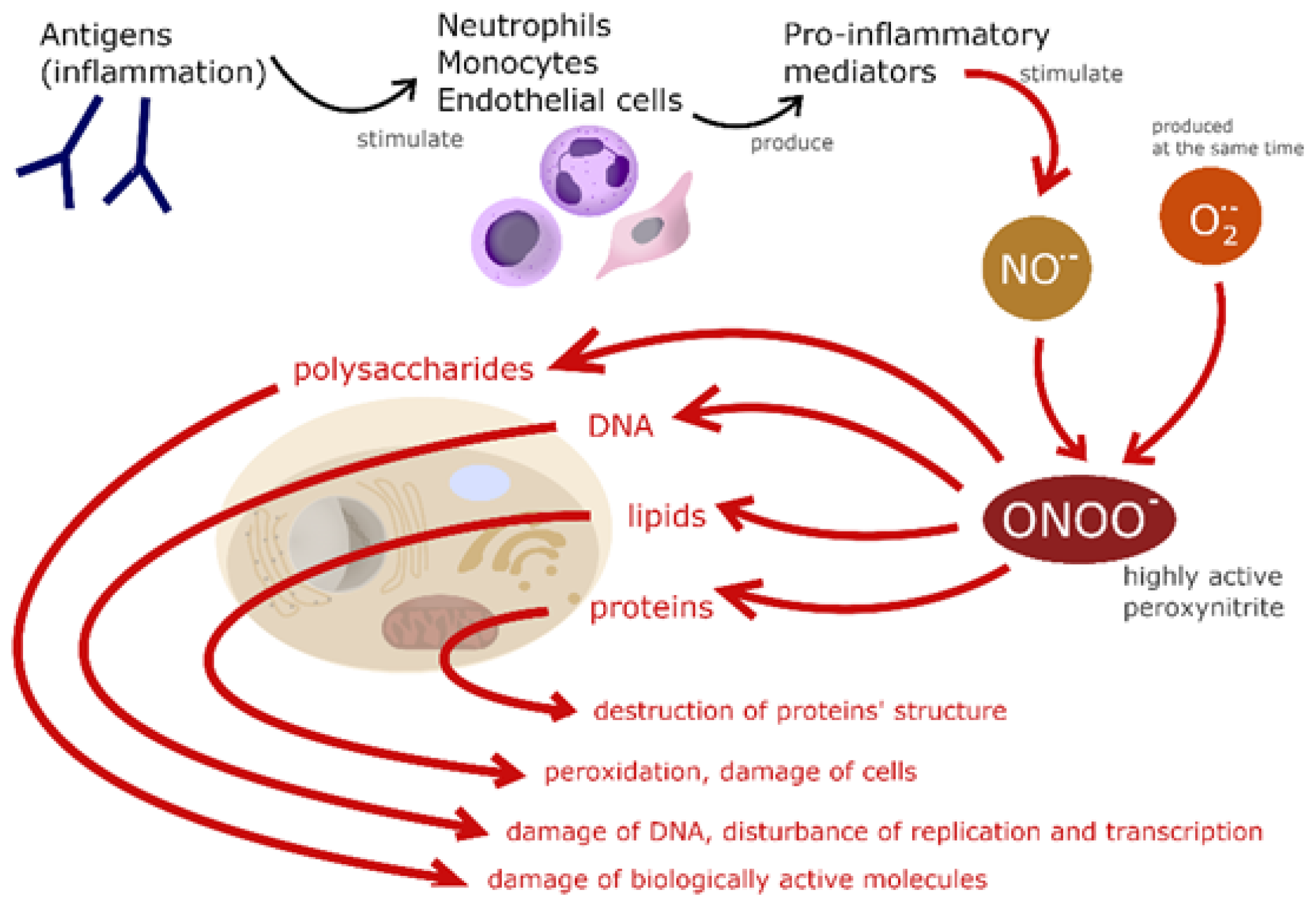
1. Introduction
2. Materials and Methods
2.1. Methods for Determining the Level of NO
2.2. Portable Devices for Measuring NO Level in Exhaled Air
3. Discussion
3.1. Respiratory System Diseases
3.1.1. Asthma
3.1.2. Allergic Rhinitis (AR)
3.1.3. Chronic Obstructive Pulmonary Disease (COPD)
3.1.4. Obstructive Sleep Apnea (OSA)
3.2. Gastrointestinal Diseases
3.2.1. Oral Cavity Pathologies
3.2.2. Diseases of the Further Sections of the Digestive System
3.3. Diseases of Other Human Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, S.R.; Gori, S.S.; Morris, J.D.; Xie, Z.; Fu, X.-A.; Nantz, M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites 2022, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Gdula-Argasińska, J.; Tyszka-Czochara, M.; Paśko, P.; Opoka, W. Rola wolnych rodników w regulacji procesów fizjologicznych. Med. Int. Rev. 2012, 25, 41–46. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Paulo, M.; Araújo, A.V.; Rodrigues, G.J.; Bendhack, L.M. Nitric oxide synthesis and biological functions of nitric oxide released from ruthenium compounds. Braz. J. Med. Biol. Res. 2011, 44, 947–957. [Google Scholar] [CrossRef]
- Tadeusiewicz, J.; Olas, B. Tlenek azotu i tlenek węgla−dwa ważne gazotransmitery. Kosmos 2014, 63, 543–554. [Google Scholar]
- Dai, Z.; Wu, Z.; Yang, Y.; Wang, J.; Satterfield, M.C.; Meininger, C.J.; Bazer, F.W.; Wu, G. Nitric oxide and energy metabolism in mammals. Biofactors 2013, 39, 383–391. [Google Scholar] [CrossRef]
- Sokołowska, M.; Włodek, L. Dobre i złe strony tlenku azotu. Folia Cardiol. 2001, 8, 467–477. [Google Scholar]
- Kröncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.K.; Daneva, Z.; Azam, M.; Li, N.; Li, P.-L.; Ritter, J.K. Role of Nitric Oxide in the Cardiovascular and Renal Systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Hodgson, J.M. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2036–2052. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous Mediators Nitric Oxide and Hydrogen Sulfide in the Mechanism of Gastrointestinal Integrity, Protection and Ulcer Healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Nitric oxide in the gastrointestinal tract: Opportunities for drug development. Br. J. Pharmacol. 2019, 176, 147–154. [Google Scholar] [CrossRef]
- Budani, M.C.; Tiboni, G.M. Novel Insights on the Role of Nitric Oxide in the Ovary: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 980. [Google Scholar] [CrossRef] [PubMed]
- Staicu, F.D.; Martínez-Soto, J.C.; Canovas, S.; Matás, C. Nitric oxide-targeted protein phosphorylation during human sperm capacitation. Sci. Rep. 2021, 11, 20979. [Google Scholar] [CrossRef] [PubMed]
- Antosova, M.; Mokra, D.; Pepucha, L.; Plevkova, J.; Buday, T.; Sterusky, M.; Bencova, A. Physiology of nitric oxide in the respiratory system. Physiol. Res. 2017, 66 (Suppl. 2), S159–S172. [Google Scholar] [CrossRef]
- Goshi, E.; Zhou, G.; He, Q. Nitric oxide detection methods in vitro and in vivo. Med. Gas Res. 2019, 9, 192–207. [Google Scholar] [CrossRef]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef]
