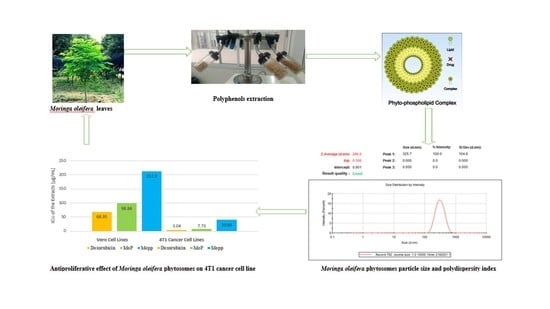
Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Total Phenolic Content by Use of Standard Curve and the Percentage Encapsulation Efficiency
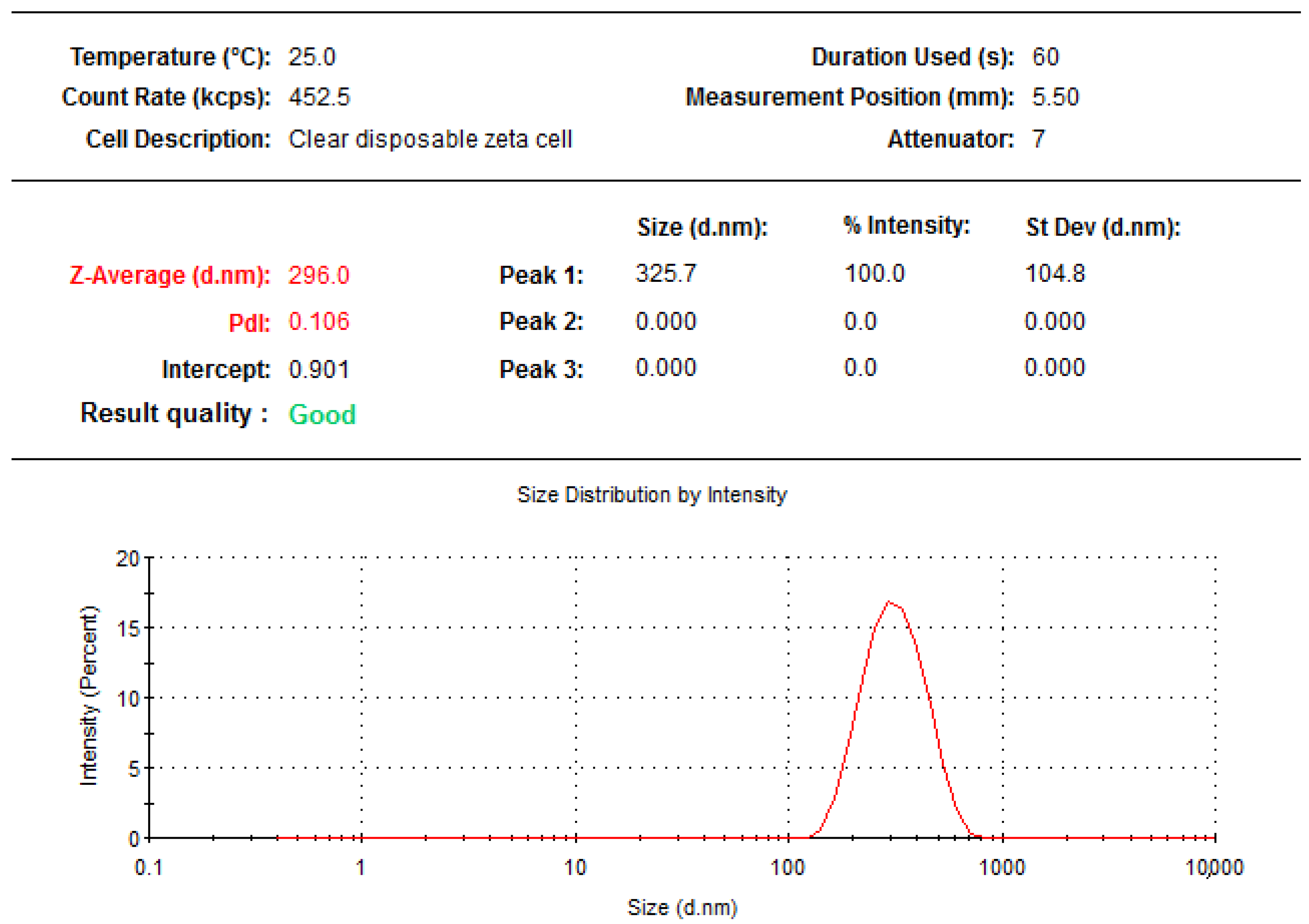
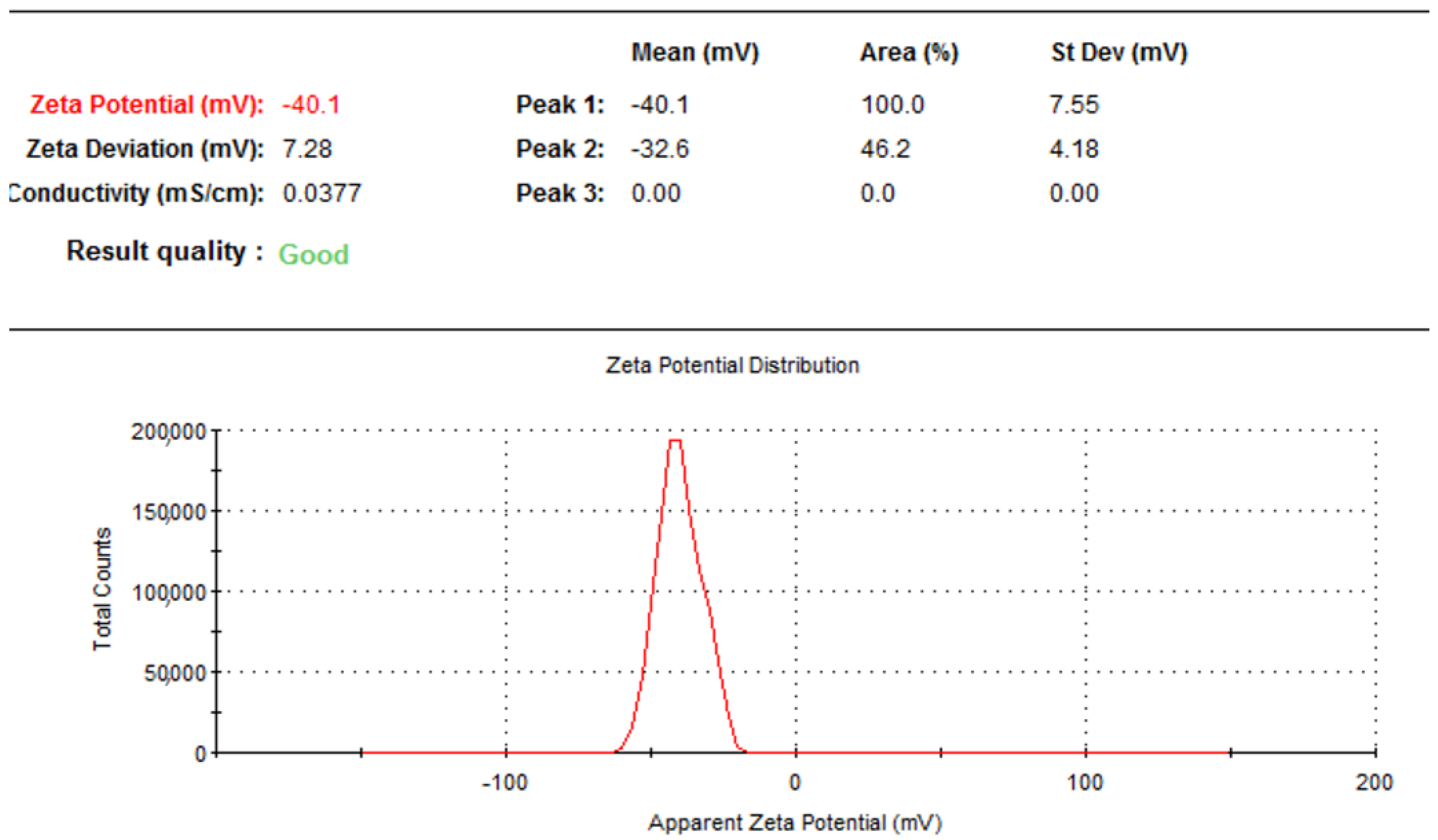
2.2. Particle Size, Polydispersity Index, and Zeta Potential of Formulated Moringa oleifera Phytosomes
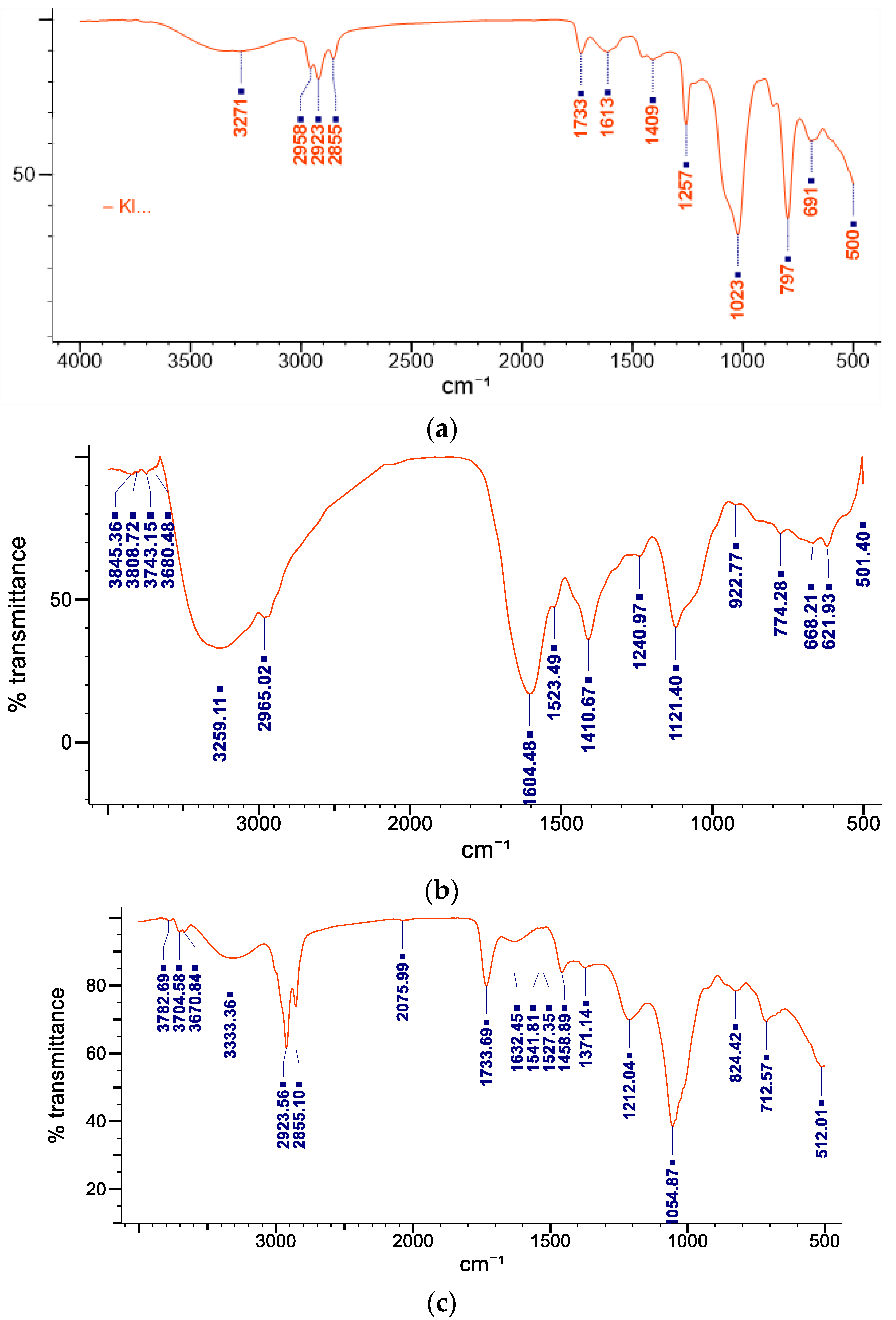
2.3. Fourier Transform Infrared Spectroscopy
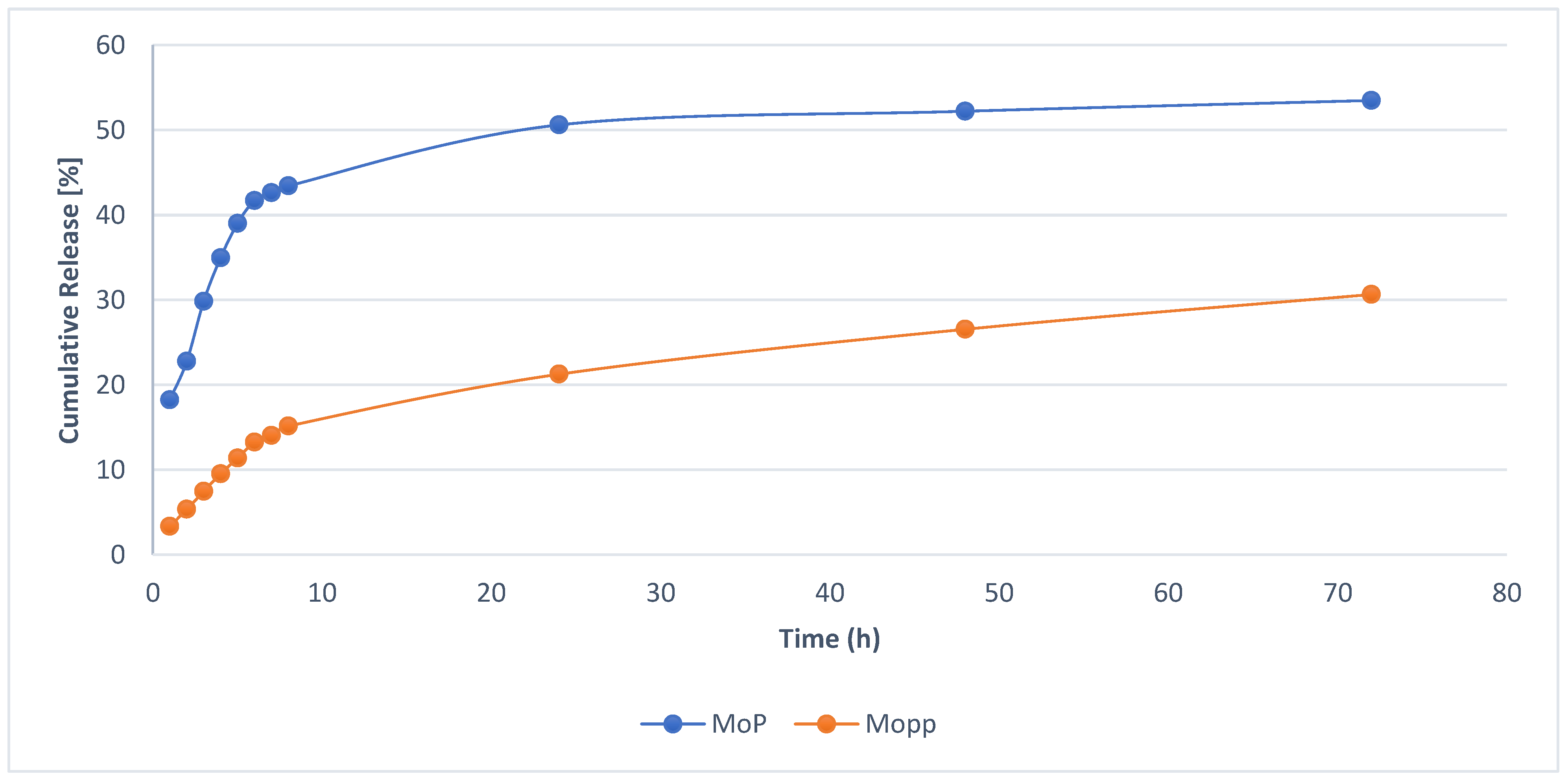
2.4. In Vitro Drug Release
2.5. In Vitro Bioaccessibility
2.6. Physical Storage Stability Test
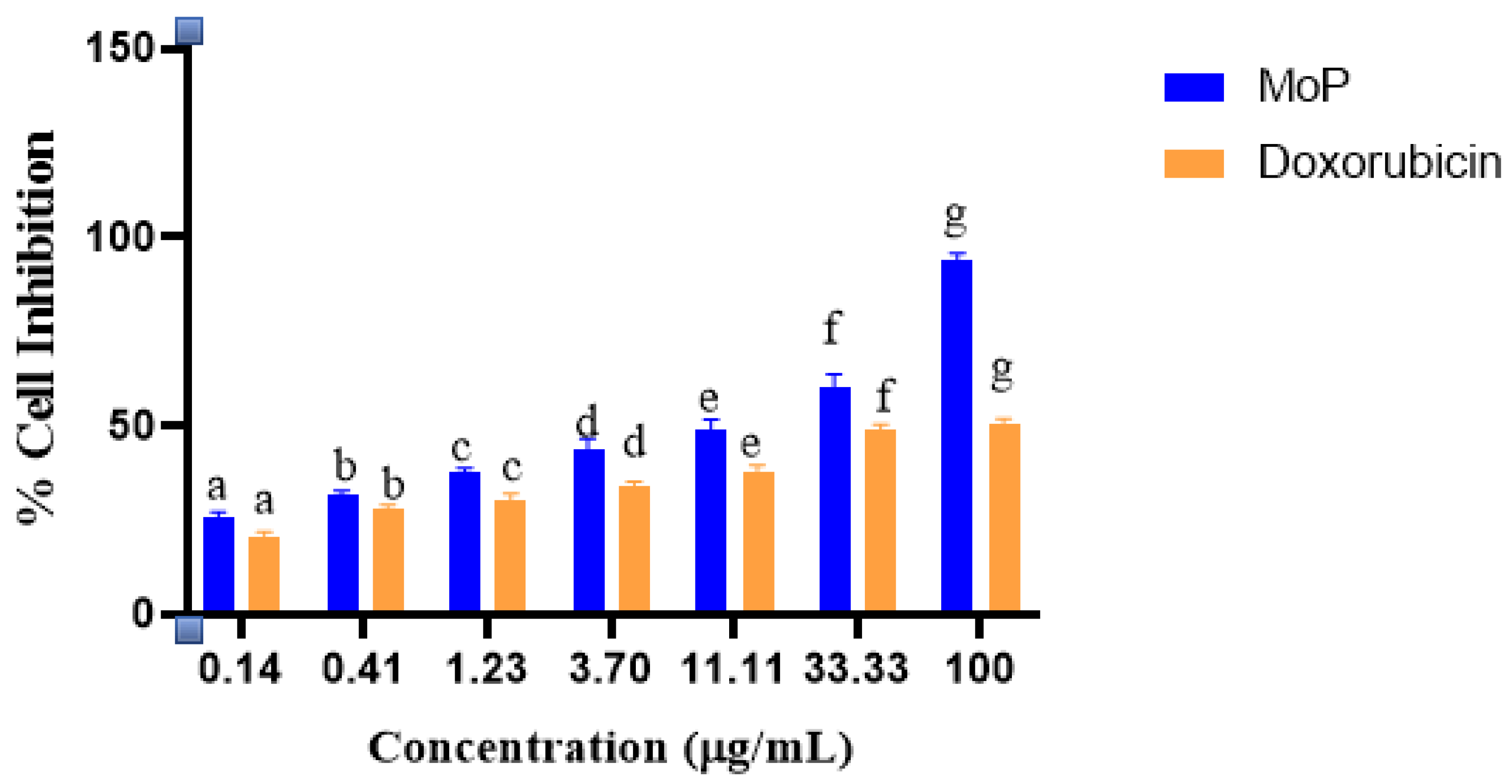
2.7. Phytosomes, Polyphenols, and Doxorubicin Effects on Vero E6 (Normal) Cell Lines
2.8. In Vivo Toxicity Studies
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Ethical Considerations
3.3. Sample Collection and Preparation
3.4. Microwave-Assisted Extraction
3.5. Estimation of Total Phenolic Content
3.6. Phytosome Synthesis
3.7. Standard Curve and Percentage Entrapment Efficiency
3.8. Particle Size Distribution, Zeta Potential, and Polydispersity Index
3.9. Fourier Transform Infrared Spectroscopy
3.10. In Vitro Drug Release of Polyphenol from Moringa oleifera Phytosomes
3.11. In Vitro Bioaccessibility Determination of MoP and Mopp
3.11.1. Simulated Salivary Fluid in Mouth Phase
3.11.2. Simulated Gastric Fluid (SGF)
3.11.3. Small Intestinal Phase
3.11.4. Measurement of Bioaccessibility
3.12. In Vitro Storage Stability Tests
3.13. Cell Viability Test
3.14. In Vivo Acute Toxicity Study Using Swiss albino Mice
3.15. Data Management and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zhang, S.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Breast cancer burden in Africa: Evidence from GLOBOCAN 2018. J. Public Health 2021, 43, 763–771. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.; McKenzie, F.; Foerster, M.; Zietsman, A.; Galukande, M.; Adisa, C.; Anele, A.; Parham, G.; Pinder, L.F.; Cubasch, H.; et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): A prospective cohort study. Lancet Glob. Health 2020, 8, 1203–1212. [Google Scholar] [CrossRef]
