Membrane Permeabilization and Antimicrobial Activity of Recombinant Defensin-d2 and Actifensin against Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans
Abstract
:1. Introduction
2. Results
2.1. Bioinformatics Analysis of Defensin-d2 and Actifensin
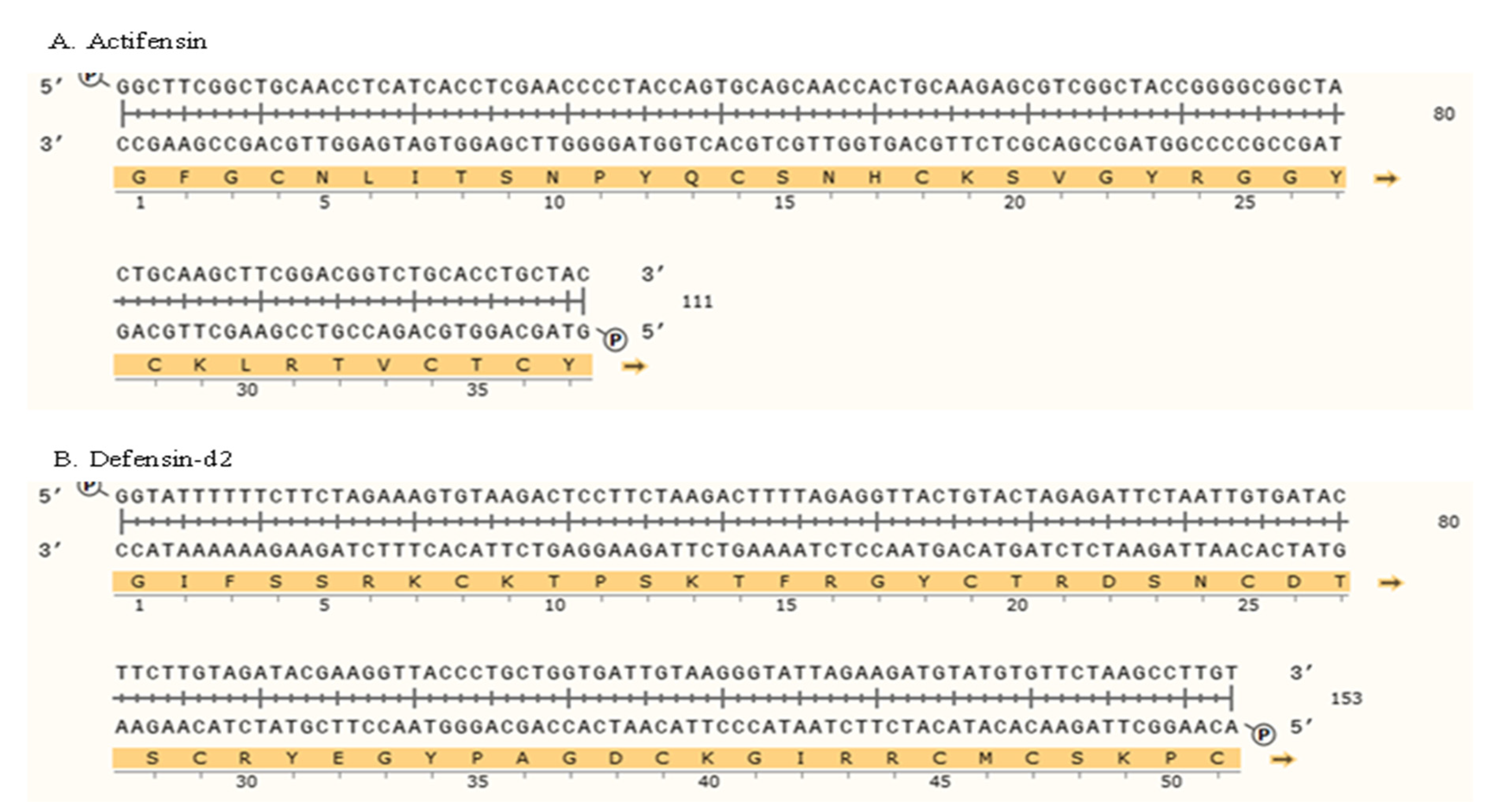
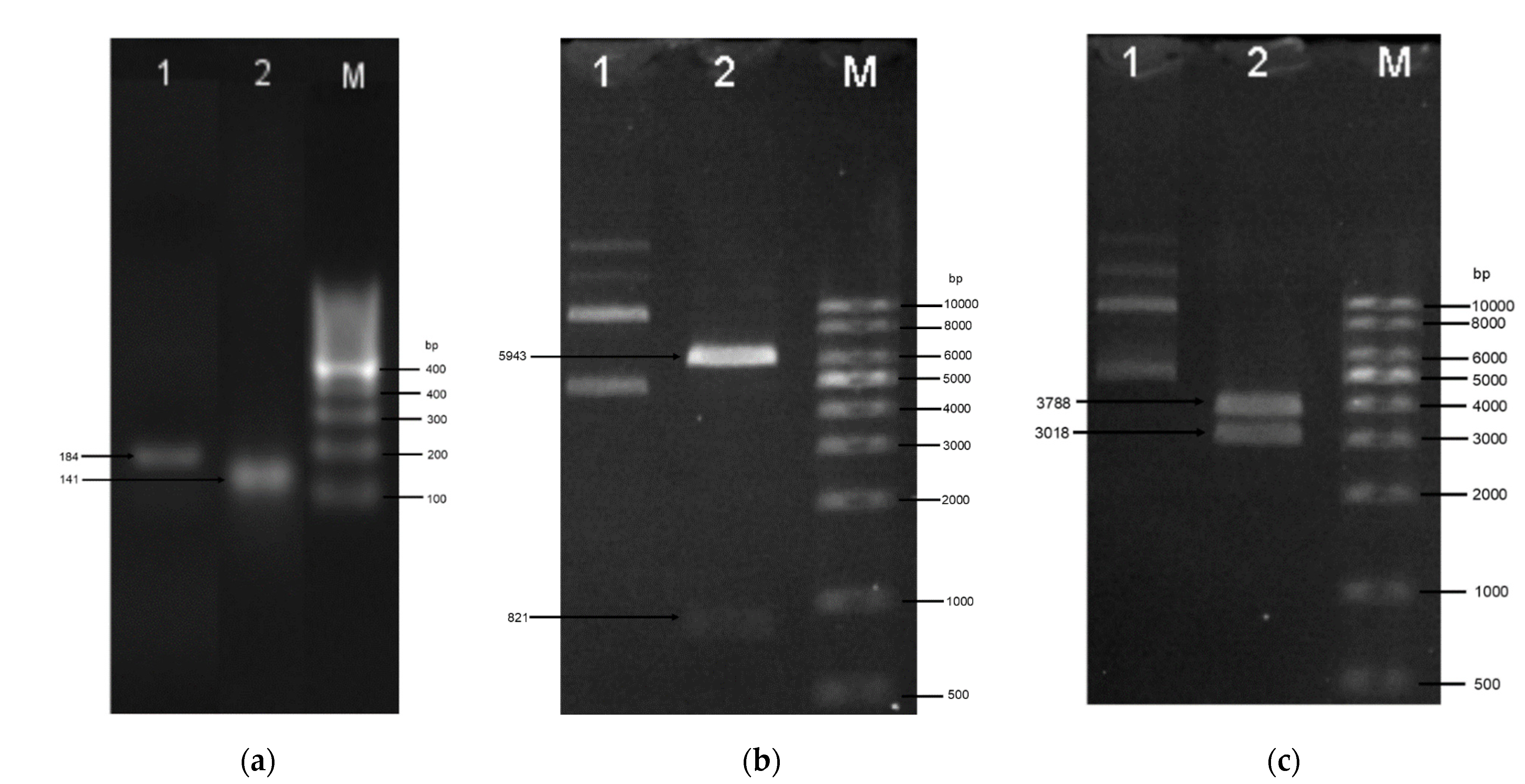
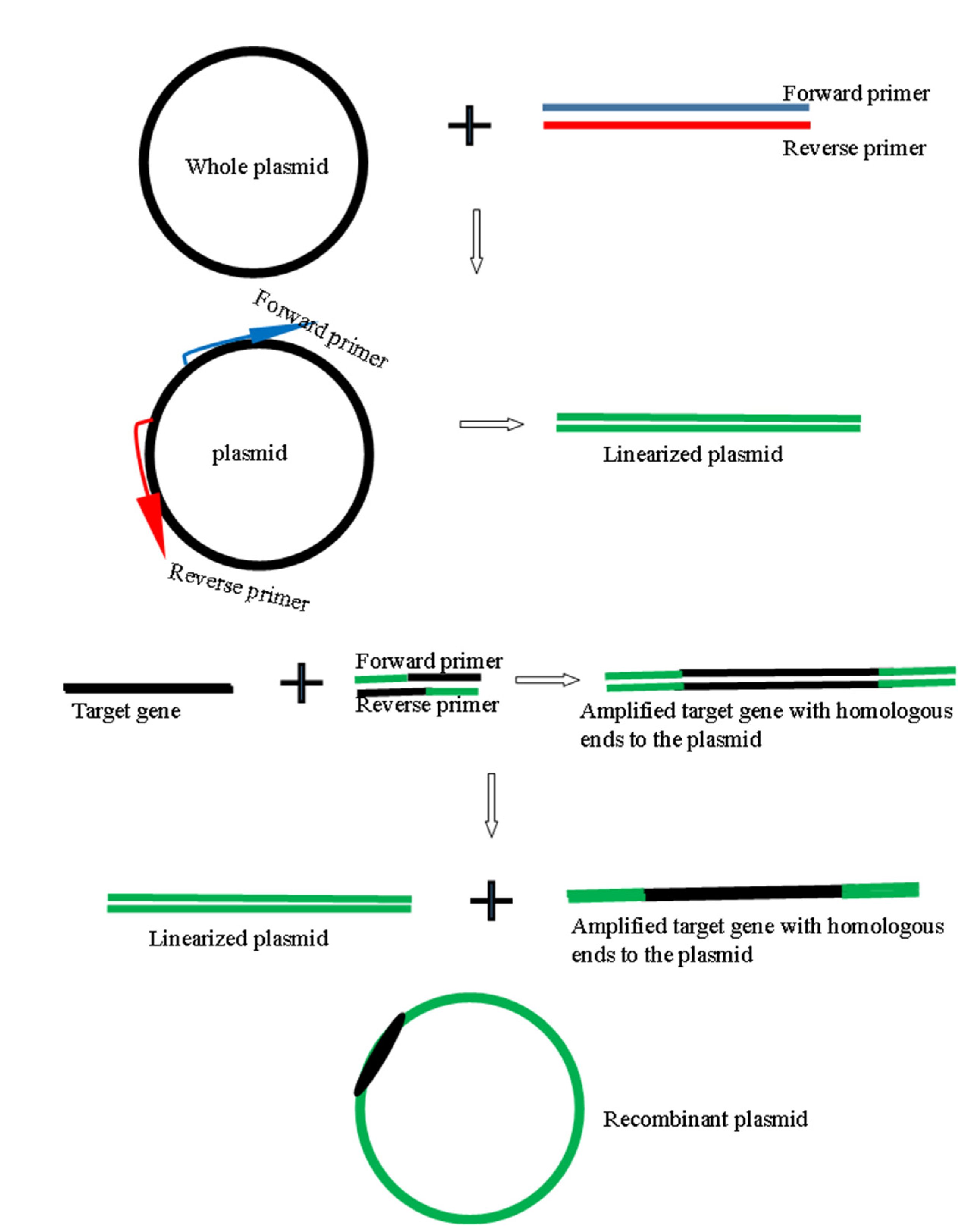
2.2. Generation of Genes and Recombinant Plasmids
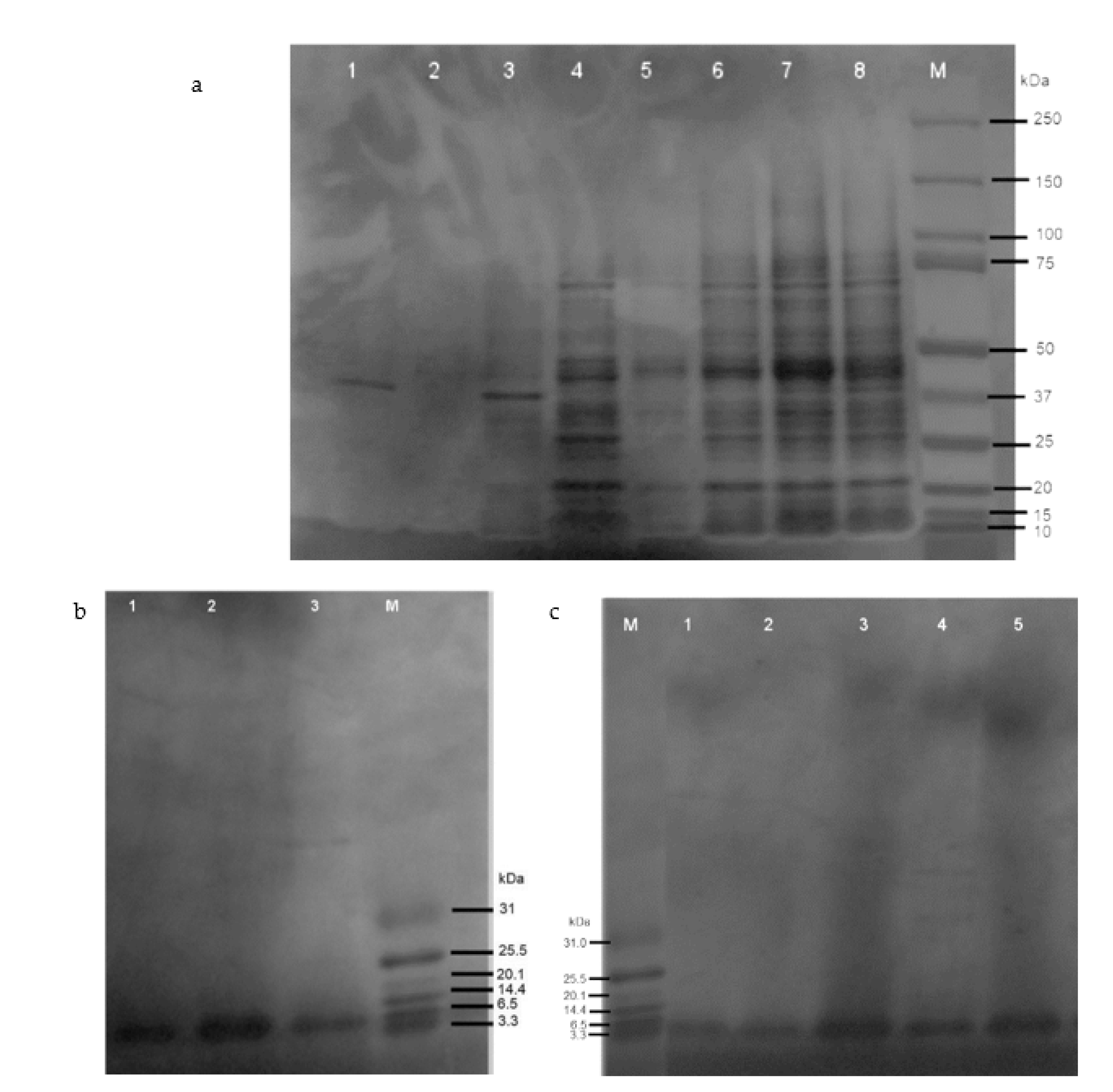
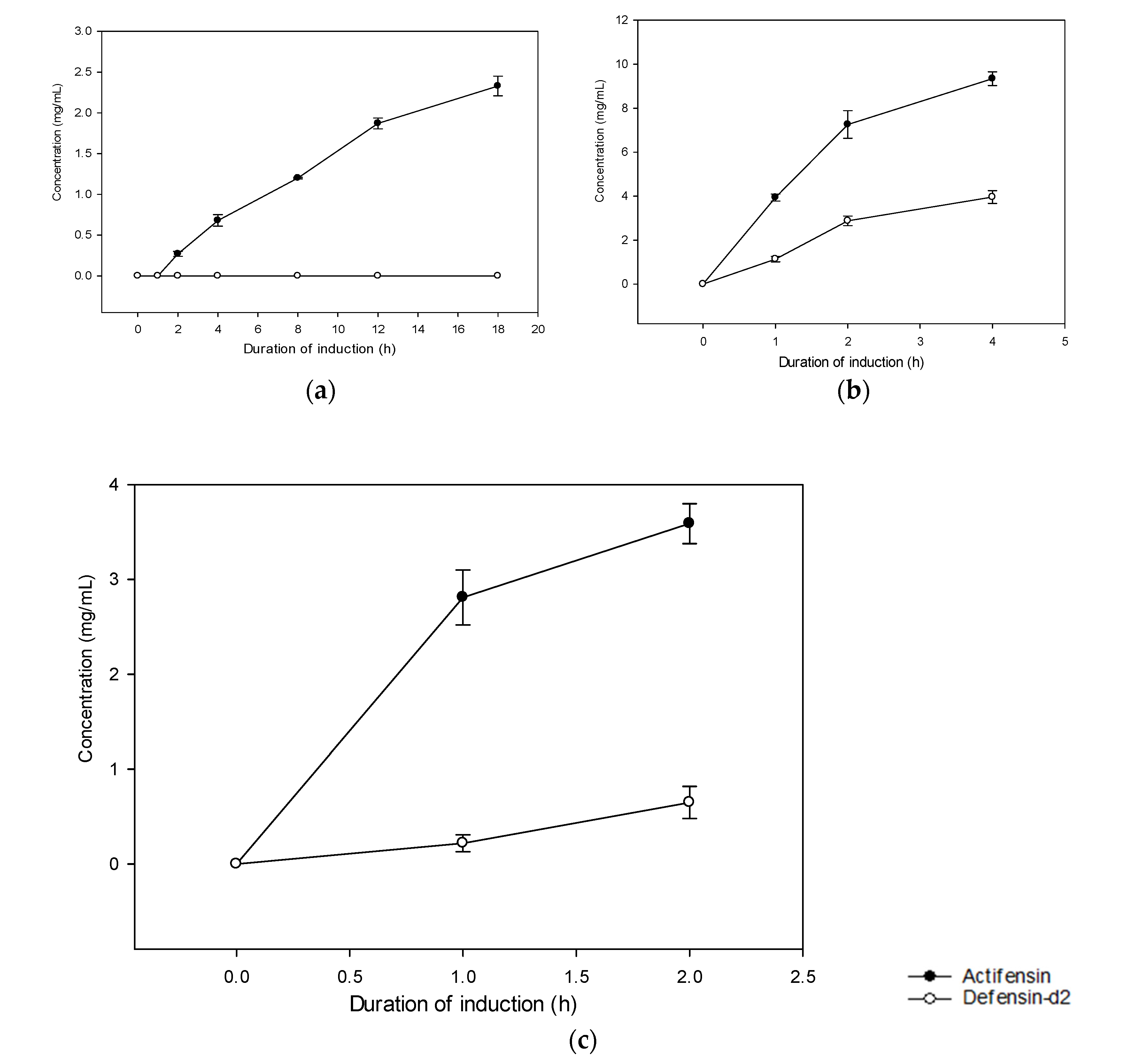
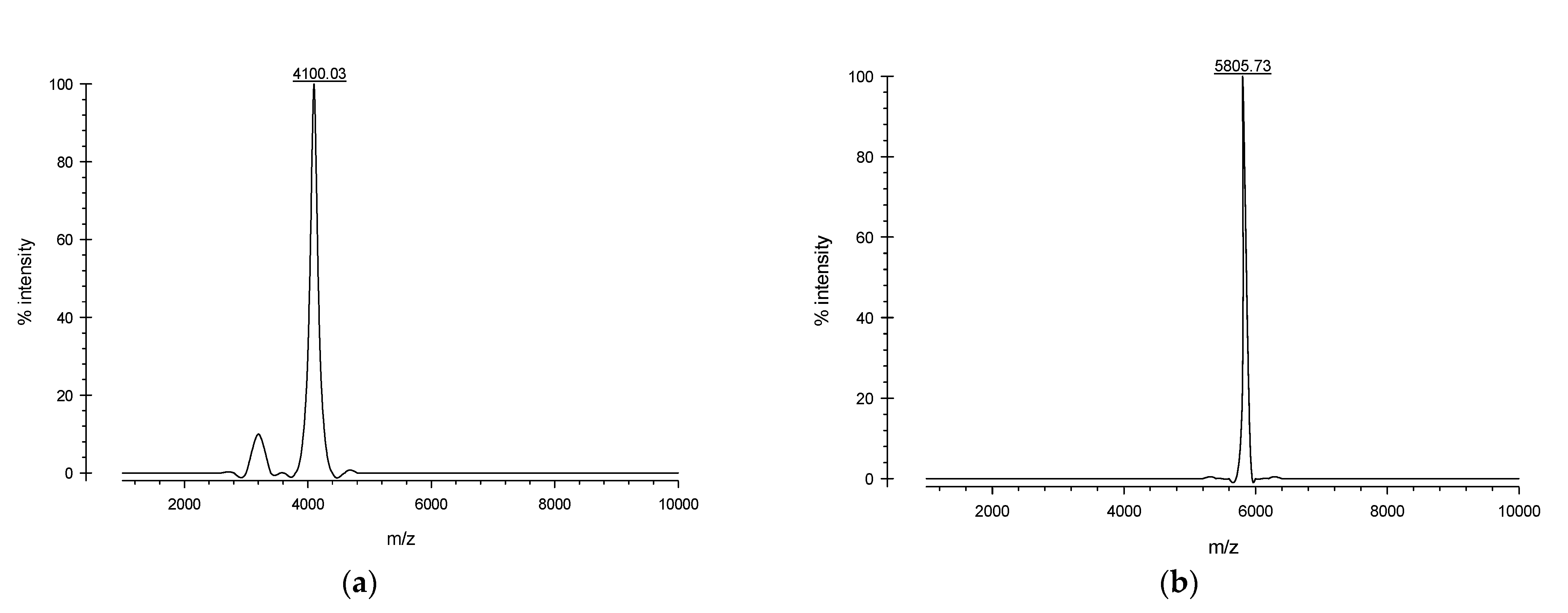
