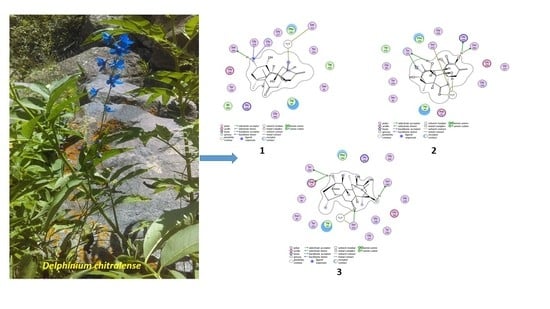
In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense
Abstract
:1. Introduction
2. Results and Discussion
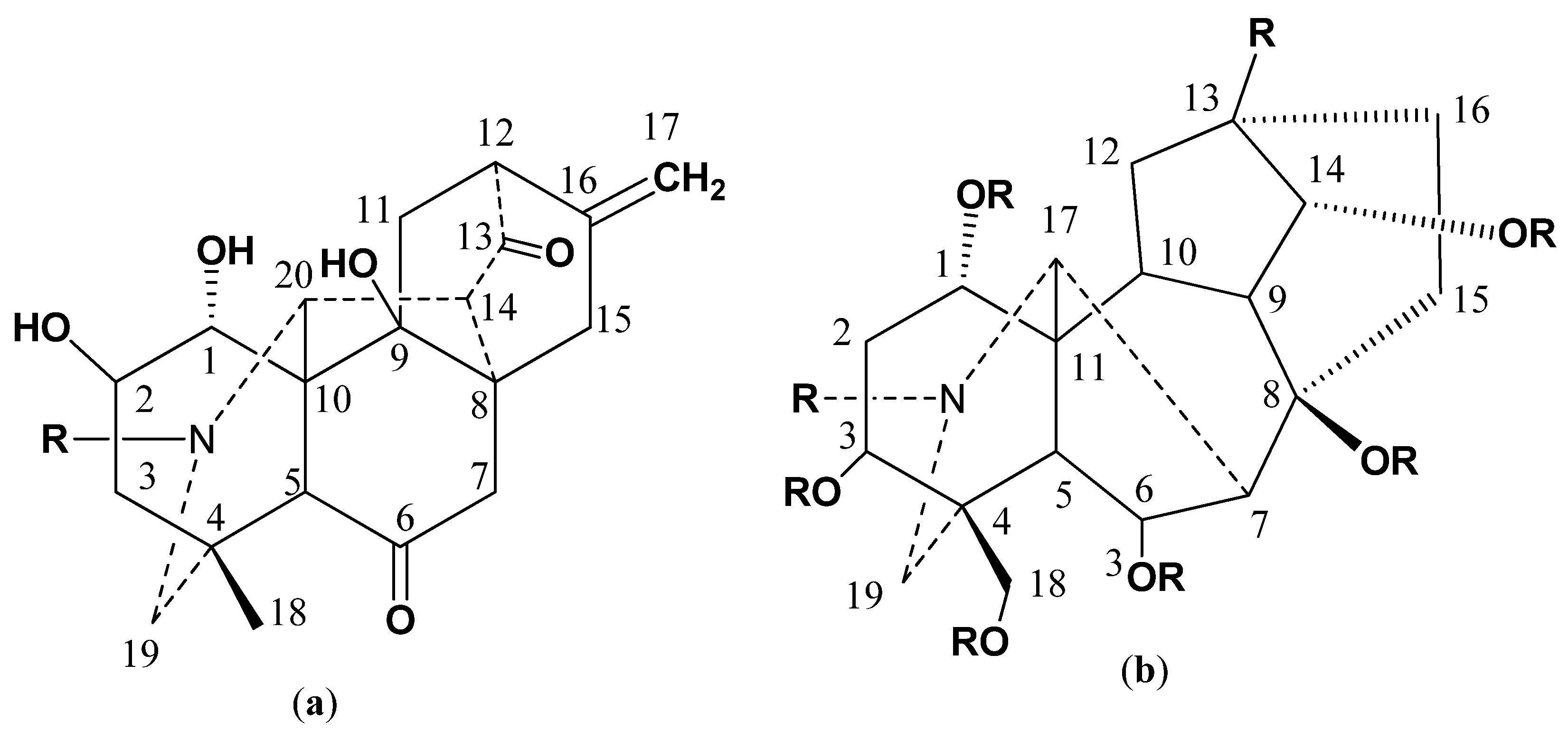
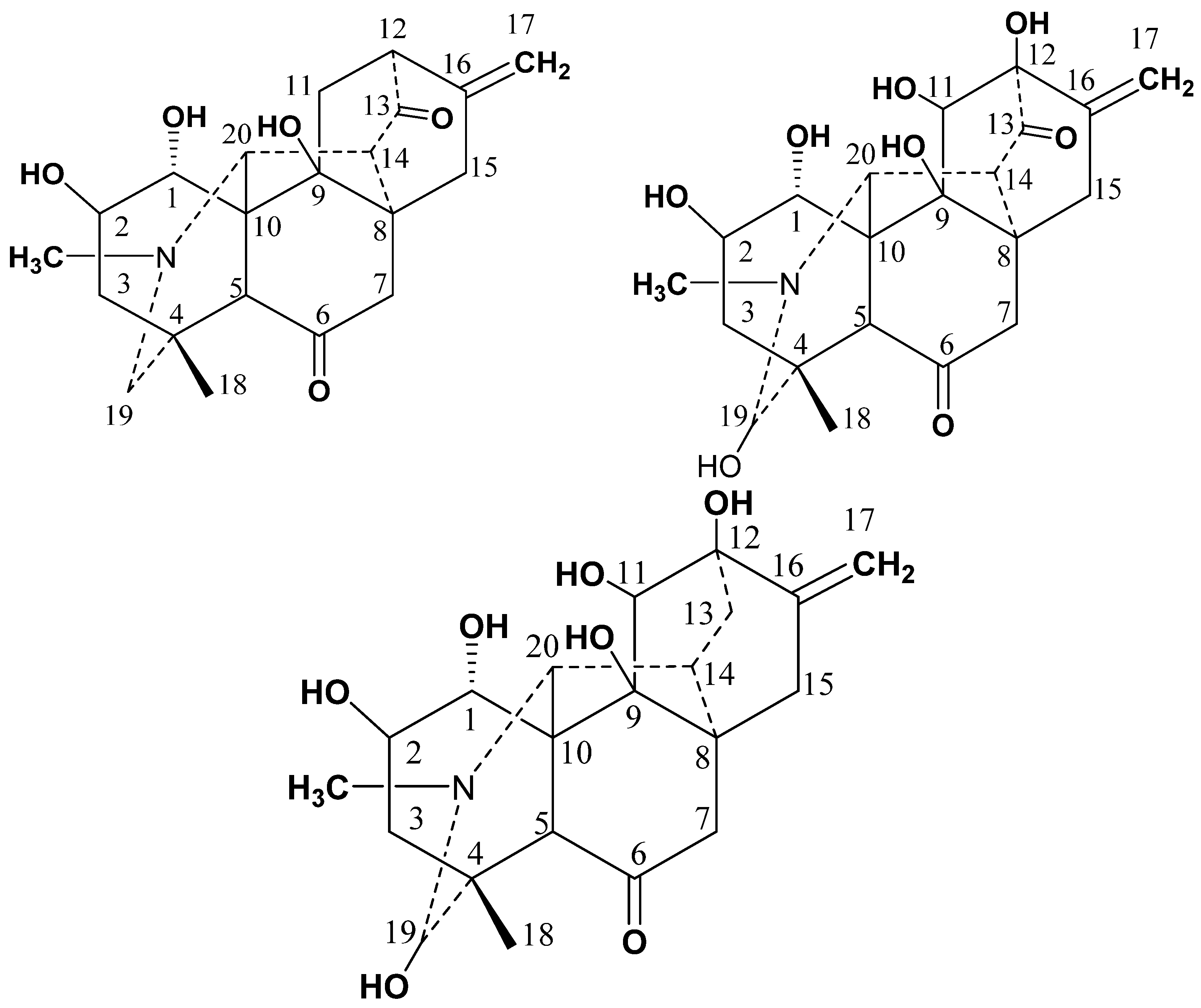
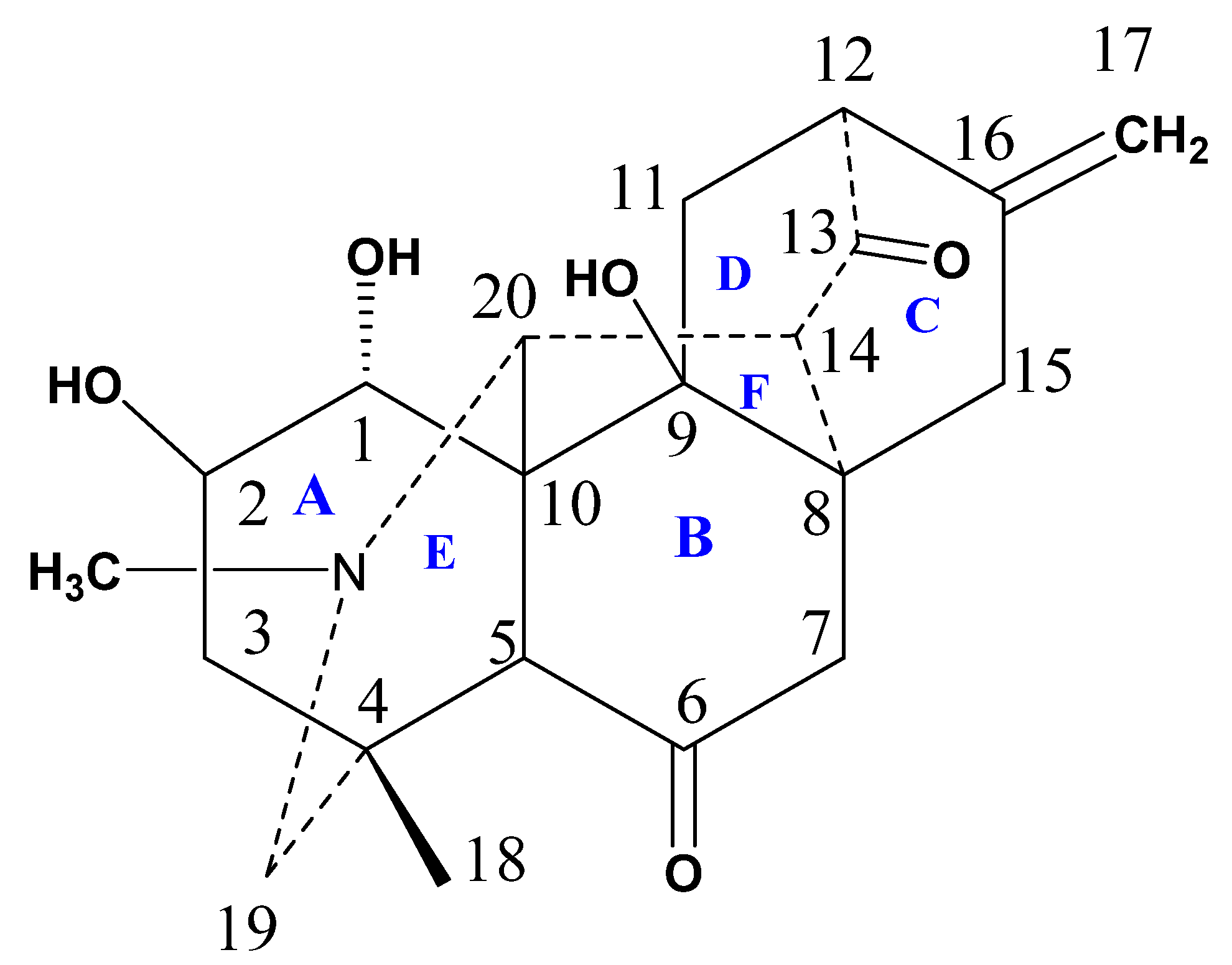
2.1. Structure Elucidation and Identification
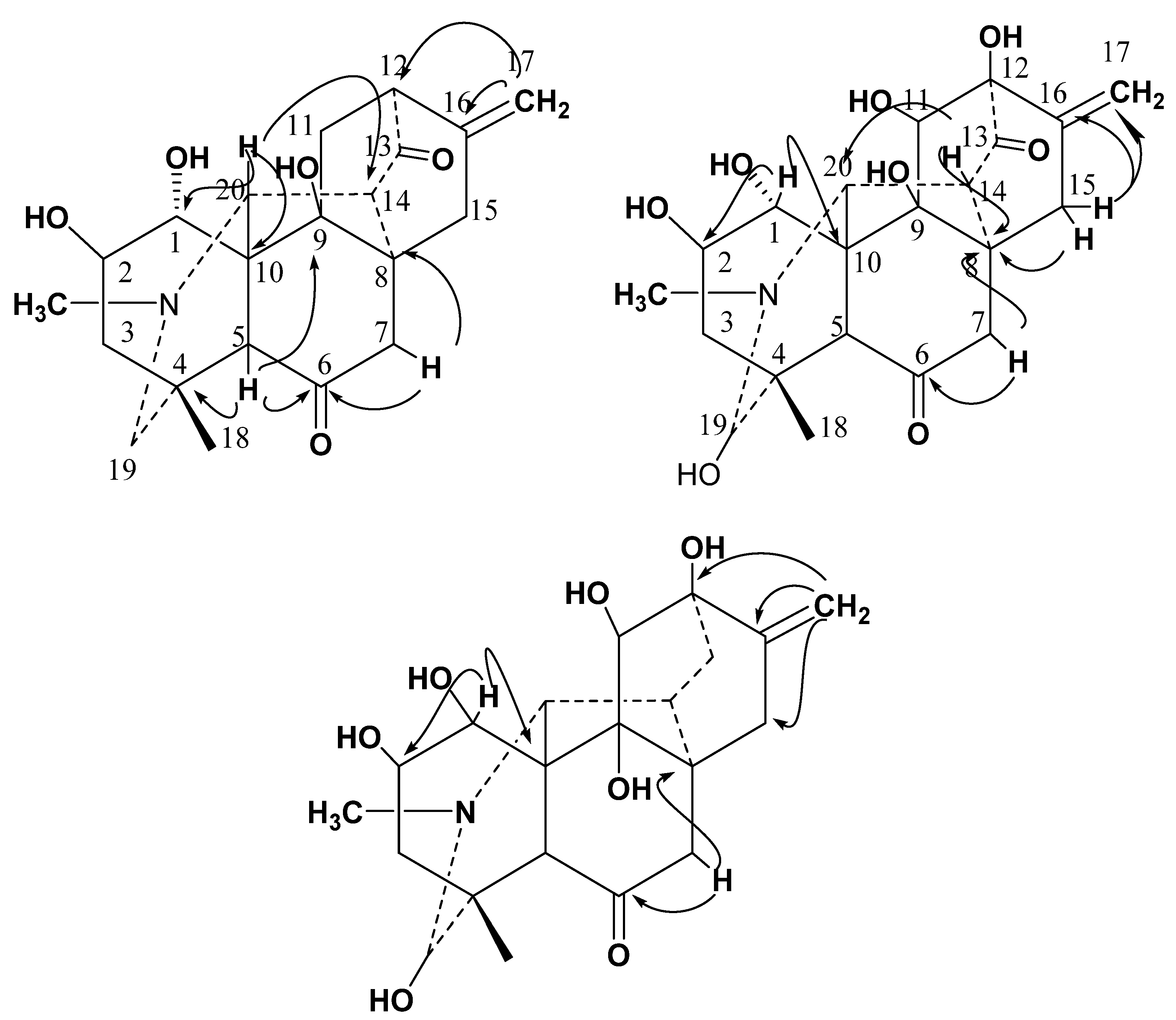
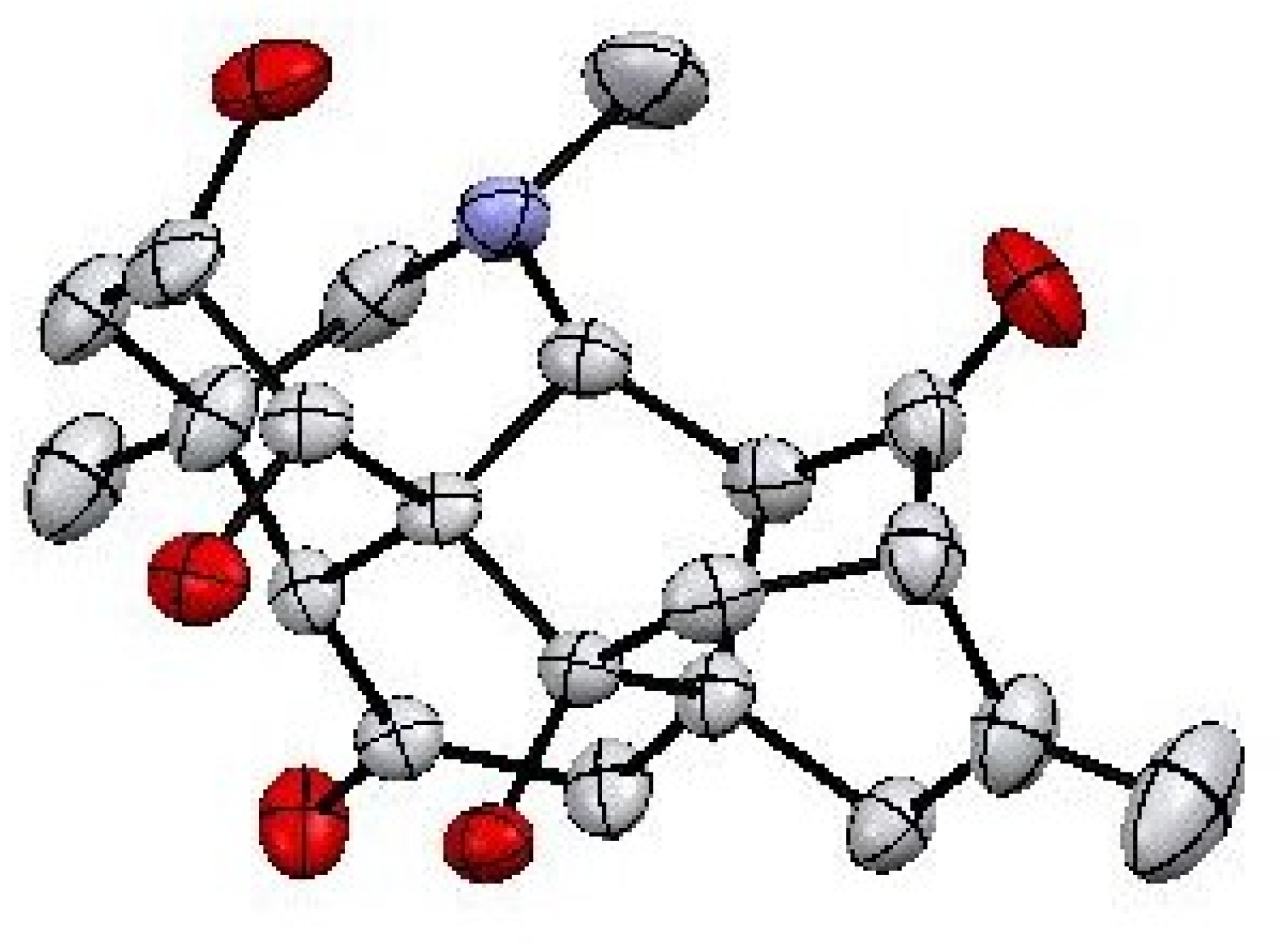
2.1.1. Crystal Structure Determination
2.1.2. DFT Calculation of Compound 1
2.2. Acetylcholinesterase and Butyrylcholinesterase Inhibition Activities
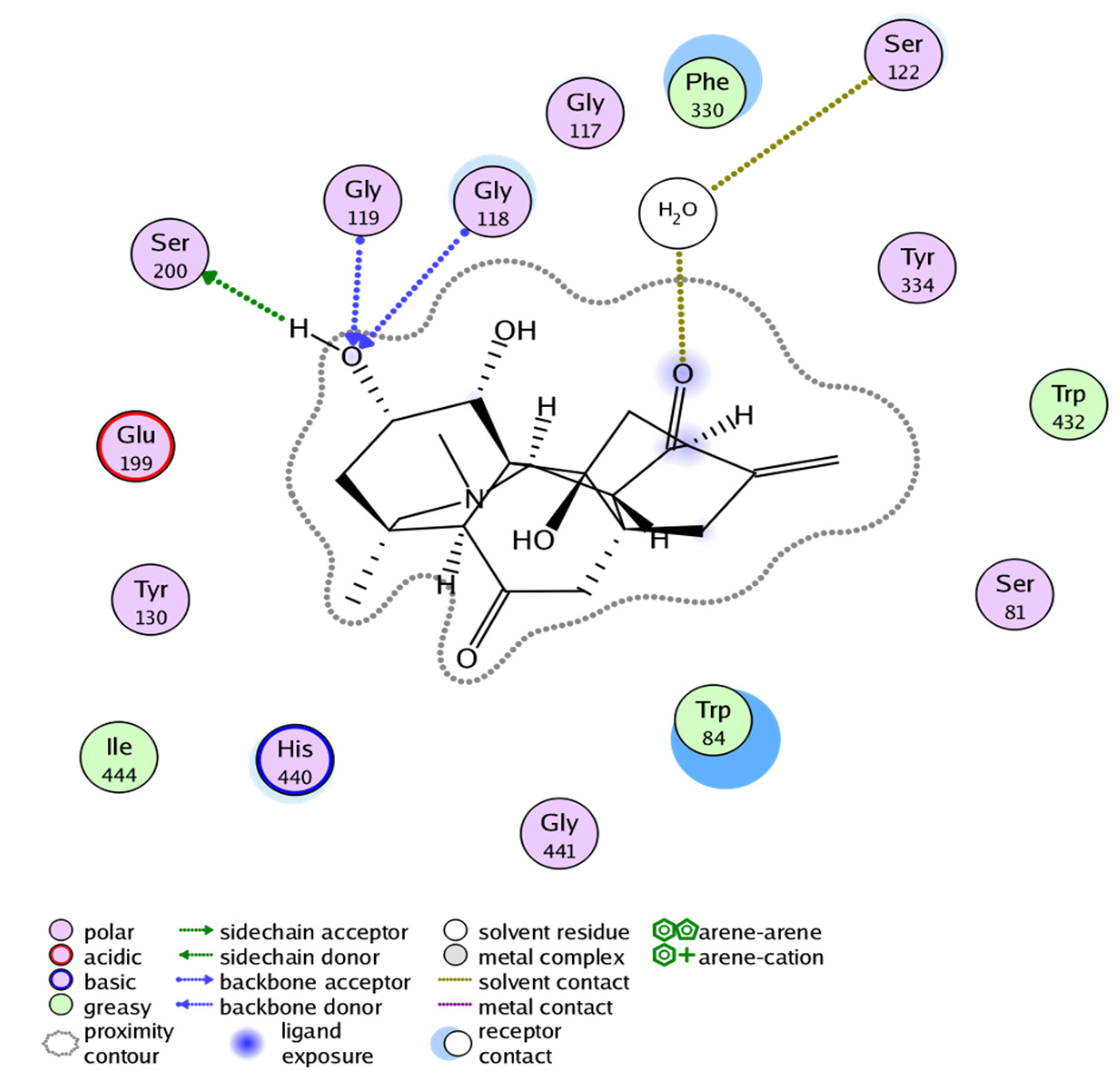
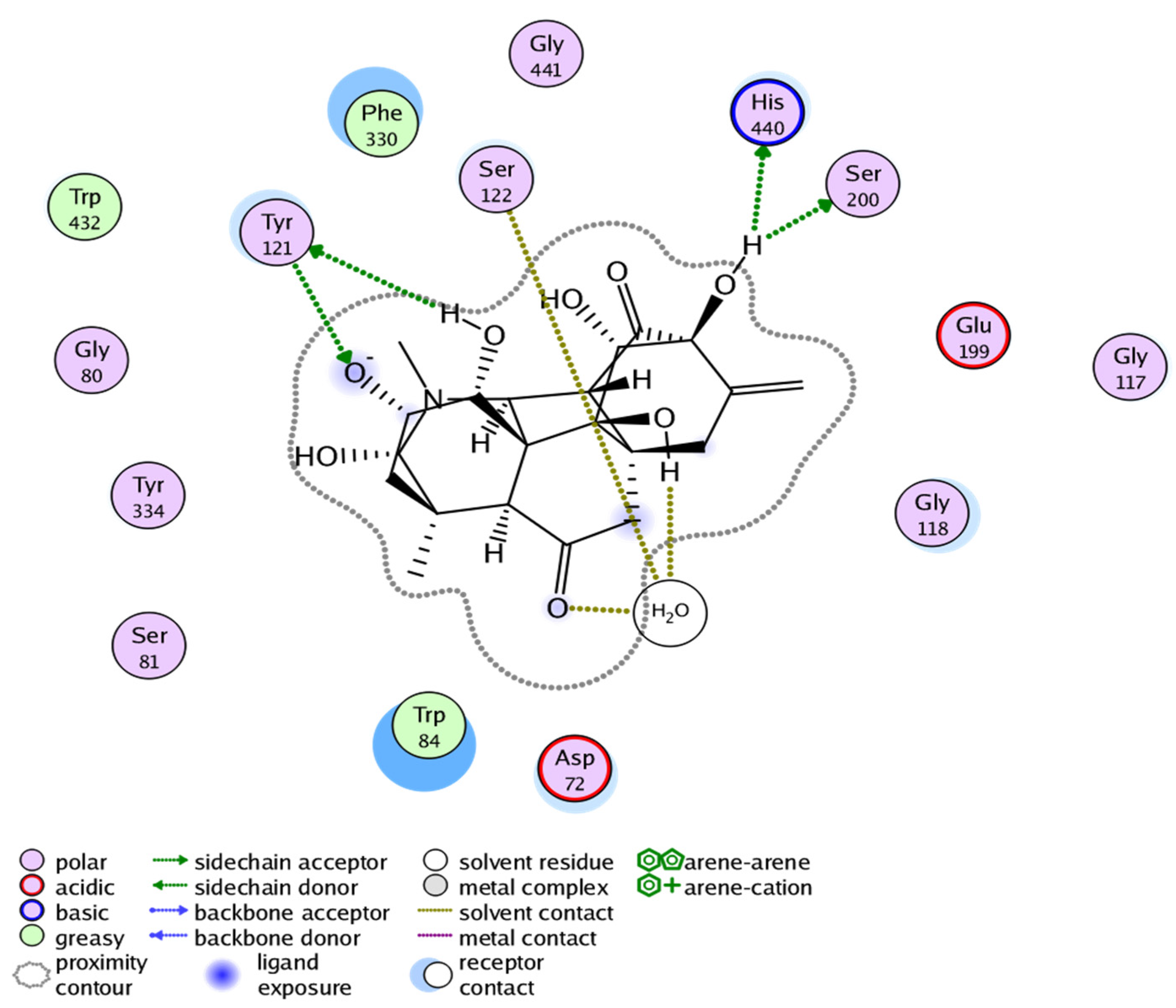
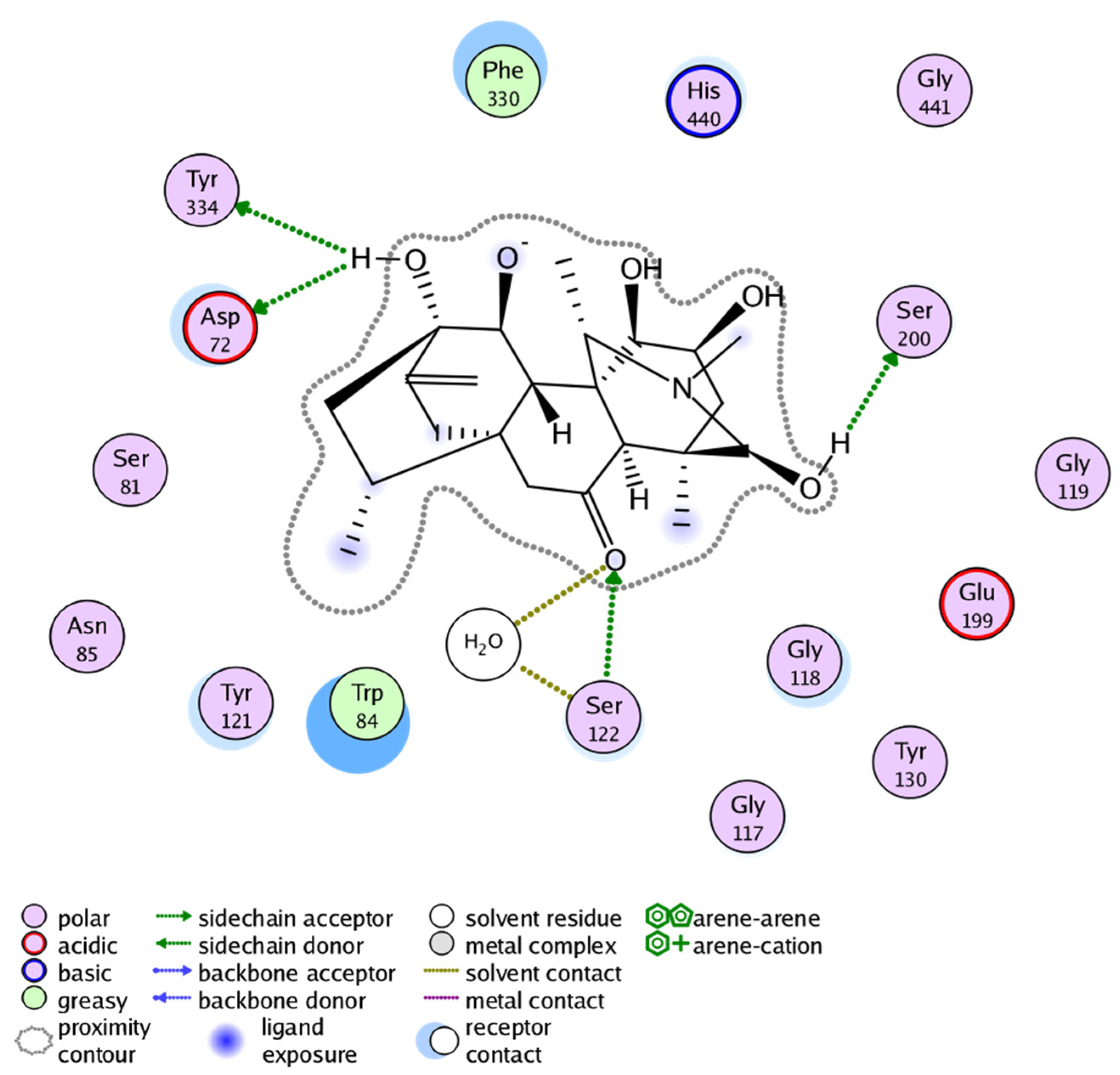
2.3. Molecular Docking Study
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Physical and Spectroscopic Data of New Compounds
3.4.1. Chitralinine-A (1)
3.4.2. Chitralinine-B (2)
3.4.3. Chitralinine-C (3)
3.5. X-ray Crystallography
3.6. Density Functional Theory (DFT) Calculations
3.7. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibition Assays
IC50 Values Determination
3.8. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shaheen, F.; Ahmad, M.; Rizvi, T.S.; Ali, L. Norditerpenoid alkaloids from Delphinium kohatense Munz. Rec. Nat. Prod. 2015, 9, 76–80. [Google Scholar]
- Qasem, A.M.A.; Zeng, Z.; Rowan, M.G.; Blagbrough, I.S. Norditerpenoid alkaloids from Aconitum and Delphinium: Structural relevance in medicine, toxicology, and metabolism. Nat. Prod. Rep. 2022, 39, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Houghton, P.J. Screening of Indian medicinal plants for acetylcholinesterase inhibitory activity. Phyther. Res. 2007, 21, 1142–1145. [Google Scholar] [CrossRef]