- Sood, R.; Masalu, N.; Connolly, R.M.; Chao, C.A.; Faustine, L.; Mbulwa, C.; Anderson, B.O.; Rositch, A.F. Invasive breast cancer treatment in Tanzania: Landscape assessment to prepare for implementation of standardized treatment guidelines. BMC Cancer 2021, 21, 527. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Lee, M.Y.; Jang, M.K. The experience of cancer-related fatigue, exercise and exercise adherence among women breast cancer survivors: Insights from focus group interviews. J. Clin. Nurs. 2020, 29, 758–769. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.G.; Nwokocha, C.R.; Anderson, M.S.; Wilson-Clarke, C.; Vaz, K.; Anderson-Jackson, L.; Brown, J. Medicinal herbs used in traditional management of breast cancer: Mechanisms of action. Medicines 2020, 7, 47. [Google Scholar] [CrossRef]
- Gaonkar, V.P.; Hullatti, K. Indian traditional medicinal plants as a source of potent Anti-diabetic agents: A review. J. Diabetes Metab. Disord. 2020, 19, 1895–1908. [Google Scholar] [CrossRef]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Wanjiru, J.N.; Anino, E.; Njuguna, D.K.; Mwangangi, R.; Jepkorir, M.; Mbugua, R.W.; Mwitari, P. Phytochemical screening and synergistic anti-proliferative activity against selected cancer cell lines of Moringa oleifera and Indigofera arrecta Leaf. Eur. J. Med. Plants 2018, 23, 1–11. [Google Scholar]
- Gaffar, S.; Apriani, R.; Herlina, T.; Gaffar, S.; Apriani, R.; Herlina, T. n-Hexane fraction of Moringa oleifera Lam. leaves induces apoptosis and cell cycle arrest on T47D breast cancer cell line. J. Pharm. Pharmacogn. Res. 2019, 7, 173–183. [Google Scholar]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.C.; Usuwanthim, K. In vitro bioassay-guided identification of anticancer properties from Moringa oleifera Lam. leaf against the MDA-MB-231 cell line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.Z.; Kausar, F.; Hassan, M.; Javaid, S.; Malik, A. Anticancer activities of phenolic compounds from Moringa oleifera leaves: In vitro and in silico mechanistic study. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 12. [Google Scholar] [CrossRef]