2.3. Expression and Purification of Recombinant Defensin-d2 and Actifensin
2.4. Antimicrobial Activity of the Recombinant Peptides
2.4.1. Minimum Inhibitory Concentrations of Recombinant Peptides
2.4.2. Synergistic Activity of Recombinant Peptides
2.4.3. Inhibitory Kinetics of Recombinant Actifensin and Defensin-d2
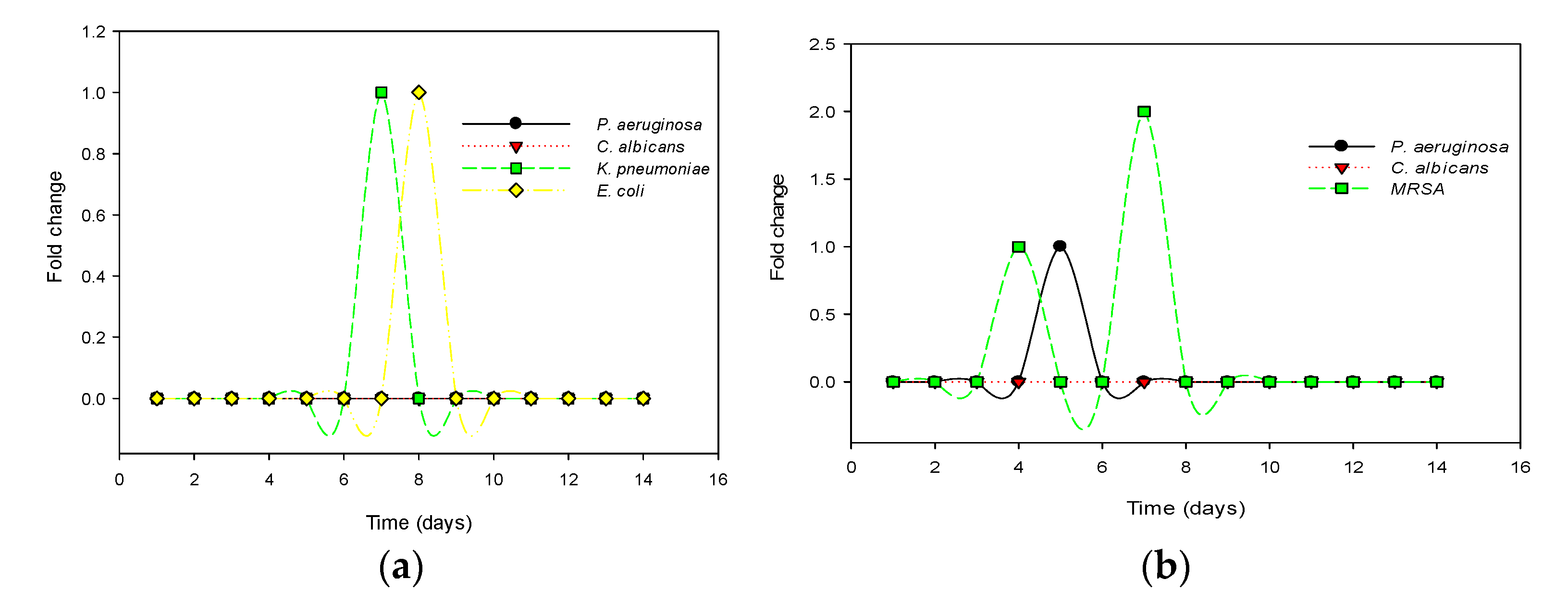
2.4.4. Resistance Potential of Test Organisms to Recombinant Actifensin and Defensin-d2
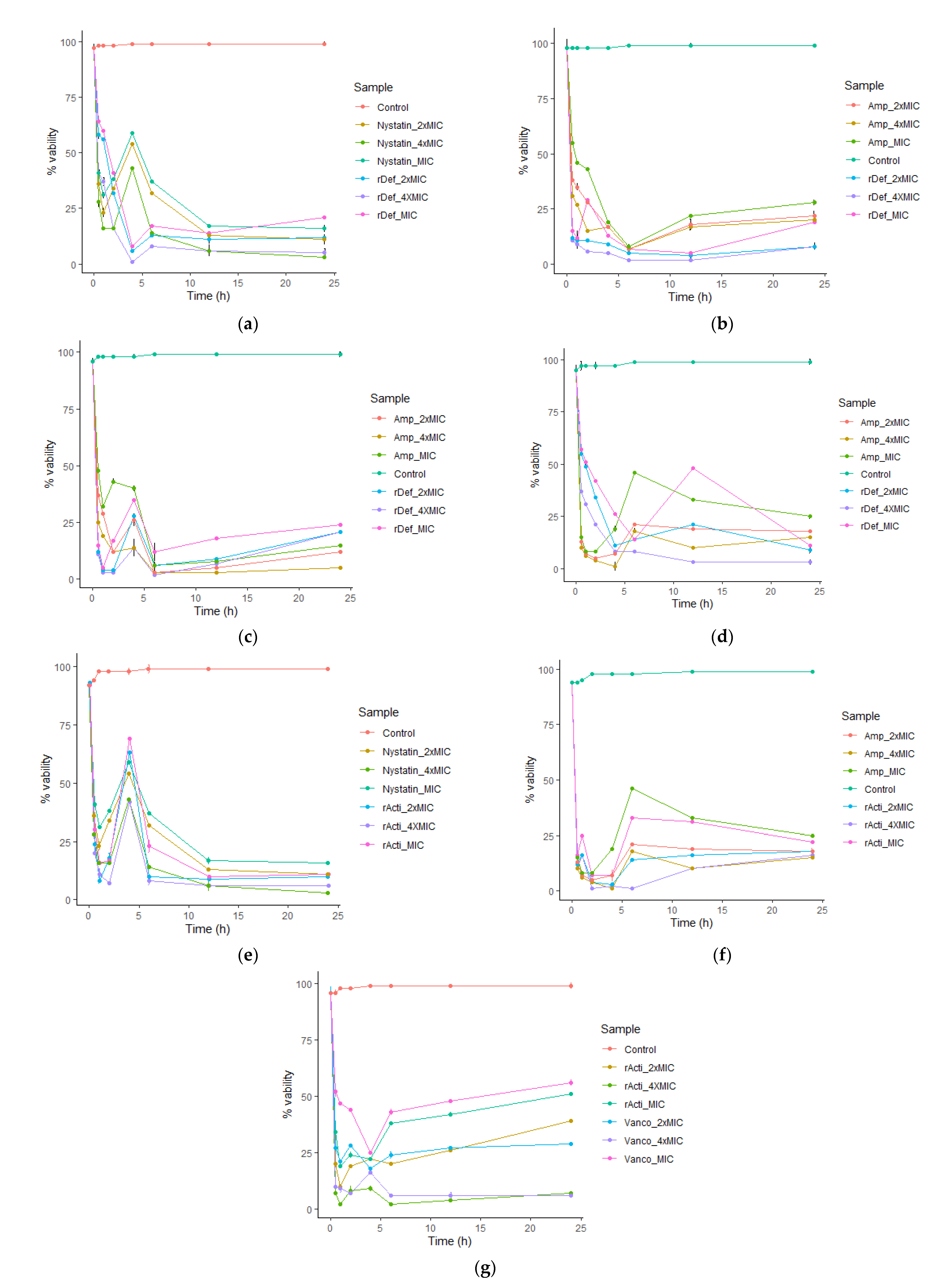
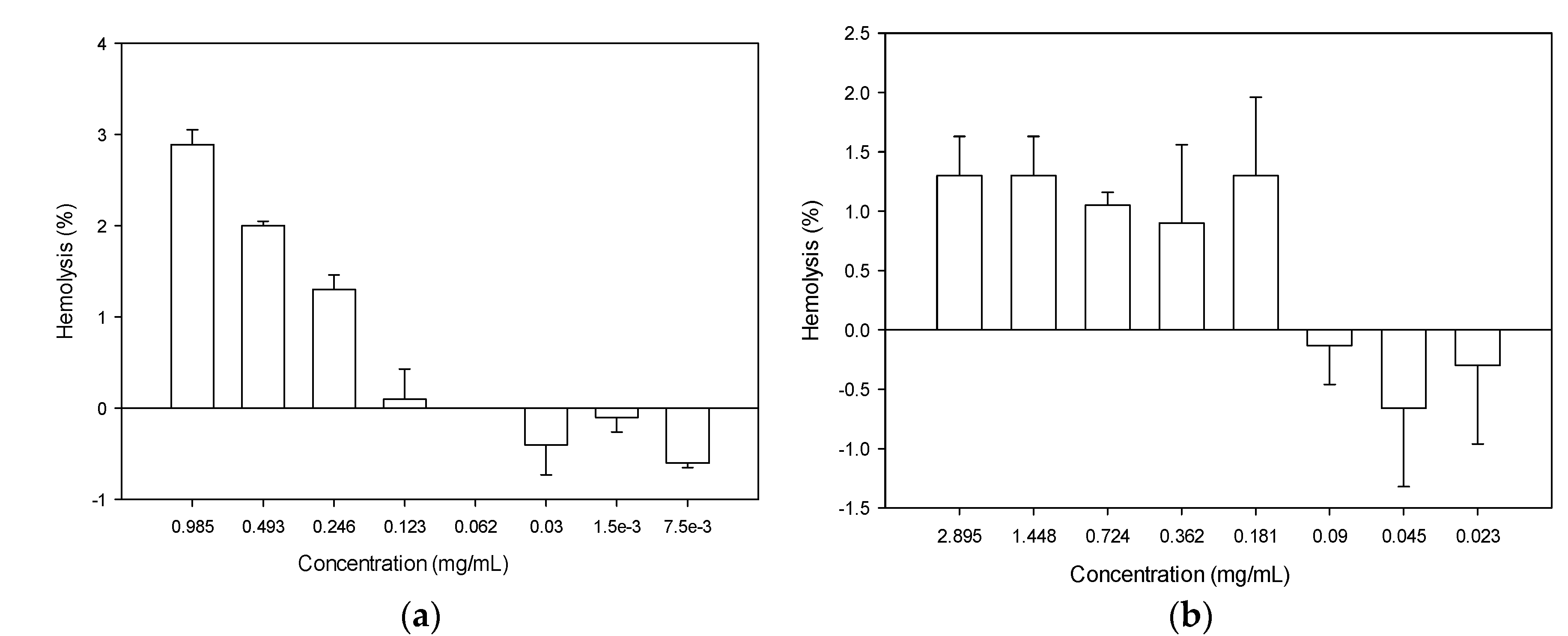
2.5. Hemolytic Activity of Recombinant Actifensin and Defensin-d2
