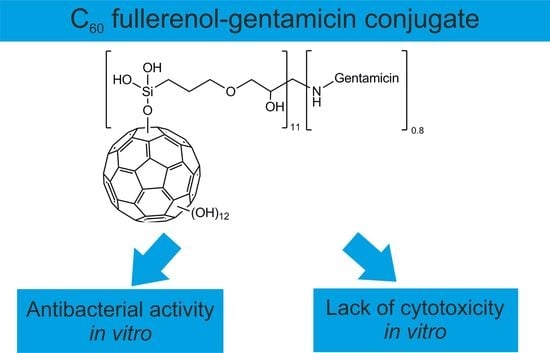
Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties
Abstract
:1. Introduction
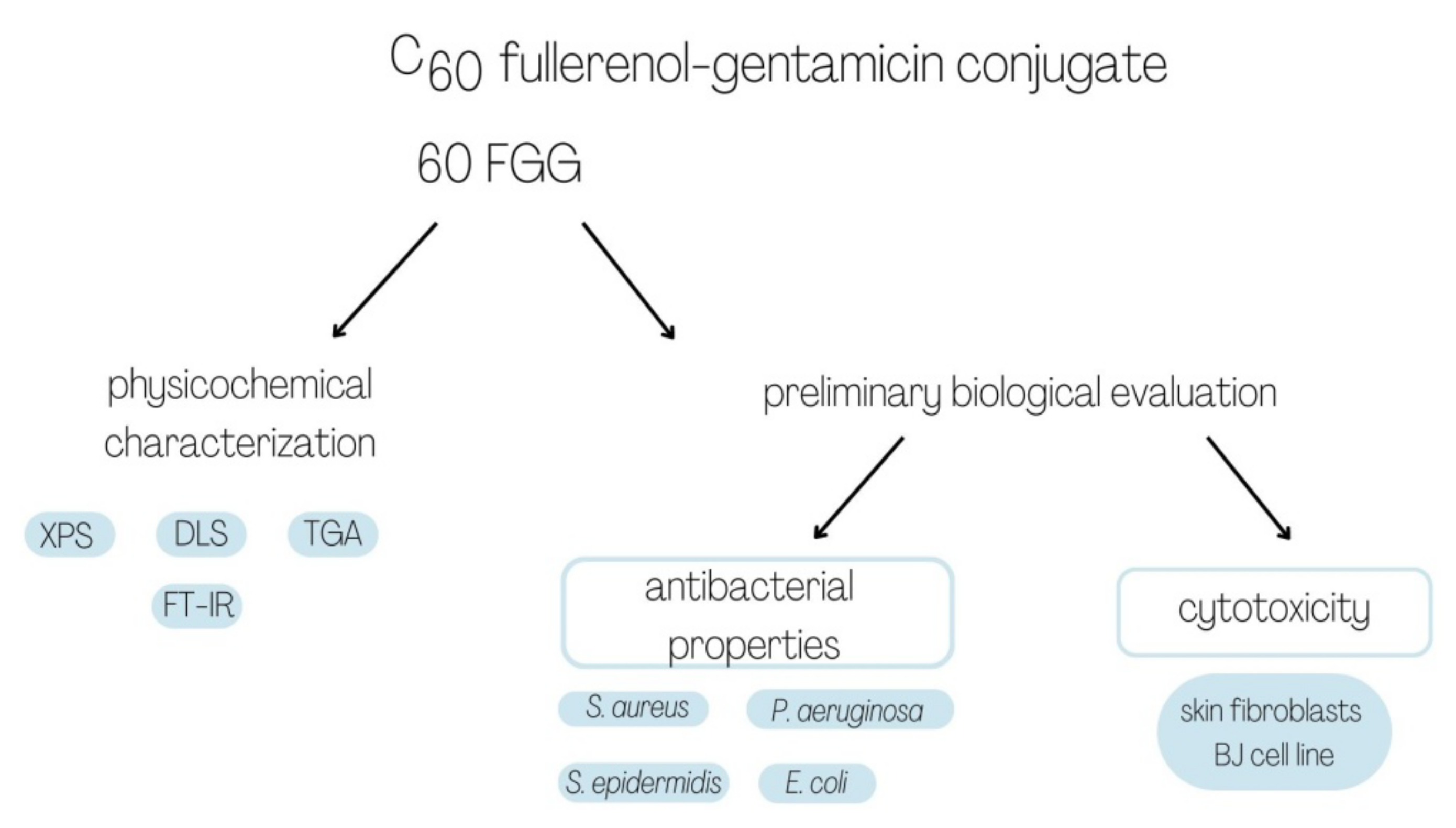
2. Results and Discussion
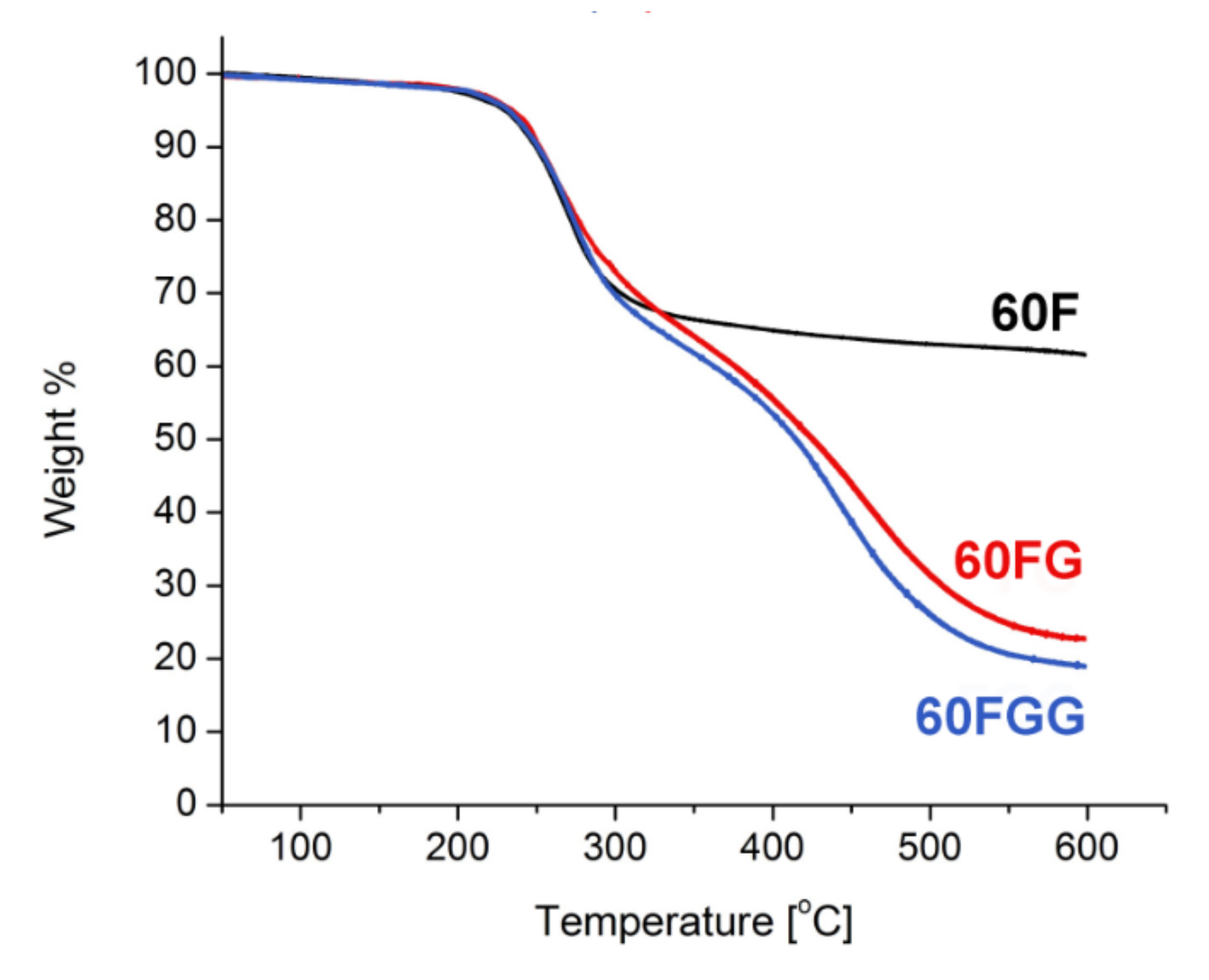
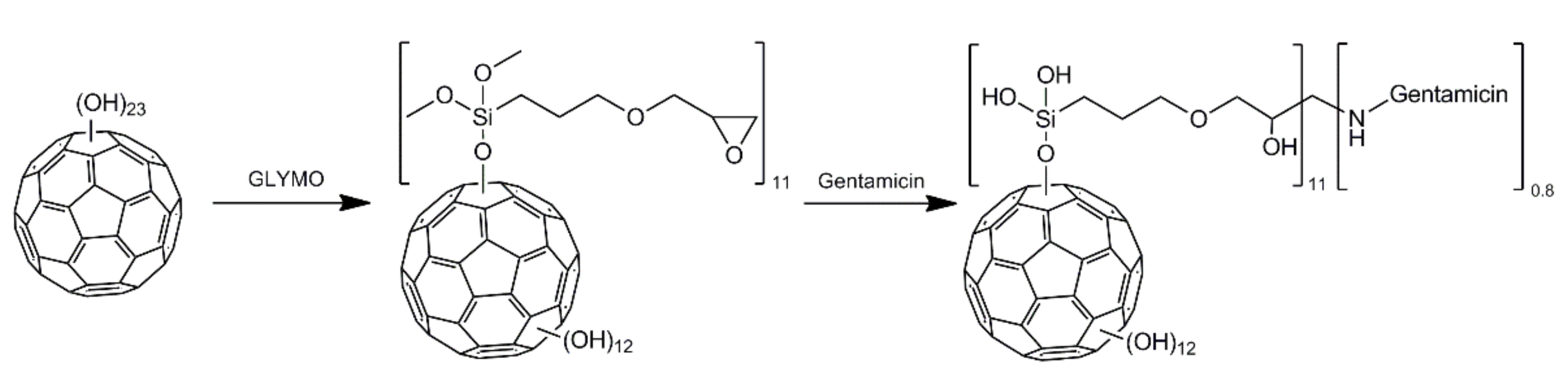
2.1. Characterization of C60 Fullerenol-Gentamicin Derivative
2.2. Antibacterial Properties of Gentamicin Functionalized C60 Fullerene Derivative
2.3. Cytotoxic Properties of Gentamicin Functionalized C60 Fullerene
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of C60 Fullerenol-Gentamicin Derivative
3.3. Biological Properties of PEG Functionalized C60 Fullerenol-Gentamicin Conjugate
3.3.1. Antibacterial Properties In Vitro
3.3.2. Cytotoxic Properties In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Lidija, M.; Roumiana, T.; Jelena, M.; Mari, M.; Kyoko, B.; Marija, T.; Branislava, J. Fullerene based nanomaterials for biomedical applications: Engineering, functionalization and characterization. Adv. Mater. Res. 2013, 633, 224–238. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Pochkaeva, E.I.; Podolsky, N.E.; Zakusilo, D.N.; Petrov, A.V.; Charykov, N.A.; Vlasov, T.D.; Penkova, A.V.; Vasina, L.V.; Murin, I.V.; Sharoyko, V.V.; et al. Fullerene derivatives with amino acids, peptides and proteins: From synthesis to biomedical application. Prog. Solid State Chem. 2020, 57, 100255. [Google Scholar] [CrossRef]
- Zhu, X.; Sollogoub, M.; Zhang, Y. Biological applications of hydrophilic C60 derivatives—A structural perspective. Eur. J. Med. Chem. 2016, 115, 438–452. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel biocompatible with animal cells composite material based on organosilicon polymers and fullerenes with Light-induced bacteriostatic properties. Nanomaterials 2021, 11, 2804. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Davydova, O.K.; Yankina, Z.Z.; Vasilchenko, A.S.; Miroshnikov, S.A.; Kornev, A.B.; Ivanchikhina, A.V.; Troshin, P.A. The activity of [60]fullerene derivatives bearing amine and carboxylic solubilizing groups against Escherichia coli: A comparative study. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lyon, D.Y.; Alvarez, P.J.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef]
- Martinez, Z.S.; Castro, E.; Seong, C.S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [Green Version]
- Marforio, T.D.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Fullerenes against COVID-19: Repurposing C60 and C70 to Clog the Active Site of SARS-CoV-2 Protease. Molecules 2022, 27, 1916. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Doi, Y.; Hashizume, M.; Kikuchi, J.I.; Konishi, T. An extremely effective DNA photocleavage utilizing functionalized liposomes with a fullerene-enriched lipid bilayer. J. Am. Chem. Soc. 2007, 129, 4140–4141. [Google Scholar] [CrossRef]
- Gao, Y.; Ou, Z.; Yang, G.; Liu, L.; Jin, M.; Wang, X.; Zhang, B.; Wang, L. Efficient photocleavage of DNA utilizing water soluble riboflavin/naphthaleneacetate substituted fullerene complex. J. Photochem. Photobiol. A Chem. 2009, 203, 105–111. [Google Scholar] [CrossRef]
- Soldatova, Y.V.; Areshidze, D.A.; Zhilenkov, A.V.; Kraevaya, O.A.; Peregudov, A.S.; Poletaeva, D.A.; Faingold, I.I.; Troshin, P.A.; Kotelnikova, R.A. Water-soluble fullerene derivatives: The inhibition effect on polyol pathway enzymes and antidiabetic potential on high-fat diet/low-dose streptozotocin-induced diabetes in rats. J. Nanoparticle Res. 