- Dobrzynska, M.; Napierala, M.; Florek, E. Flavonoid nanoparticles: A promising approach for cancer therapy. Biomolecules 2020, 10, 1268. [Google Scholar] [CrossRef]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharmacother. 2018, 108, 457–466. [Google Scholar] [CrossRef]
- Velidandi, A.; Dahariya, S.; Pabbathi, N.P.P.; Kalivarathan, D.; Baadhe, R.R. A review on synthesis, applications, toxicity, risk assessment and limitations of plant extracts synthesized silver nanoparticles. NanoWorld J. 2020, 6, 35–60. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 26, 3702518. [Google Scholar] [CrossRef]
- Chi, C.; Zhang, C.; Liu, Y.; Nie, H.; Zhou, J.; Ding, Y. Phytosome-nanosuspensions for silybin-phospholipid complex with increased bioavailability and hepatoprotection efficacy. Eur. J. Pharm. Sci. 2020, 144, 105212. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Nematollahi, M.H. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literature. Int. J. Nanomed. 2021, 16, 6983. [Google Scholar] [CrossRef]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, C.; Zhao, Y.; Chang, Y.; Guo, L. Comparison of bioactive phenolic compounds and antioxidant activities of different parts of Taraxacum mongolicum. Molecules 2020, 25, 3260. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Dave, V.; Paliwal, S.; Sharma, M.; Potdar, M.B.; Tyagi, A. Phytosomes—Nanoarchitectures’ promising clinical applications and therapeutics. Nanopharmaceutical Adv. Deliv. Syst. 2021, 187–216. [Google Scholar] [CrossRef]
- Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Cinci, L.; Landucci, E.; Bilia, A.R.; Bergonzi, M.C. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules 2019, 24, 1688. [Google Scholar] [CrossRef] [Green Version]