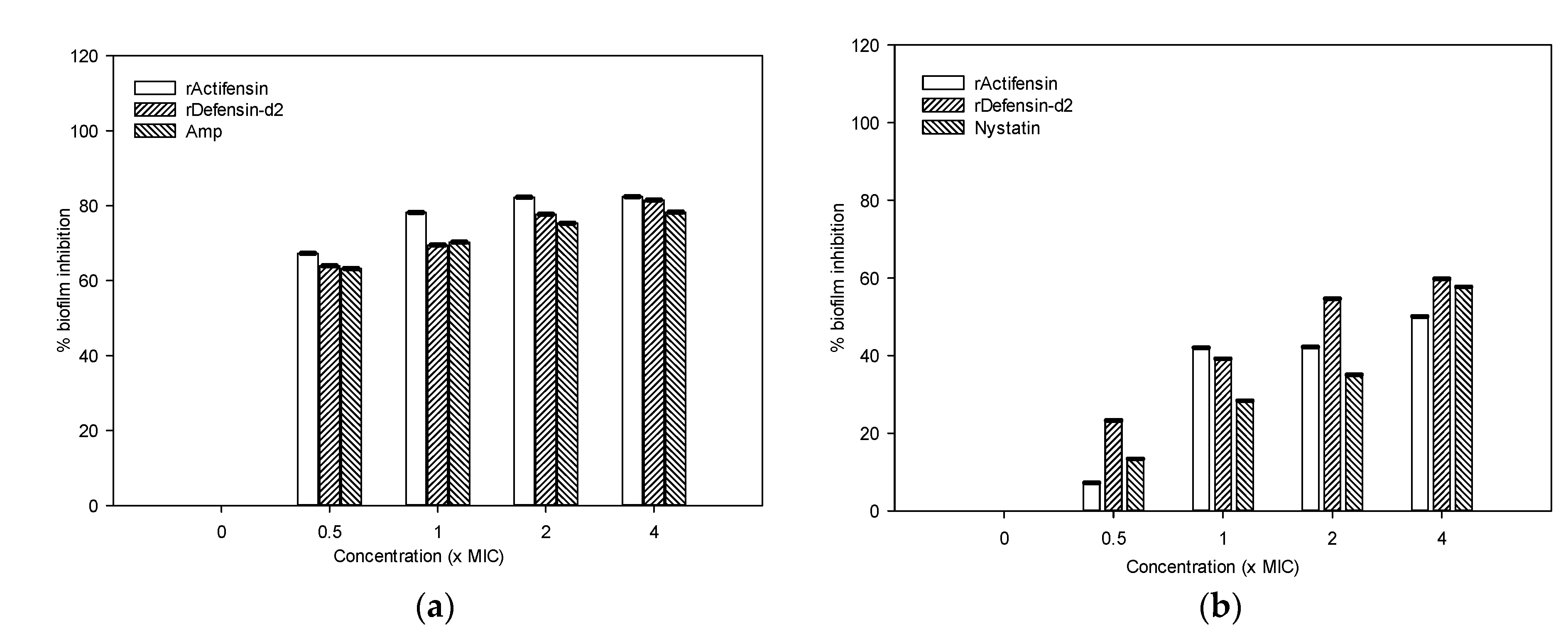
2.6. Biofilm-Inhibition Potential of Recombinant Actifensin and Defensin-d2
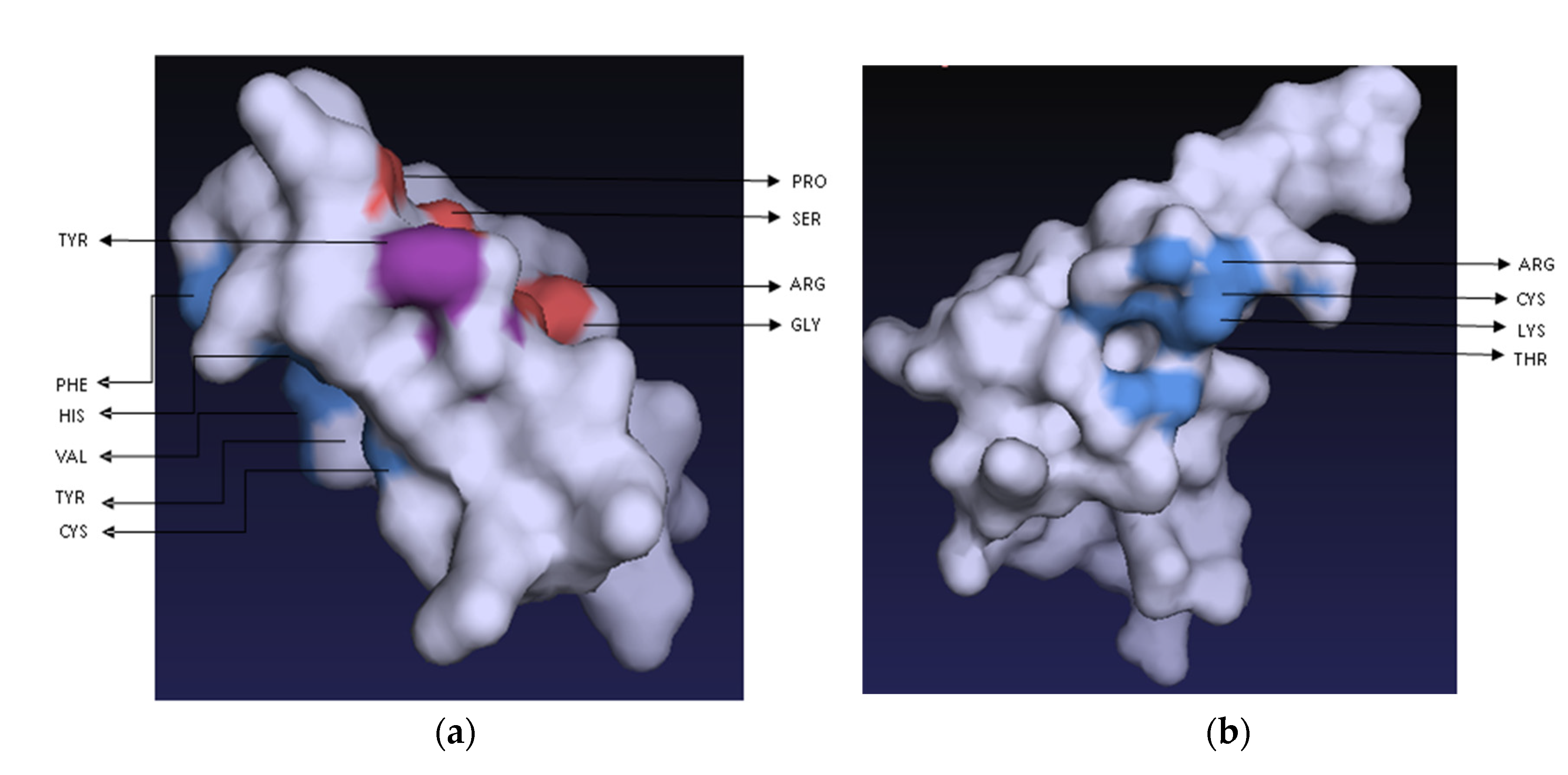
2.7. Peptide–Ligand Interactions of Recombinant Actifensin and Defensin-d2
2.8. Membrane Permeability by Recombinant Defensin-d2 and Actifensin
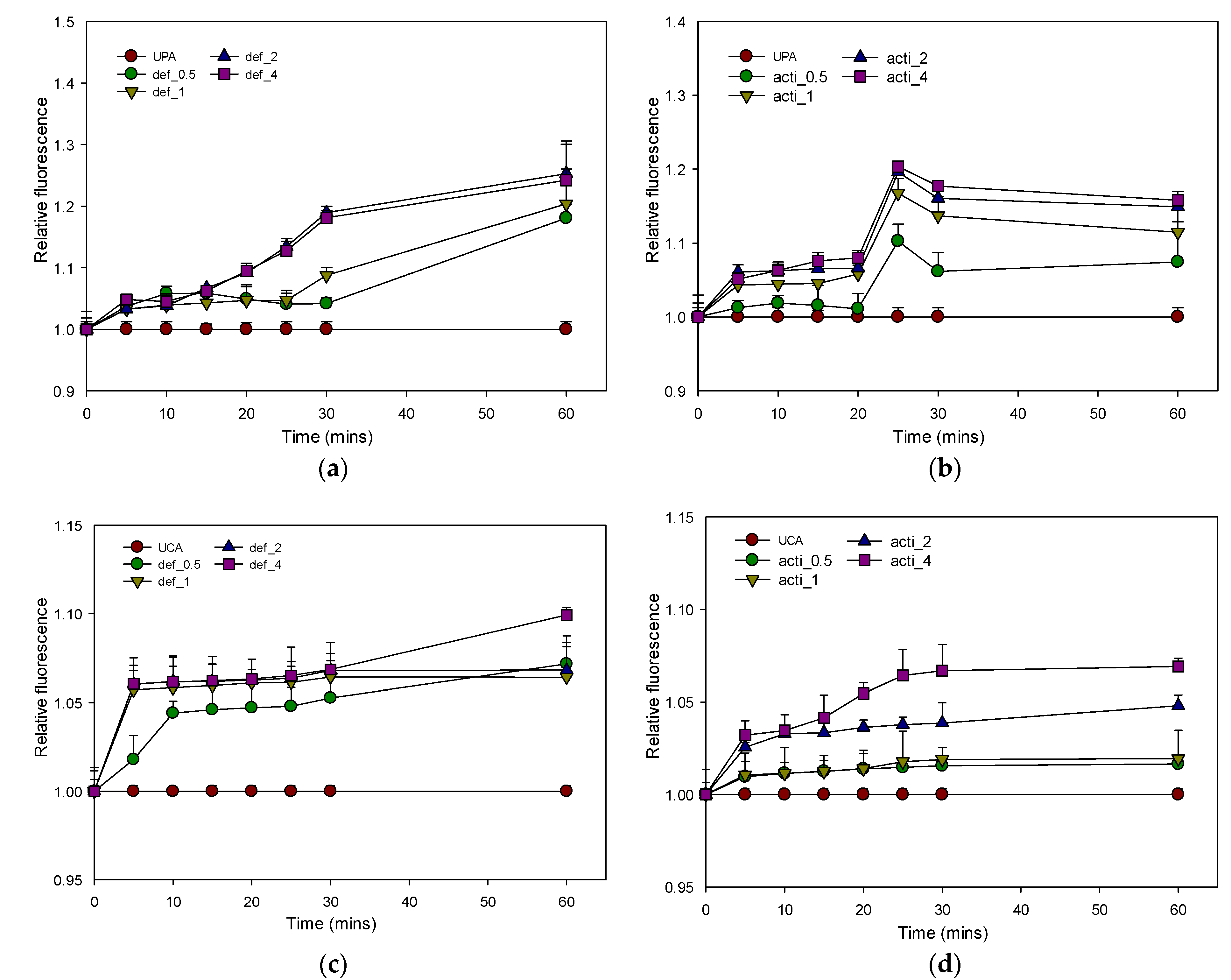
2.8.1. Outer-Membrane Permeability by Recombinant Defensin-d2 and Actifensin
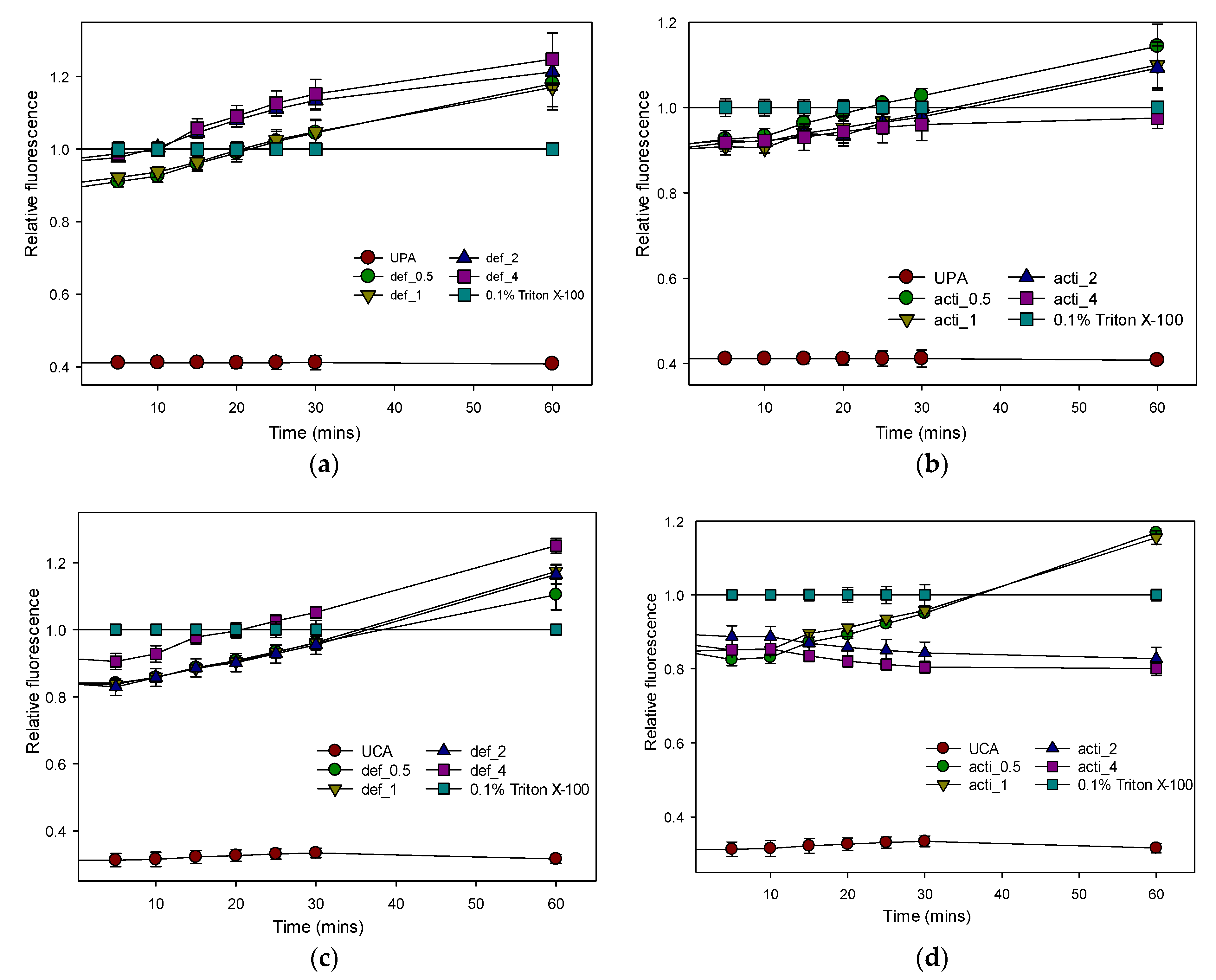
2.8.2. Plasma-Membrane Permeability by Recombinant Defensin-d2 and Actifensin