- Korn, S.; Wilk, M.; Voigt, S.; Weber, S.; Keller, T.; Buhl, R. Measurement of Fractional Exhaled Nitric Oxide: Comparison of Three Different Analysers. Respiration 2020, 99, 1–8. [Google Scholar] [CrossRef]
- Kumor, M.; Przybyłowski, T.; Maskey-Warzęchowska, M.; Hildebrand, K.; Fangrat, A.; Bielicki, P.; Górska, K.; Kościuch, J.; Kucińska, J.; Chazan, R. Powtarzalność pomiaru stężenia tlenku azotu w powietrzu wydechowym (FENO) przeprowadzonego z wykorzystaniem zestawu NIOX u zdrowych osób. Pneumonol. Alergol. Pol. 2004, 72, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Vitale, C.; Vatrella, A.; Molino, A.; Bianco, A.; Mazzarella, G. Fractional exhaled nitric oxide-measuring devices: Technology update. Med. Devices Evid. Res. 2016, 9, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Selby, A.; Clayton, B.; Grundy, J.; Pike, K.; Drew, K.; Raza, A.; Kurukulaaratchy, S.; Arshad, H.; Roberts, G. Are exhaled nitric oxide measurements using the portable NIOX MINO repeatable? Respir. Res. 2010, 11, 43. [Google Scholar] [CrossRef]
- Rouatbi, S.; Ali Chouchene, M.; Sfaxi, I.; Ben Rejeb, M.; Tabka, Z.; Ben Saad, H. Fraction of Exhaled Nitric Oxide (FeNO) Norms in Healthy Tunisian Adults. BioMed Res. Int. 2014, 2014, 269670. [Google Scholar] [CrossRef] [PubMed]
- Sonnappa, S. Exhaled Nitric Oxide Measurements From Different Analyzers. Chest 2010, 138, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Barański, K.; Kocot, K.; Melaniuk-Wolny, E.; Zajusz-Zubek, E.; Kowalska, M. The Effect of Physical Activity on Spirometry and Fractional Exhaled Nitric Oxide in Adolescents—Longitudinal Study. Sustainability 2021, 13, 5770. [Google Scholar] [CrossRef]
- Amaral, R.; Jácome, C.; Almeida, R.; SáSousa, A.; Pinho, B.; Guedes, R.; Jacinto, T.; Fonseca, J. Reproducibility of the Vivatmo pro measurements for exhaled nitric oxide values. Eur. Respir. J. 2019, 54, PA3909. [Google Scholar] [CrossRef]
- Korn, S.; Telke, I.; Kornmann, O.; Buhl, R. Measurement of exhaled nitric oxide: Comparison of different analysers. Respirology 2010, 15, 1203–1208. [Google Scholar] [CrossRef]
- Taylor, D.; Palmay, R.; Cowan, J.; Herbison, G. Long term performance characteristics of an electrochemical nitric oxide analyser. Respir. Med. 2011, 105, 211–217. [Google Scholar] [CrossRef]
- Bushe, C.; Kamada, A.; Hafner, R. FeNO Variability When Using Different Analyzers at the Joint ATS/ERS Guideline Cutoff. Respiration 2020, 99, 93. [Google Scholar] [CrossRef]
- Schiller, B.; Hammer, J.; Barben, J.; Trachsel, D. Comparability of a hand-held nitric oxide analyser with online and offline chemiluminescence-based nitric oxide measurement. Pediatr Allergy Immunol. 