- Parashar, P.; Rana, P.; Dwivedi, M.; Saraf, S.A. Dextrose modified bilosomes for peroral delivery: Improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome Res. 2018, 29, 251–263. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: Recent developments and challenges. Drug Deliv. Transl. Res. 2013, 5, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the biopharmaceutics classification system. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef] [Green Version]
- USP. UP. 34-NF 29. In The United State Pharmacopeia and the National Formulary; The United States Pharmacopeial Convection: Rockville, MD, USA, 2011; p. 20852. [Google Scholar]
- Bhardwaj, U.; Burgess, D.J. Physicochemical properties of extruded and non-extruded liposomes containing the hydrophobic drug dexamethasone. Int. J. Pharm. 2010, 388, 181–189. [Google Scholar] [CrossRef]
- Xu, X.; Khan, M.A.; Burgess, D.J. A two-stage reverse dialysis in vitro dissolution testing method for passive targeted liposomes. Int. J. Pharm. 2012, 426, 211–218. [Google Scholar] [CrossRef]
- Ruiz-Henestrosa, V.M.P.; Ribourg, L.; Kermarrec, A.; Anton, M.; Pilosof, A.; Viau, M.; Meynier, A. Emulsifiers modulate the extent of gastric lipolysis during the dynamic in vitro digestion of submicron chia oil/water emulsions with limited impact on the final extent of intestinal lipolysis. Food Hydrocoll. 2022, 124, 107336. [Google Scholar] [CrossRef]
- Guan, W.; Ma, Y.; Ding, S.; Liu, Y.; Song, Z.; Liu, X.; Tang, L.; Wang, Y. The technology for improving stability of nanosuspensions in drug delivery. J. Nanoparticle Res. 2022, 24, 14. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, X.; Yu, F.; Quan, Y.; Cheng, Y.; Yuan, H. DOX loaded aggregation-induced emission active polymeric nanoparticles as a fluorescence resonance energy transfer traceable drug delivery system for self-indicating cancer therapy. Acta Biomater. 2019, 85, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Kaul, V.