- Hostettmann, K.; Borloz, A.; Urbain, A.; Marston, A. Natural Product Inhibitors of Acetylcholinesterase. Curr. Org. Chem. 2006, 10, 825–847. [Google Scholar] [CrossRef]
- Ranjan, N.; Ranjan, N.; Kumari, M. Acetylcholinesterase inhibition by medicinal plants: A Review. Ann. Plant Sci. 2017, 6, 1640–1644. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Cai, L.; Ding, Z. An overview of the chemical constituents from the genus Delphinium reported in the last four decades. RSC Adv. 2020, 10, 13669–13686. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, D.; Chen, Y.; Xu, J.; Xu, Y.; Yue, X.; Jia, J.; Li, H.; Chen, L. Alkaloids of Delphinium grandiflorum and their implication to H2O2-induced cardiomyocytes injury. Bioorganic Med. Chem. 2021, 37, 116113. [Google Scholar] [CrossRef]
- Xu, J.B.; Li, Y.Z.; Huang, S.; Chen, L.; Luo, Y.Y.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from the whole herb of Delphinium grandiflorum L. Phytochemistry 2021, 190, 112866. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Jian, X.X.; Ding, J.Y.; Deng, H.Y.; Chao, R.B.; Chen, Q.H.; Chen, D.L.; Wang, F.P. Further studies on structure-cardiac activity relationships of diterpenoid alkaloids. Nat. Prod. Commun. 