2009, 20, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Berg, J.; Lindgren, P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009, 64, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Boot, J.D.; de Ridder, L.; de Kam, M.L.; Calderon, C.; Mascelli, M.A.; Diamant, Z. Comparison of exhaled nitric oxide measurements between NIOX MINO electrochemical and Ecomedics chemiluminescence analyzer. Respir. Med. 2008, 102, 1667–1671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pizzimenti, S.; Bugiani, M.; Piccioni, P.; Heffler, E.; Carosso, A.; Guida, G.; Rolla, G. Exhaled nitric oxide measurements: Correction equation to compare hand-held device to stationary analyzer. Respir. Med. 2008, 102, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- de Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm. Pharmacol. Ther. 2008, 21, 689–693. [Google Scholar] [CrossRef]
- Maniscalco, M.; de Laurentiis, G.; Weitzberg, E.; Lundberg, J.O.; Sofia, M. Validation study of nasal nitric oxide measurements using a hand-held electrochemical analyser. Eur. J. Clin. Investig. 2008, 38, 197–200. [Google Scholar] [CrossRef]
- Khalili, B.; Boggs, P.B.; Bahna, S.L. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy 2007, 62, 1171–1174. [Google Scholar] [CrossRef]
- Ito, Y.; Adachi, Y.; Itazawa, T.; Okabe, Y.; Adachi, Y.S.; Katsumuma, T.; Miyawaki, T. Comparison of exhalation time methods (6 sec vs. 10 sec) of a hand-held exhaled nitric oxide analyzer. Pediatr. Pulmonol. 2010, 45, 1005–1008. [Google Scholar] [CrossRef]
- Antus, B.; Horvath, I.; Barta, I. Assessment of exhaled nitric oxide by a new hand-held device. Respir. Med. 2010, 104, 1377–1380. [Google Scholar] [CrossRef]
- Ziętkowski, Z.; Ziętkowska, E.; Bodzenta-Łukaszyk, A. Kliniczne znaczenie pomiarów stężenia tlenku azotu w powietrzu wydychanym w chorobach układu oddechowego. Alerg. Astma Immunol. 2009, 14, 215–222. [Google Scholar]
- Kowalczyk, A.; Krogulska, A. Usefulness of measurement of nitric oxide in exhaled air in diagnostics and treatment of allergic rhinitis and asthma in children and adolescents. Dev. Period. Med. 2018, 22, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hanibuchi, M.; Saijo, A.; Mitsuhashi, A.; Takeji, T.; Kitagawa, T. The clinical usefulness of a new hand-held device for fractional exhaled nitric oxide measurement, NIOX VERO®, for diagnosing the etiology of cough. J. Med. Investig. 2020, 67, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, M.; Lundberg, J.O. Hand-held nitric oxide sensor NIOX MINO® for the monitoring of respiratory disorders. Expert Rev. Respir. Med. 2010, 4, 715–721. [Google Scholar] [CrossRef]
- Rachel, M.; Biesiadecki, M.; Aebisher, D.; Galiniak, S. Exhaled nitric oxide in pediatric patients with respiratory disease. J. Breath Res. 2019, 13, 046007. [Google Scholar] [CrossRef]
- Bieńkowska-Haba, M. Tlenek azotu wytwarzany przez leukocyty płucne w astmie oskrzelowej. Postępy Hit Med. Dosw. 2005, 59, 584–601. [Google Scholar]
- Menzies-Gow, A.; Mansur, A.H.; Brightling, C.E. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur. Respir. J. 2020, 55, 1901633. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Dinh-Xuan, A.T.; Annesi-Maesano, I.; Berger, P.; Chambellan, A.; Chanez, P.; Chinet, T.; Degano, B.; Delclaux, C.; Demange, V.; Didier, A.; et al. Contribution of exhaled nitric oxide measurement in airway inflammation assessment in asthma. A position paper from the French Speaking Respiratory Society. Rev. Mal. Respir. 2015, 32, 193–215. [Google Scholar] [CrossRef]