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Pandey, M.M.; Singhvi, G. UV Spectrophotometric method for characterization of curcumin loaded nanostructured lipid nanocarriers in simulated conditions: Method development, in-vitro and ex-vivo applications in topical delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 17392. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy, 1st ed.; Oregon Medical Press: Princeton, MN, USA, 2001; p. 851. [Google Scholar]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green tea polyphenol EGCG attenuates MDSCs-mediated immunosuppression through canonical and non-canonical pathways in a 4T1 murine breast cancer model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, P.; Hou, X.; Yan, F.; Jiang, Z.; Shi, J.; Feng, N. Hybrid curcumin–phospholipid complex-near-infrared dye oral drug delivery system to inhibit lung metastasis of breast cancer. Int. J. Nanomed. 2019, 14, 3311. [Google Scholar] [CrossRef] [Green Version]
- Komath, S.; Garg, A.; Wahajuddin, M. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles-storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Du, F.; He, M.; Bai, L.; Gu, Y.Y.; Yang, L.L.; Liu, Y.J. Studies of anticancer activity in vitro and in vivo of iridium (III) polypyridyl complexes-loaded liposomes as drug delivery system. Eur. J. Med. Chem. 2019, 178, 390–400. [Google Scholar] [CrossRef]
- del Pilar Sánchez-Camargo, A.; Ballesteros-Vivas, D.; Buelvas-Puello, L.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Cifuentes, A.; Ferreira, S.R.; Gutiérrez, L.F. Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. LWT 2021, 137, 110414. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, J.J.; Liu, C.Y.; Bai, W.S.; Cheng, N. A modified Folin-Ciocalteu method for the microdetermination of total phenolic content in honey. Int. Food Res. J. 2020, 27, 576–584. [Google Scholar]