2015, 10, 2075–2084. [Google Scholar] [CrossRef] [Green Version]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L.; Lim, A.C.; Gordon, H.L.; Yarns, B.C.; Melrose, R.J. Cholinergic receptor binding in unimpaired older adults, mild cognitive impairment, and Alzheimer’s disease dementia. Alzheimer’s Res. Ther. 2022, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- MH, O.; PJ, H.; WK, W.; JH, C. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 2004, 11, 544–548. [Google Scholar] [CrossRef]
- Schulz, V. Ginkgo extract or cholinesterase inhibitors in patients with dementia: What clinical trials and guidelines fail to consider. Phytomedicine 2003, 10 (Suppl. 4), 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Ahmad, S.; Ali, M.; Latif, A.; Shah, S.A.A.; Naz, H.; ur Rahman, N.; Shaheen, F.; Wadood, A.; Khan, H.U.; et al. Norditerpenoid alkaloids of Delphinium denudatum as cholinesterase inhibitors. Bioorganic Chem. 2018, 78, 427–435. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Khan, H.U.; Khan, F.A.; Ali, M.; Latif, A.; Shaheen, F.; Ahmad, M. Selective dual cholinesterase inhibitors from Aconitum laeve. J. Asian Nat. Prod. Res. 2017, 20, 172–181. [Google Scholar] [CrossRef]
- Mitić, M.; Lazarević-Pašti, T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, H.; Khan, H.U.; Shahzad, A.; Khan, E.; Ali Shah, S.A.; Ali, M.; Wadud, A.; Ghufran, M.; Naz, H.; et al. Crystal structure, phytochemical study and enzyme inhibition activity of Ajaconine and Delectinine. J. Mol. Struct. 2016, 1123, 441–448. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, S.; Khan, E.; Shahzad, A.; Ali, M.; Tahir, M.N.; Shaheen, F.; Ahmad, M. Isolation, crystal structure determination and cholinesterase inhibitory potential of isotalatizidine hydrate from Delphinium denudatum. Pharm. Biol. 2017, 55, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.M.R.; Santos, H.S.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Rodrigues, A.S.; Silva, C.B.; Gusmão, G.O.M.; Freire, P.T.C.; Bento, R.R.F. Vibrational Spectroscopy of Xanthoxyline Crystals and DFT Calculations. Braz. J. Phys. 2012, 42, 180–185. [Google Scholar] [CrossRef]
- Ali Altaf, A.; Shahzad, A.; Gul, Z.; Rasool, N.; Gul, Z.; Badshah, A.; Lal, B.; Khan, E.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Tsuneda, T.; Song, J.W.; Suzuki, S.; Hirao, K. On Koopmans’ theorem in density functional theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]
- Clare, B.W. Frontier orbital energies in quantitative structure-activity relationships: A comparison of quantum chemical methods. Theor. Chim. Acta 1994, 87, 415–430. [Google Scholar] [CrossRef]
- Clare, B.W. Charge transfer complexes and frontier orbital energies in QSAR: A congeneric series of electron acceptors. J. Mol. Struct. Theochem 1995, 337, 139–150. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave, C.B. Comparison of DFT methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr A short history of SHELX. Acta Crystallogr. Sect. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Wang, H.; Wang, L.; Liu, A. DFT study of new bipyrazole derivatives and their potential activity as corrosion inhibitors. J. Mol. Model. 2006, 13, 147–153. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Latif, A.; Ali, M.; Khan, F.A.; Tahir, M.N.; Shaheen, F.; Wadood, A.; Ahmad, M. Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum. Bioorganic Med. Chem. 2017, 25, 3368–3376. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Batista, J.; Rocha, T.; Rocha, J.B.T.; Emanuelli, T.; Pereira, M.E. Effects of early undernutrition on kinetic parameters of brain acetylcholinesterase from adult rats. Acta Neurobiol. Exp. 1993, 53, 431. [Google Scholar]
- Burlingham, B.T.; Widlanski, T.S. An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. J. Chem. Educ. 2003, 80, 214–218. [Google Scholar] [CrossRef]
- Henchman, R.H.; Tai, K.; Shen, T.; Andrew McCammon, J. Properties of water molecules in the active site gorge of acetylcholinesterase from computer simulation. Biophys. J. 2002, 82, 2671. [Google Scholar] [CrossRef]
- Daoud, I.; Melkemi, N.; Salah, T.; Ghalem, S. Combined QSAR, molecular docking and molecular dynamics study on new Acetylcholinesterase and Butyrylcholinesterase inhibitors. Comput. Biol. Chem. 2018, 74, 304–326. [Google Scholar] [CrossRef]
- Geromichalos, G.D.; Lamari, F.N.; Papandreou, M.A.; Trafalis, D.T.; Margarity, M.; Papageorgiou, A.; Sinakos, Z. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J. Agric. Food Chem. 2012, 60, 6131–6138. [Google Scholar] [CrossRef]