- Cameli, P.; Bargagli, E.; Bergantini, L.; D’Alessandro, M.; Pieroni, M.; Fontana, G.A.; Sestini, P.; Refini, R.M. Extended Exhaled Nitric Oxide Analysis in Interstitial Lung Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6187. [Google Scholar] [CrossRef]
- Kharitonov, S.A.; Wells, A.U.; O’Connor, B.J.; Cole, P.J.; Hansell, D.M.; Logan- Sinclair, R.B.; Barnes, P.J. Elevated levels of exhaled nitric oxide in bronchiectasis. Am. J. Respir. Crit. Care Med. 1995, 151, 1889–1893. [Google Scholar] [CrossRef]
- Antosova, M.; Bencova, A.; Mokra, D.; Plevkova, J.; Pepucha, L.; Buday, T. Exhaled and Nasal Nitric Oxide–Impact for Allergic Rhinitis. Physiol. Res. 2020, 69 (Suppl. 1), S123–S130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, Y.J.; Lee, E.; Yang, S.I.; Kim, Y.H.; Jung, Y.H.; Seo, J.H.; Kwon, J.W.; Kim, H.B.; Lee, S.Y.; et al. Allergic Rhinitis in Preschool Children and the Clinical Utility of FeNO. Allergy Asthma Immunol. Res. 2017, 9, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Vo-Thi-Kim, A.; Van-Quang, T.; Nguyen-Thanh, B.; Dao-Van, D.; Duong-Quy, S. The effect of medical treatment on nasal exhaled nitric oxide (NO) in patients with persistent allergic rhinitis: A randomized control study. Adv. Med. Sci. 2020, 65, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Rachel, M. Comparison of fractional exhaled nitric oxide in asthmatics with and without allergic rhinitis. Biomarkers 2021, 26, 174–183. [Google Scholar] [CrossRef]
- Malerba, M.; Radaeli, A.; Olivini, A.; Damiani, G.; Ragnoli, B.; Montuschi, P.; Ricciardolo, F.L. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. BioMed Res. Int. 2014, 2014, 271918. [Google Scholar] [CrossRef]
- Turner, S.W.; Chang, A.B.; Yang, I.A. Clinical utility of exhaled nitric oxide fraction in the management of asthma and COPD. Breathe 2019, 15, 306–316. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, T.O.; Chang, J.; Shin, H.J.; Kwon, Y.S.; Lim, S.C.; Kim, Y.I. Clinical Features of Chronic Obstructive Pulmonary Disease with High Fractional Exhaled Nitric Oxide. Tuberc. Respir. Dis. 2020, 83, 234–241. [Google Scholar] [CrossRef]
- Zhou, A.; Zhou, Z.; Deng, D.; Zhao, Y.; Duan, J.; Cheng, W.; Liu, C.; Chen, P. The Value of FENO Measurement for Predicting Treatment Response in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 2257–2266. [Google Scholar] [CrossRef]
- Przybyłowski, T. Tlenek azotu w powietrzu wydechowym u chorych na obturacyjny bezdech podczas snu. Pneumonol. Alergol. Pol. 2006, 74, 25. [Google Scholar]
- Rubinstein, I. Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 1995, 105, 175–177. [Google Scholar] [CrossRef]