- Wijiani, N.; Isadiartuti, D.; Rijal, M.A.S.; Yusuf, H. Characterization and dissolution study of micellar curcumin-spray dried powder for oral delivery. Int. J. Nanomed. 2020, 15, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Far, A.H.; Godugu, K.; Salaheldin, T.A.; Darwish, N.H.; Saddiq, A.A.; Mousa, S.A. Nanonutraceuticals: Anticancer activity and improved safety of chemotherapy by costunolide and its nanoformulation against colon and breast cancer. Biomedicines 2021, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.R.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Biol. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, L.; Su, L.; Wu, X.; Wang, Y.; Liu, L.; Lin, X. Design of a zero-order sustained release PLGA microspheres for palonosetron hydrochloride with high encapsulation efficiency. Int. J. Pharm. 2020, 575, 119006. [Google Scholar] [CrossRef]
- Thiruvengadam, V.; Bansod, A.V. Characterization of silver nanoparticles synthesized using chemical method and its antibacterial property. Biointerface Res. Appl. Chem. 2020, 10, 7257–7264. [Google Scholar]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting drug release kinetics from nanocarriers inside dialysis bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa E.R. tablets. Dev. Drugs J. 2017, 6, 1–8. [Google Scholar]
- Lang, Y.; Li, B.; Gong, E.; Shu, C.; Si, X.; Gao, N.; Meng, X. Effects of α-casein and β-casein on the stability, antioxidant activity and bioaccessibility of blueberry anthocyanins with an in vitro simulated digestion. Food Chem. 2021, 334, 127526. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Fahmy, U.A.; Badr-Eldin, S.M.; Ahmed, O.A.; Asfour, H.Z.; Aldawsari, H.M.; Mohamed, A.I. Optimized icariin phytosomes exhibit enhanced cytotoxicity and apoptosis-inducing activities in ovarian cancer cells. J. Pharm. 2012, 12, 346. [Google Scholar] [CrossRef]
- Laure, O.D.M.; Epoh, N.J.; Tadjoua, H.T.; Yousuf, S.; Telefo, P.B.; Tapondjou, L.A.; Choudhary, M.I. Acute and sub-acute toxicity of the aqueous extract from the stem bark of Tetrapleura tetrapteura Taub. (Fabaceae) in mice and rats. J. Ethnopharmacol. 2019, 236, 42–49. [Google Scholar]