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Chen, Z.; Yao, L.; Zhao, D.; Yang, X.; He, M.; Yu, Q. Inhibition of a thermophilic deoxyribonucleic acid polymerase by fullerene derivatives. J. Enzym. Inhib. Med. Chem. 2007, 22, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease, A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [Green Version]
- Vorobyov, V.; Kaptsov, V.; Gordon, R.; Makarova, E.; Podolski, I.; Sengpiel, F. Neuroprotective effects of hydrated fullerene C60: Cortical and hippocampal EEG interplay in an amyloid-infused rat model of alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- Golomidov, I.; Bolshakova, O.; Komissarov, A.; Sharoyko, V.; Slepneva, E.; Slobodina, A.; Latypova, E.; Zherebyateva, O.; Tennikova, T.; Sarantseva, S. The neuroprotective effect of fullerenols on a model of Parkinson’s disease in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2020, 523, 446–451. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. (UK) 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Nakamura, S.; Mashino, T. Water-Soluble Fullerene Derivatives for Drug Discovery. J. Nippn. Med. Sch. 2012, 79, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Rašović, I.; Porfyrakis, K. Functionalisation of fullerenes for biomedical applications. Compr. Nanosci. Nanotechnol. 2019, 1–5, 109–122. [Google Scholar] [CrossRef]
- Zaręba, N.; Więcławik, K.; Kizek, R.; Hosnedlova, B.; Kepinska, M. The Impact of Fullerenes as Doxorubicin Nano-Transporters on Metallothionein and Superoxide Dismutase Status in MCF-10A Cells. Pharmaceutics 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Paraskar, A.; Soni, S.; Mashelkar, R.A.; Sengupta, S. Fullerenol-cytotoxic conjugates for cancer chemotherapy. ACS Nano 2009, 3, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, S.