- Sekosan M i wsp. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope 1996, 106, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Nguyen-Traxler, A.; Lee, E.M.; Yip, J.S.; Weinstock, J.V.; Chan, W.W.; Ngo, P.; Weinstein, B.J.; Bonis, P.A. Assessment of fractionated exhaled nitric oxide as a biomarker for the treatment of eosinophilic esophagitis. Allergy Asthma Proc. 2013, 33, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Ibe, T.; Fukusumi, M.; Mouri, A.; Hamamoto, Y. Influence of oral care on fractional exhaled nitric oxide. Asia Pac. Allergy 2018, 8, e23. [Google Scholar] [CrossRef] [PubMed]
- Wyszyńska, M.; Rosak, P.; Czelakowska, A.; Białożyt-Bujak, E.; Kasperski, J.; Łopaciński, M.; Al Khatib, N.; Skucha-Nowak, M. Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report. Healthcare 2022, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Thansamay, S.; Yang, K.; Luo, T.; Chen, S. Measurement of Exhaled Nitric Oxide in Cirrhotic Patients with Esophageal and Gastric Varices. BioMed Res. Int. 2019, 2019, 9673162. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Czelakowska, A.; Rój, R.; Zając, M.; Mielnik, M.; Kasperski, J.; Skucha-Nowak, M. Measurement of the Level of Nitric Oxide in Exhaled Air in Patients Using Acrylic Complete Dentures and with Oral Pathologies. Coatings 2021, 11, 169. [Google Scholar] [CrossRef]
- Kasperski, J.; Wyszyńska, M.; Kustra, S.; Czecior, E.; Misiołek, M.; Kasperska-Zając, A. Letter to the Editor: Dres Helicobacter Pylori infection increase the levels of exhaled nitric oxide? Eur. J. Inflamm. 2013, 11, 279–282. [Google Scholar] [CrossRef]
- Koek, G.H.; Verleden, G.M.; Evenepoel, P.; Rutgeerts, P. Activity related increase of exhaled nitric oxide in Crohn’s disease and ulcerative colitis: A manifestation of systemic involvement? Respir. Med. 2002, 96, 530–535. [Google Scholar] [CrossRef]
- Ikonomi, E.; Rothstein, R.D.; Ehrlich, A.C.; Friedenberg, F.K. Measurement of Fractional Exhaled Nitric Oxide as a Marker of Disease Activity in Inflammatory Bowel Disease. J. Gastroenterol. Pancreatol. Liver Disord. 2016, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Reher, V.G.; Zenóbio, E.G.; Costa, F.O.; Reher, P.; Soares, R.V. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J. Oral Sci. 2007, 49, 271–276. [Google Scholar] [CrossRef]
- Humberto, J.S.M.; Pavanin, J.V.; Rocha, M.J.A.D.; Motta, A.C.F. Cytokines, cortisol, and nitric oxide as salivary biomarkers in oral lichen planus: A systematic review. Braz. Oral Res. 2018, 32, e82. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Iwase, M.; Nagumo, M. Elevated production of salivary nitric oxide in oral mucosal diseases. J. Oral Pathol. Med. 1999, 28, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Gurel, A.; Altinyazar, H.C.; Unalacak, M.; Armutcu, F.; Koca, R. Purine catabolic enzymes and nitric oxide in patients with recurrent aphthous ulceration. Oral Dis. 2007, 13, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Senthil Eagappan, A.R.; Rao, V.A.; Sujatha, S.; Senthil, D.; Sathiyajeeva, J.; Rajaraman, G. Evaluation of salivary nitric oxide level in children with early childhood caries. Dent. Res. J. 2016, 13, 338–341. [Google Scholar] [CrossRef]