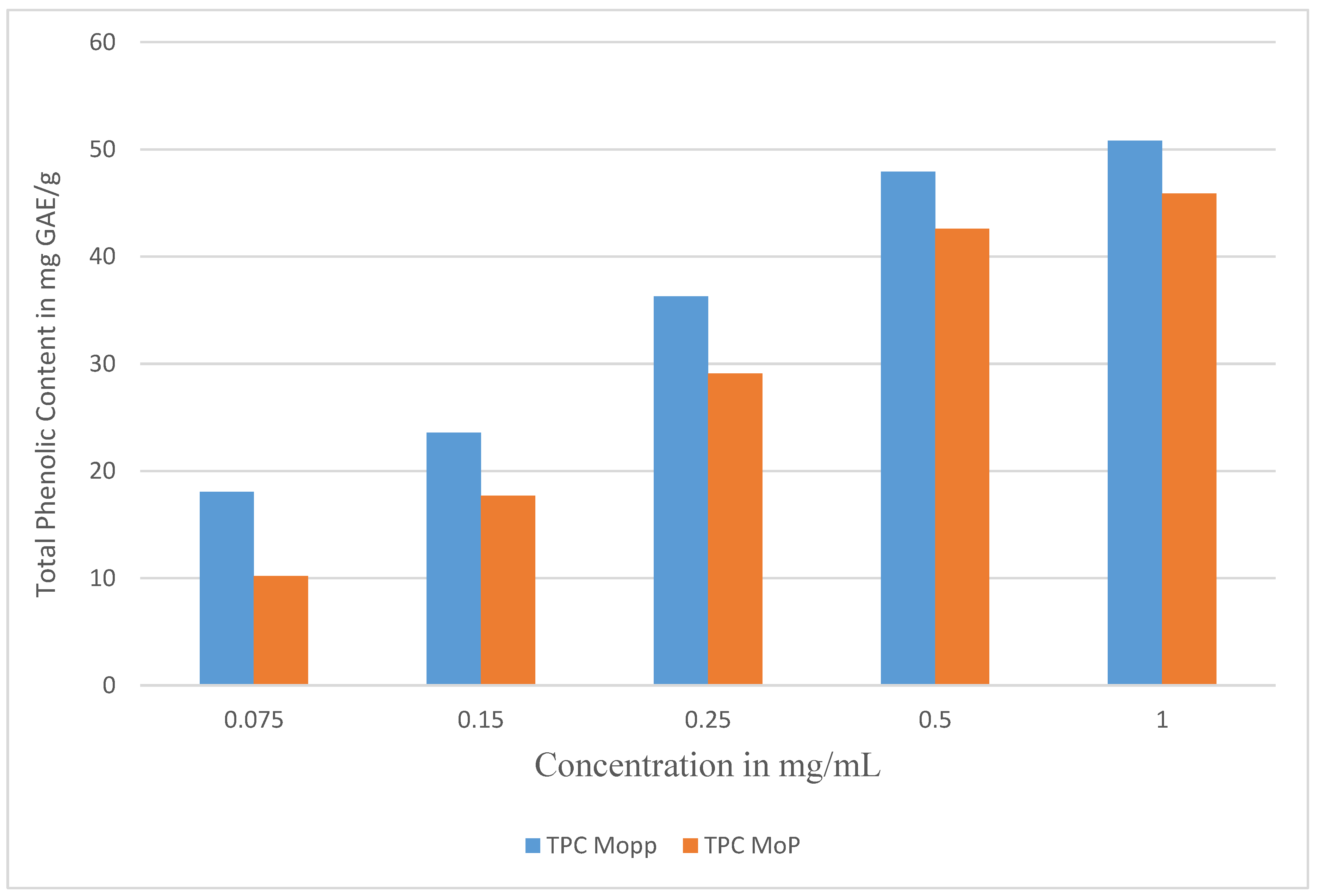
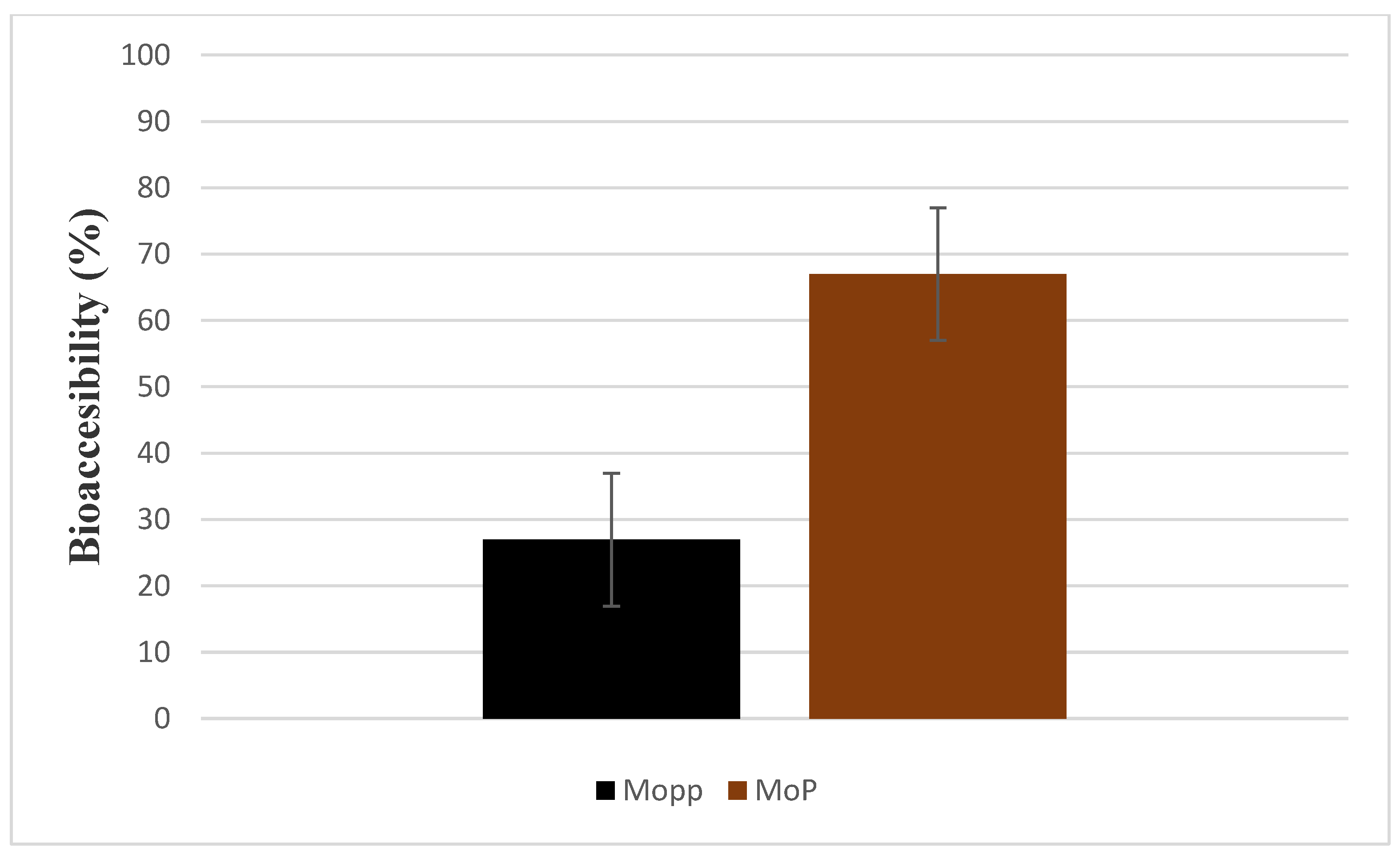

| Concentration mg/mL | TPC Polyphenols in GAE/g | TPC Phytosome GAE/g | % Encapsulation Efficiency |
|---|---|---|---|
| 1 | 50.81 ± 0.02 | 45.89 ± 0.27 | 90.32 ± 0.11 |
| 0.5 | 47.93 ± 0.13 | 42.60 ± 0.33 | 88.88 ± 0.41 |
| 0.25 | 36.29 ± 0.05 | 29.11 ± 1.13 | 80.21 ± 2.26 |
| 0.15 | 23.59 ± 0.17 | 17.71 ± 0.71 | 75.07 ± 2.5 |
| 0.075 | 18.07 ± 1.12 | 10.19 ± 0.13 | 56.39 ± 1.35 |
| S/No. | Frequency Range (cm−1) | Functional Group Identified |
|---|---|---|
| 1 | 3271 | hydroxyl compound |
| 2 | 2958, 2923, and 2855 | CH and CH2 stretching aliphatic group |
| 3 | 1733 | carbonyl group |
| 4 | 1613 | C=C unsaturated compounds |
| 5 | 1409 | stretching -C=O inorganic carbonate |
| 6 | 1257 | C-N amide 111 band |
| 7 | 1023 | C-O-C group |
| 8 | 797 | C-H |
| 9 | 691 | C-S linkage |
| Time | Square Root of Time | Log | Cumulative Percentage Drug Release ± SD | Log Cumulative Percentage Drug Release | Cumulative Percent Drug Remaining | Log cumulative Percent Drug Remaining |
|---|---|---|---|---|---|---|
| h | Time | |||||
| 1 | 1 | 0 | 18.23 ± 0.01 | 1.26 ± 0.01 | 81.77 ± 0.41 | 1.91 ± 0.72 |
| 2 | 1.41 | 0.3 | 22.77 ± 0.03 | 1.36 ± 0.06 | 77.23 ± 0.91 | 1.89 ± 0.11 |
| 3 | 1.73 | 0.48 | 29.83 ± 0.07 | 1.47 ± 0.04 | 70.17 ± 2.36 | 1.85 ± 0.51 |
| 4 | 2 | 0.6 | 32.96 ± 0.18 | 1.52 ± 0.02 | 67.04 ± 1.28 | 1.83 ± 0.02 |
| 5 | 2.24 | 0.7 | 40.01 ± 0.15 | 1.6 ± 0.13 | 59.99 ± 1.37 | 1.78 ± 0.31 |
| 6 | 2.45 | 0.78 | 41.67 ± 0.90 | 1.62 ± 0.20 | 58.31 ± 2.21 | 1.77 ± 0.95 |
| 7 | 2.65 | 0.85 | 42.63 ± 1.07 | 1.63 ± 0.73 | 57.37 ± 1.02 | 1.76 ± 0.91 |
| 8 | 2.83 | 0.9 | 43.43 ± 0.47 | 1.64 ± 1.07 | 56.57 ± 1.50 | 1.75 ± 0.07 |
| 24 | 4.9 | 1.38 | 50.6 ± 1.25 | 1.7 ± 0.60 | 49.4 ± 1.23 | 1.69 ± 0.09 |
| 48 | 6.93 | 1.68 | 52.21 ± 0.64 | 1.72 ± 0.29 | 47.79 ± 0.91 | 1.68 ± 0.32 |
| 72 | 8.49 | 1.86 | 53.49 ± 1.02 | 1.73 ± 0.11 | 46.51 ± 0.26 | 1.67 ± 0.02 |