2.8.3. Inner-Membrane Depolarization by Recombinant Defensin-d2 and Actifensin
2.8.4. Effect of Recombinant Defensin-d2 and Actifensin on ROS Production
3. Discussion
3.1. Bioinformatics Analysis of the Peptides
3.2. Generation of Recombinant Plasmids and Protein Expression
3.3. Antimicrobial Activity of Purified Recombinant Peptides
3.4. Toxicity of the Recombinant Peptides
3.5. Cell Permeability of Recombinant Actifensin and Defensin-d2
4. Materials and Methods
4.1. Test Organisms
4.2. Bioinformatics Analysis
4.3. Generation of Gene Constructs
PCR Amplification of Plasmid and Inserts
4.4. Gene Cloning
4.4.1. Transformation of E. coli and Clone Verification
4.4.2. Colony PCR and Plasmid Sequencing
4.5. Expression and Optimization of Recombinant Defensin-d2 and Actifensin
Peptide Cleavage and Affinity Purification
4.6. Antimicrobial Activity of the Recombinant Peptides
4.6.1. Minimum Inhibitory Concentrations of Recombinant Peptides
4.6.2. Synergistic Activity of Recombinant Peptides
4.6.3. Inhibitory Kinetics Analysis
4.6.4. Resistance Potential of Test Organisms to Recombinant Peptides
4.7. Hemolytic Activity of the Recombinant Peptides
4.8. Biofilm-Formation Inhibition by the Recombinant Peptides
4.9. Peptide–Ligand Interaction of the Recombinant Peptides
4.10. Cell Permeability by the Recombinant Peptides
4.10.1. Outer-Membrane-Permeabilization Assay
4.10.2. Plasma-Membrane-Permeability Assay
4.10.3. Inner-Membrane-Depolarization Assay
4.10.4. Determination of Reactive-Oxygen-Species (ROS) Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations: The Review on Antimicrobial Resistance. 2016. Available online: https://apo.org.au/node/63983 (accessed on 6 June 2022).