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Raza, K. Fullerenol-Based Intracellular Delivery of Methotrexate: A Water-Soluble Nanoconjugate for Enhanced Cytotoxicity and Improved Pharmacokinetics. AAPS PharmSciTech 2018, 19, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 fullerene enhances cisplatin anticancer activity and overcomes tumor cell drug resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Park, S.K.; Cho, Y.S.; Lee, H.S.; Kim, K.R.; Kim, M.G.; Chung, W.H. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear. Res. 2006, 211, 46–53. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-Spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Hafeez, H.; Choi, D.K.; Lee, C.M.; Jesuraj, P.J.; Kim, D.H.; Song, A.; Chung, K.B.; Song, M.; Ma, J.F.; Kim, C.S.; et al. Replacement of n-type layers with a non-toxic APTES interfacial layer to improve the performance of amorphous Si thin-film solar cells. RSC Adv. 2019, 9, 7536–7542. [Google Scholar] [CrossRef] [Green Version]
- Goswami, T.H.; Singh, R.; Alam, S.; Mathur, G.N. Thermal analysis: A unique method to estimate the number of substituents in fullerene derivatives. Thermochim. Acta 2004, 419, 97–104. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Kadish, K.M.; Boulas, P.; Chen, E.C.M. Relationship between the electron affinities and half-wave reduction potentials of fullerenes, aromatic hydrocarbons, and metal complexes. J. Phys. Chem. 1995, 99, 8843–8850. [Google Scholar] [CrossRef]
- Karadeniz, K.; Aki, H.; Sen, M.Y.; Çalikoʇlu, Y. Ring opening of epoxidized soybean oil with compounds containing two different functional groups. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 725–731. [Google Scholar] [CrossRef]