| Formulations | Zero-Order (R2) | First-Order (R2) | Higuchi (R2) | Korsmeyer–Peppas (R2) |
|---|---|---|---|---|
| Phytosome | 0.5203 | 0.59 | 0.8877 | 0.9306 |
| Number in Days | Average Particle Size in nm | Zeta Potential in mV | Polydispersity Index |
|---|---|---|---|
| 1 | 220.3 ± 0.12 | −38.3 ± 1.14 | 0.11 ± 0.02 |
| 5 | 227.9 ± 1.11 | −40.9 ± 3.56 | 0.13 ± 0.11 |
| 10 | 229.6 ± 0.20 | −41.1 ± 2.48 | 0.14 ± 0.04 |
| 15 | 231.7 ± 1.34 | 41.4 ± 1.52 | 0.17 ± 0.16 |
| 20 | 236.5 ± 2.53 | −42.7 ± 31 | 0.19 ± 0.03 |
| 25 | 239.6 ± 2.46 | −42.8 ± 2.53 | 0.19 ± 0.07 |
| Concentration of Mopp and MoP | Weight (g) Day 1 | Weight (g) Day 7 | Weight (g) Day 14 |
|---|---|---|---|
| 50 mg/kg Polyphenols | 21.60 ± 1.21 | 25.50 ± 0.32 | 27.0 ± 2.71 |
| 300 mg/kg Polyphenol Group 1 | 24.12 ± 0.10 | 28.15 ± 0.37 | 29.20 ± 1.33 |
| 300 mg/kg Polyphenol Group 2 | 23.65 ± 0.22 | 26.60 ±0.49 | 27.61 ± 1.42 |
| 2000 mg/kg Polyphenols | 23.39 ± 0.28 | 26.1 ± 1.68 | 27.67 ± 2.79 |
| 50 mg/kg Phytosome Complex | 24.67 ± 1.36 | 28.20 ± 1.32 | 28.50 ± 2.45 |
| 300 mg/kg Phytosome Complex | 25.0 ± 1.31 | 27.67 ± 1.53 | 28.28 ± 0.27 |
| 300 mg/kg Phytosome Complex | 22.0 ± 0.62 | 25.14 ± 1.02 | 25.67 ± 1.38 |
| 2000 mg/kg Phytosome Complex | 26.65 ± 2.29 | 26.51 ± 1.92 | 27.06 ± 0.03 |
| Control | 23.00 ± 3.02 | 25.15 ± 2.58 | 27.11 ± 1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430. https://doi.org/10.3390/molecules27144430
Wanjiru J, Gathirwa J, Sauli E, Swai HS. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules. 2022; 27(14):4430. https://doi.org/10.3390/molecules27144430
Chicago/Turabian StyleWanjiru, Jecinta, Jeremiah Gathirwa, Elingarami Sauli, and Hulda Shaid Swai. 2022. "Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines" Molecules 27, no. 14: 4430. https://doi.org/10.3390/molecules27144430
APA StyleWanjiru, J., Gathirwa, J., Sauli, E., & Swai, H. S. (2022). Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules, 27(14), 4430. https://doi.org/10.3390/molecules27144430