- Berg, L.; Da Andersson, C.D.; Artursson, E.; Hörnberg, A.; Tunemalm, A.K.; Linusson, A.; Ekström, F. Targeting acetylcholinesterase: Identification of chemical leads by high throughput screening, structure determination and molecular modeling. PLoS ONE 2011, 6, e26039. [Google Scholar] [CrossRef] [Green Version]
- Babitha, P.; Sahila, M.; Bandaru, S.; Nayarisseri, A.; Sureshkumar, S. Molecular Docking and Pharmacological Investigations of Rivastigmine-Fluoxetine and Coumarin–Tacrine hybrids against Acetyl Choline Esterase. Bioinformation 2015, 11, 378–386. [Google Scholar] [CrossRef] [Green Version]

| Position | Compound 1 (150 MHz) | Compound 2 (150 MHz) | Compound 3 (125 MHz) |
|---|---|---|---|
| 1 | 78.4 | 75.4 | 75.7 |
| 2 | 75.9 | 73.3 | 73.3 |
| 3 | 40.7 | 34.0 | 34.0 |
| 4 | 36.7 | 32.5 | 32.6 |
| 5 | 59.9 | 49.7 | 48.9 |
| 6 | 209.4 | 209.4 | 209.6 |
| 7 | 49.7 | 44.0 | 44.2 |
| 8 | 44 | 36.7 | 36.7 |
| 9 | 78.6 | 78.3 | 83.8 |
| 10 | 39.7 | 43.6 | 44.0 |
| 11 | 32.3 | 80.7 | 80.7 |
| 12 | 53.4 | 89.3 | 78.3 |
| 13 | 209.4 | 209.5 | 34.8 |
| 14 | 57.9 | 48.7 | 40.7 |
| 15 | 34.9 | 30.8 | 26.9 |
| 16 | 142 | 141.9 | 142 |
| 17 | 110.3 | 110.4 | 110.4 |
| 18 | 27.3 | 29.4 | 29.7 |
| 19 | 63.1 | 94.2 | 94.2 |
| 20 | 70.3 | 70.1 | 70.1 |
| 21 | 48 | 38.7 | 38.7 |
| Crystal Parameter Compound 1 | |||
|---|---|---|---|
| Empirical formula | C21H27NO5 | Density (mg m−3) | 1.153 |
| Formula weight | 373.43 | (h, k, l) min | (−31, −5, −15) |