- Sangle, V.A.; Chaware, S.J.; Kulkarni, M.A.; Ingle, Y.C.; Singh, P.; Pooja, V.K. Elevated tissue nitric oxide in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2018, 22, 35–39. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Czecior, E.; Namyslowski, G. Effect of tonsillectomy on the level of exhaled nitric oxide (NO) in patients with recurrent tonsillitis. Respir. Med. 2010, 104, 1757–1759. [Google Scholar] [CrossRef][Green Version]
- Kasperska-Zajac, A.; Brzoza, Z.; Czecior, E.; Rogala, B.; Polok, A.; Namyslowski, G. Elevated levels of exhaled nitric oxide in recurrent tonsillitis. Eur. Respir. J. 2008, 31, 909–910. [Google Scholar] [CrossRef]
- Rizzi, M.; Radovanovic, D.; Airoldi, A.; Cristiano, A.; Frassanito, F.; Gaboardi, P.; Saad, M.; Atzeni, F.; Sarzi-Puttini, P.; Santus, P. Rationale underlying the measurement of fractional exhaled nitric oxide in systemic sclerosis patients. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 1), 125–132. [Google Scholar]
- Tiev, K.P.; Coste, J.; Ziani, M.; Aubourg, F.; Cabane, J.; Dinh-Xuan, A.T. Diagnostic value of exhaled nitric oxide to detect interstitial lung disease in systemic sclerosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2009, 26, 32–38. [Google Scholar]
- Oświęcimska, J.; Ziora, K.; Ziora, D.; Machura, E.; Smerdziński, S.; Pyś-Spychała, M.; Kasperski, J.; Zamłyński, J.; Kasperska-Zajac, A. Elevated levels of exhaled nitric oxide in patients with anorexia nervosa. Eur. Child Adolesc. Psychiatry 2014, 23, 845–850. [Google Scholar] [CrossRef]

| 10 Years | NO in Exhaled Air in Medicine | NO in Exhaled Air in Oral Cavity Pathologies | NO in Exhaled Air in Stomatology |
|---|---|---|---|
| Review and systematic review | 53 | 0 | 2 |
| All article types | 545 | 5 | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszyńska, M.; Nitsze-Wierzba, M.; Czelakowska, A.; Kasperski, J.; Żywiec, J.; Skucha-Nowak, M. An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring. Molecules 2022, 27, 4279. https://doi.org/10.3390/molecules27134279
Wyszyńska M, Nitsze-Wierzba M, Czelakowska A, Kasperski J, Żywiec J, Skucha-Nowak M. An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring. Molecules. 2022; 27(13):4279. https://doi.org/10.3390/molecules27134279
Chicago/Turabian StyleWyszyńska, Magdalena, Monika Nitsze-Wierzba, Aleksandra Czelakowska, Jacek Kasperski, Joanna Żywiec, and Małgorzata Skucha-Nowak. 2022. "An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring" Molecules 27, no. 13: 4279. https://doi.org/10.3390/molecules27134279
APA StyleWyszyńska, M., Nitsze-Wierzba, M., Czelakowska, A., Kasperski, J., Żywiec, J., & Skucha-Nowak, M. (2022). An Evidence-Based Review of Application Devices for Nitric Oxide Concentration Determination from Exhaled Air in the Diagnosis of Inflammation and Treatment Monitoring. Molecules, 27(13), 4279. https://doi.org/10.3390/molecules27134279