- Elhadidy, M.; Ali, M.M.; El-Shibiny, A.; Miller, W.G.; Elkhatib, W.F.; Botteldoorn, N.; Dierick, K. Antimicrobial Resistance Patterns and Molecular Resistance Markers of Campylobacter Jejuni Isolates from Human Diarrheal Cases. PLoS ONE 2020, 15, e0227833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, A.; Khan, R.S.; Shehryar, K.; Imran, A.; Ali, F.; Attia, S.; Shah, S.; Mii, M. Antimicrobial Peptides as Effective Tools for Enhanced Disease Resistance in Plants. PCTOC 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Orrapin, S.; Intorasoot, A.; Roytrakul, S.; Dechsupa, N.; Kantapan, J.; Onphat, Y.; Srimek, C.; Sitthidet Tharinjaroen, C.; Anukool, U.; Butr-Indr, B.; et al. A Novel Recombinant Javanicin with Dual Antifungal and Anti-Proliferative Activities. Sci. Rep. 2019, 9, 18417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Ferreira, J.D.C.; de Oliveira-Silva, R.L.; de Jesus Pires, C.; Aburjaile, F.F.; et al. Plant Antimicrobial Peptides: State of the Art, In Silico Prediction and Perspectives in the Omics Era. Bioinform. Biol. Insights 2020, 14, 1177932220952739. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, N.; Bilal, M.; Iqbal, H. Medicinal Potentialities of Plant Defensins: A Review with Applied Perspectives. Medicines 2019, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Segura, A.; Moreno, M.; Molina, A.; García-Olmedo, F. Novel Defensin Subfamily from Spinach (Spinacia Oleracea). FEBS Lett. 1998, 435, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Sathoff, A.E.; Sathoff, A.E.; Lewenza, S.; Lewenza, S.; Samac, D.A.; Samac, D.A. Plant Defensin Antibacterial Mode of Action against Pseudomonas Species. BMC Microbiol. 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; O’Connor, P.M.; Hill, C.; Stanton, C.; Paul Ross, R. Actinomyces Produces Defensin-like Bacteriocins (Actifensins) with a Highly Degenerate Structure and Broad Antimicrobial Activity. J. Bacteriol. 2020, 202, e00529–e00539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Chittpurna; Ashish; Sharma, V.; Patil, P.B.; Korpole, S. Identification, Purification and Characterization of Laterosporulin, a Novel Bacteriocin Produced by Brevibacillus Sp. Strain GI-9. PLoS ONE 2012, 7, e31498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shea, E.F.; O’Connor, P.M.; O’Sullivan, O.; Cotter, P.D.; Ross, R.P.; Hill, C. Bactofencin A, a New Type of Cationic Bacteriocin with Unusual Immunity. mBio 2013, 4, e00498–e00513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, Z.; Wang, X.; Wang, J. A Recombinant Fungal Defensin-like Peptide-P2 Combats Multidrug-Resistant Staphylococcus aureus and Biofilms. Appl. Microbiol. Biotechnol. 2019, 103, 5193–5213. [Google Scholar] [CrossRef]
- Sinha, R.; Shukla, P. Antimicrobial Peptides: Recent Insights on Biotechnological Interventions and Future Perspectives. Protein Pept. Lett. 2018, 26, 79–87. [Google Scholar] [CrossRef]
- Lay, F.T.; Ryan, G.F.; Caria, S.; Phan, T.K.; Veneer, P.K.; White, J.A.; Kvansakul, M.; Hulett, M.D. Structural and Functional Characterization of the Membrane-Permeabilizing Activity of Nicotiana occidentalis Defensin NoD173 and Protein Engineering to Enhance Oncolysis. FASEB J. 2019, 33, 6470–6482. [Google Scholar] [CrossRef]
- Zorko, M.; Jerala, R. Production of Recombinant Antimicrobial Peptides in Bacteria. Methods Mol. Biol. 2010, 618, 61–76. [Google Scholar]
- Dias, R.D.O.; Franco, O.L. Cysteine-Stabilized Aβ Defensins: From a Common Fold to Antibacterial Activity. Peptides 2015, 72, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedemann, C.; Kumar, A.; Lang, A.; Ohlenschläger, O. Cysteines and Disulfide Bonds as Structure-Forming Units: Insights From Different Domains of Life and the Potential for Characterization by NMR. Front. Chem. 2020, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Carvalho, A.; Moreira Gomes, V. Plant Defensins and Defensin-Like Peptides—Biological Activities and Biotechnological Applications. Curr. Pharm. Des. 2012, 17, 4270–4293. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant Defensins from a Structural Perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef]
- Alaei, L.; Ashengroph, M.; Moosavi-Movahedi, A.A. Chapter Eight—The concept of protein folding/unfolding and its impacts on human health. Adv. Prot. Chem. Struc. Biol. 2021, 126, 227–278. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. The Proteomics Protocols Handbook—Chapter 52: Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.P.; Brooks, B.R.; Thirumalai, D. Effects of PH on Proteins: Predictions for Ensemble and Single-Molecule Pulling Experiments. J. Am. Chem. Soc. 2012, 134, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guruprasad, K.; Reddy, B.V.B.; Pandit, M.W. Correlation between Stability of a Protein and Its Dipeptide Composition: A Novel Approach for Predicting in vivo Stability of a Protein from Its Primary Sequence. Prot. Eng. 1990, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. Structures of Dipeptides: The Head-to-Tail Story. Acta Crystallogr B 2010, 66, 84–93. [Google Scholar] [CrossRef]
- Ikai, A. Thermostability and Aliphatic Index of Globular Proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar]
- Panda, S.; Chandra, G. Physicochemical Characterization and Functional Analysis of Some Snake Venom Toxin Proteins and Related Non-Toxin Proteins of Other Chordates. Bioinformation 2012, 8, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Bryksin, A.V.; Matsumura, I. Overlap Extension PCR Cloning: A Simple and Reliable Way to Create Recombinant Plasmids. Biotechniques 2010, 48, 463–465. [Google Scholar] [CrossRef]
- Bhat, S.; Bialy, D.; Sealy, J.E.; Sadeyen, J.R.; Chang, P.; Iqbal, M. A Ligation and Restriction Enzyme Independent Cloning Technique: An Alternative to Conventional Methods for Cloning Hard-to-Clone Gene Segments in the Influenza Reverse Genetics System. Virol. J. 2020, 17, 82. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [Green Version]
- Al Kashgry, N.A.T.; Abulreesh, H.H.; El-Sheikh, I.A.; Almaroai, Y.A.; Salem, R.; Mohamed, I.; Waly, F.R.; Osman, G.; Mohamed, M.S.M. Utilization of a Recombinant Defensin from Maize (Zea mays L.) as a Potential Antimicrobial Peptide. AMB Express 2020, 10, 208. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Xu, C.; Sun, W.; Wang, P.; Li, D.; Yin, T.; Zhuge, Q. Characterization, Expression Profiling, and Functional Analysis of PtDef, a Defensin-Encoding Gene from Populus trichocarpa. Front. Microbiol. 2020, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, S.; Wang, X.; Gu, Q.; Li, P. Antimicrobial Activity and Preliminary Mode of Action of PlnEF Expressed in Escherichia coli against Staphylococci. Prot. Expr. Purif. 2018, 143, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ma, J.; Chen, X.; Tan, Y.; Chen, P.; Zhu, X.; Liu, L. Expression and Purification of Recombinant Lactobacillus casei Bacteriocin and Analysis of Its Antibacterial Activity. CYTA J. Food 2020, 18, 301–308. [Google Scholar] [CrossRef]
- Madi-Moussa, D.; Coucheney, F.; Drider, D. Expression of Five Class II Bacteriocins with Activity against Escherichia coli in Lacticaseibacillus paracasei CNCM I-5369, and in a Heterologous Host. Biotechnol. Rep. 2021, 30, e00632. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: http://Www.Who.Int/Medicines/Publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.Pdf?Ua=1, (accessed on 6 June 2022).