| Temperature (K) | 29.6 | (h, k, l) max | (31, 9, 15) |
| Wavelength (Å) | 0.71073 | Theta (max) | 26.0 |
| Crystal system | Monoclinic | R (reflection) | 0.053(2408) |
| Space group | C2 | wR2 | 0.185 |
| A | 25.726 (5) Å | No of measured, independent and observed [I > 2σ(I)] reflections | 8474, 3521, 2408 |
| B | 7.5766 (12) Å | Rint | 0.053 |
| C | 12.654 (2) Å | (sin θ/λ)max (Å−1) | 0.617 |
| Volume Å3 | 2150.4 (6) Å3 | No. of reflections | 3521 |
| μ (mm−1) | 0.08 | No. of parameters | 270 |
| Z | 4 | No. of restraints | 1 |
| Crystal size (mm) | 0.43 × 0.22 × 0.18 | Absolute structure parameter | −0.7 (10) |
| Compound-1 | 6-31G(d)/B3LYP | 6-311 + G(d,p)/wB97XD |
|---|---|---|
| EHOMO (au) | −0.225 | −0.319 |
| ELUMO (au) | −0.034 | −0.027 |
| ΔE = (ELUMO-EHOMO) (au) | 0.191 | 0.292 |
| IE = = −EHUMO (au) | 0.225 | 0.319 |
| EA = −ELUMO (au) | 0.034 | 0.027 |
| Global Hardness(η) = 1/2 (ELOMO-EHOMO) | 0.095 | 0.146 |
| Chemical Potential μ = 1/2 (EHOMO + ELUMO) | −0.095 | −0.146 |
| Global Electrophilicity ω = μ2/2η | 0.048 | 0.073 |
| S. No | Compounds | AChE ± SEM a (μM) | BChE ± SEM a (μM) | Type of Inhibition |
|---|---|---|---|---|
| 1 | Chitralinine A | 13.86 ± 0.35 | 28.17 ± 0.92 | Competitive |
| 2 | Chitralinine B | 11.64 ± 0.08 | 24.31± 0.33 | Competitive |
| 3 | Chitralinine-C | 12.11 ± 0.82 | 26.35± 0.06 | Competitive |
| 6 | Allanzanthane A | 8.23 ± 0.01 | 18 ± 0.06 | |
| 7 | Galanthamine b | 10.12 ±0.06 | 20.62 ± 0.08 |
| Inhibitors | MOE Score | MOE Interactions Residues | Gorge Site Residues of Target |
|---|---|---|---|
| 1 | −14.457 | Ser200, Gly119, Gly118, Ser122 | 121(288)297(290)120(118)121(119)204(201)447(440)334(327)203(200)86(84)72(70)124(121)286(279) |
| 2 | −15.591 | Tyr121, Ser122, His440, Ser200 | |
| 3 | −16.400 | Tyr334, Asp72, Ser122, Ser200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Ahmad, M.; Almehmadi, M.; Shah, S.A.A.; Khan, F.A.; Khan, N.M.; Khan, A.; Zainab; Halawi, M.; Ahmad, H. In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense. Molecules 2022, 27, 4348. https://doi.org/10.3390/molecules27144348
Ahmad S, Ahmad M, Almehmadi M, Shah SAA, Khan FA, Khan NM, Khan A, Zainab, Halawi M, Ahmad H. In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense. Molecules. 2022; 27(14):4348. https://doi.org/10.3390/molecules27144348
Chicago/Turabian StyleAhmad, Shujaat, Manzoor Ahmad, Mazen Almehmadi, Syed Adnan Ali Shah, Farman Ali Khan, Nasir Mehmood Khan, Asifullah Khan, Zainab, Mustafa Halawi, and Hanif Ahmad. 2022. "In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense" Molecules 27, no. 14: 4348. https://doi.org/10.3390/molecules27144348
APA StyleAhmad, S., Ahmad, M., Almehmadi, M., Shah, S. A. A., Khan, F. A., Khan, N. M., Khan, A., Zainab, Halawi, M., & Ahmad, H. (2022). In Vitro and In Silico Investigation of Diterpenoid Alkaloids Isolated from Delphinium chitralense. Molecules, 27(14), 4348. https://doi.org/10.3390/molecules27144348