- Sharma, A.; Srivastava, S. Anti-Candida Activity of Two-Peptide Bacteriocins, Plantaricins (Pln E/F and J/K) and Their Mode of Action. Fungal Biol. 2014, 118, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis Bacteriocin EntV Inhibits Hyphal Morphogenesis, Biofilm Formation, and Virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef] [Green Version]
- Sathoff, A.E.; Velivelli, S.; Shah, D.M.; Samac, D.A. Plant Defensin Peptides Have Antifungal and Antibacterial Activity against Human and Plant Pathogens. Phytopathology 2019, 109, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, J.; Oesch, G.; Kuster, S.P. Bacteriostatic versus Bactericidal Antibiotics for Patients with Serious Bacterial Infections: Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2015, 70, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Lambert, D.G. Drugs and Receptors. Continuing Education in Anaesthesia. Crit. Care Pain 2004, 4, 181–184. [Google Scholar]
- Wyllie, D.J.A.; Chen, P.E. Taking the Time to Study Competitive Antagonism. Br. J. Pharmacol. 2007, 150, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, M.; Nobile, C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbe Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pontes, J.T.C.; Toledo Borges, A.B.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.R.; Liao, C.C.; Chen, Y.R.; Chak, K.F. Induction of Membrane Permeability in Escherichia coli Mediated by Lysis Protein of the ColE7 Operon: Research Letter. FEMS Microbiol. Lett. 2009, 298, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Tachikawa, A.; Terauchi, S.; Yamashita, K.; Kataoka, M.; Terada, H.; Shinohara, Y. Multiple Effects of DiS-C3(5) on Mitochondrial Structure and Function. Eur. J. Biochemist. 2004, 271, 3573–3579. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, J.; Gao, H.; Wang, Z.; Dong, N.; Ma, Q.; Shan, A. Antimicrobial Properties and Membrane-Active Mechanism of a Potential α-Helical Antimicrobial Derived from Cathelicidin PMAP-36. PLoS ONE 2014, 9, e86364. [Google Scholar]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of Action of Antimicrobial Peptide P5 Truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express 2019, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Hu, C.; Cao, B.; Qu, X.; Guan, P.; Mu, Y.; Han, L.; Huang, X. A Semisynthetic Borrelidin Analogue BN-3b Exerts Potent Antifungal Activity against Candida albicans through ROS-Mediated Oxidative Damage. Sci. Rep. 2020, 10, 5081. [Google Scholar] [CrossRef] [Green Version]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Imani Fooladi, A.A.; Amani, J.; et al. The Antifungal Peptide MCh-AMP1 Derived from Matricaria chamomilla Inhibits Candida albicans Growth via Inducing ROS Generation and Altering Fungal Cell Membrane Permeability. Front. Microbiol. 2020, 10, 3150. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amerikova, M.; Pencheva El-Tibi, I.; Maslarska, V.; Bozhanov, S.; Tachkov, K. Antimicrobial Activity, Mechanism of Action, and Methods for Stabilisation of Defensins as New Therapeutic Agents. Biotechnol. Biotechnol. Equipment 2019, 33, 671–682. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The Proteomics Server for in-Depth Protein Knowledge and Analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 Improves Signal Peptide Predictions Using Deep Neural Networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, F.; Clote, P. Disulfide Connectivity Prediction Using Secondary Structure Information and Diresidue Frequencies. Bioinformatics 2005, 21, 2336–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, P.; Longden, L.; Bleasby, A. EMBOSS: The European Molecular Biology Open Software Suite. Trends Genet 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Froger, A.; Hall, J.E. Transformation of Plasmid DNA into E. coli Using the Heat Shock Method. J. Vis. Exp. 2007, 253. [Google Scholar] [CrossRef]
- Packeiser, H.; Lim, C.; Balagurunathan, B.; Wu, J.; Zhao, H. An Extremely Simple and Effective Colony PCR Procedure for Bacteria, Yeasts, and Microalgae. Appl. Biochem. Biotechnol. 2013, 169, 695–700. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals; Approved Standard, M31 A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition, CLSI document M07-A9; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard. 9th Edition Document M2-A9; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Oddo, A.; Hansen, P.R. Hemolytic activity of antimicrobial peptides. Methods Mol. Biol. 2017, 1548, 427–435. [Google Scholar] [PubMed]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The Antimicrobial Peptide SAAP-148 Combats Drug-Resistant Bacteria and Biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Srivastava, H.K.; Raghava, G.P.S. A Web Server for Analysis, Comparison and Prediction of Protein Ligand Binding Sites. Biol. Direct 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivák, R.; Hoksza, D. P2Rank: Machine Learning Based Tool for Rapid and Accurate Prediction of Ligand Binding Sites from Protein Structure. J. Cheminform. 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D. PrankWeb: A Web Server for Ligand Binding Site Prediction and Visualization. Nucleic Acids Res. 2019, 47, W345–W349. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Shen, T.; Liu, Y.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Yan, Z.; Liao, C.; Wang, C. Enhancing the Antibacterial Activity of Antimicrobial Peptide PMAP-37(F34-R) by Cholesterol Modification. BMC Vet. Res. 2020, 16, 419. [Google Scholar] [CrossRef]
- Chang, C.K.; Kao, M.C.; Lan, C.Y. Antimicrobial Activity of the Peptide Lfcinb15 against Candida albicans. J. Fungi 2021, 7, 519. [Google Scholar] [CrossRef]


| Source | Peptide | Amino Acid Sequence | Length | MW * (Da) | pI * | Net Charge | GRAVY | II * | Signal Peptide | AI * |
|---|---|---|---|---|---|---|---|---|---|---|
| Spinacia oleracea | Defensin-d2 | GIFSSRKCKTPSKTFKGICTRDSNCDTSCRYEGYPAGDCKGIRRRCMCSKPC | 52 aa | 5809.73 | 9.3 | +7.6 | −0.810 | 55.68 (unstable) | + | 24.42 |
| Actinomyces ruminicola | Actifensin | GFGCNLITSNPYQCSNHCKSVGYRGGYCKLRTVCTCY | 37 aa | 4097.70 | 8.89 | +3.8 | −0.243 | 8.22 (stable) | + | 47.30 |
| Test Organism | MIC (µg/mL) | MBC/MFC (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Actifensin | Defensin-d2 | Ampicillin | Nystatin | Vancomycin | Actifensin | Defensin-d2 | |
| MRSA | 23 | - | NA | NA | 4 | - | ND |
| E. coli | - | 30 | 5000 | NA | NA | ND | 246 |
| P. aeruginosa | 1448 | 7.5 | 10,000 | NA | NA | 1448 | 123 |
| K. pneumoniae | - | 30 | 5000 | NA | NA | ND | - |
| C. albicans | 23 | 7.5 | NA | 1290 | NA | 724 | 63 |
| Test Organism | Actifensin Alone (µg/mL) | Actifensin Combination (µg/mL) | Defensin-d2 Alone (µg/mL) | Defensin-d2 Combination (µg/mL) | FIC Index Value | Remark |
|---|---|---|---|---|---|---|
| P. aeruginosa | 1448 | 1448 | 7.5 | 63 | >4 | Antagonistic |
| C. albicans | 23 | 181 | 7.5 | 63 | >4 | Antagonistic |
| Tag | Sequences 5′–3′ | Target | Amplicon Size (bp) |
|---|---|---|---|
| Primers for colony PCR | |||
| ptxb1_F | AACTGCCAGGAATTGGGGAT | pTXB1 | 730 |
| ptxb1_R | GCTTCCAAGAAACGCACCAG | ||
| Dfn_F | AGTGTAAGACTCCTTCTAAGACTTT | defensin | 102 |
| Dfn_R | CCTTACAATCACCAGCAGGGT | ||
| Afn_F | CTTCGGCTGCAACCTCATCA | actifensin | 103 |
| Afn_R | GGTGCAGACCGTCCGAAG | ||
| Primers for cloning | |||
| Def_F | AAGAAGGAGATATACGGTATTTTTTCTTCTAGAAAGTGTAAGACTCCTTCTAAGAC | defensin | 184 |
| Def_R | TCTCCCGTGATGCAGACAAGGCTTAGAACACATACATCTTCTAATACC | ||
| Acti_F | AAGAAGGAGATATACGGCTTCGGCTGCAACCT | actifensin | 141 |
| Acti_R | ATCTCCCGTGATGCAGTAGCAGGTGCAGACCGT | ||
| Vector_F | TGCATCACGGGAGATGCACT | pTXB1 | 6654 |
| Vector_R | GTATATCTCCTTCTTAAAGTTAAACAAAATTATTTCTAGAGGGGA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbala, I.D.; Macharia, R.W.; Bargul, J.L.; Magoma, G. Membrane Permeabilization and Antimicrobial Activity of Recombinant Defensin-d2 and Actifensin against Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans. Molecules 2022, 27, 4325. https://doi.org/10.3390/molecules27144325
Gbala ID, Macharia RW, Bargul JL, Magoma G. Membrane Permeabilization and Antimicrobial Activity of Recombinant Defensin-d2 and Actifensin against Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans. Molecules. 2022; 27(14):4325. https://doi.org/10.3390/molecules27144325
Chicago/Turabian StyleGbala, Ifeoluwa D., Rosaline W. Macharia, Joel L. Bargul, and Gabriel Magoma. 2022. "Membrane Permeabilization and Antimicrobial Activity of Recombinant Defensin-d2 and Actifensin against Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans" Molecules 27, no. 14: 4325. https://doi.org/10.3390/molecules27144325
APA StyleGbala, I. D., Macharia, R. W., Bargul, J. L., & Magoma, G. (2022). Membrane Permeabilization and Antimicrobial Activity of Recombinant Defensin-d2 and Actifensin against Multidrug-Resistant Pseudomonas aeruginosa and Candida albicans. Molecules, 27(14), 4325. https://doi.org/10.3390/molecules27144325