- Mijović, J.; Andjelić, S. A Study of Reaction Kinetics by Near-Infrared Spectroscopy. 1. Comprehensive Analysis of a Model Epoxy/Amine System. Macromolecules 1995, 28, 2787–2796. [Google Scholar] [CrossRef]
- Komartin, R.S.; Balanuca, B.; Necolau, M.I.; Cojocaru, A.; Stan, R. Composite materials from renewable resources as sustainable corrosion protection coatings. Polymers 2021, 13, 3792. [Google Scholar] [CrossRef]
- Brant, J.A.; Labille, J.; Robichaud, C.O.; Wiesner, M. Fullerol cluster formation in aqueous solutions: Implications for environmental release. J. Colloid Interface Sci. 2007, 314, 281–288. [Google Scholar] [CrossRef]
- Mbizvo, G.K.; Bennett, K.; Simpson, C.R.; Susan, E.; Chin, R.F.M. Epilepsy-related and other nca l Puses of mortality in people with epilepsy: A systematic review of systematic reviews. Epilepsy Res. 2019, 157, 106192. [Google Scholar] [CrossRef]
- Afreen, S.; Kokubo, K.; Muthoosamy, K.; Manickam, S. Hydration or hydroxylation: Direct synthesis of fullerenol from pristine fullerene [C60] via acoustic cavitation in the presence of hydrogen peroxide. RSC Adv. 2017, 7, 31930–31939. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Klimek, K.; Przekora, A.; Benko, A.; Niemiec, W.; Blazewicz, M.; Ginalska, G. The use of calcium ions instead of heat treatment for β-1,3-glucan gelation improves biocompatibility of the β-1,3-glucan/HA bone scaffold. Carbohydr. Polym. 2017, 164, 170–178. [Google Scholar] [CrossRef]
- Nurzynska, A.; Klimek, K.; Palka, K.; Szajnecki, Ł.; Ginalska, G. Curdlan-based hydrogels for potential application as dressings for promotion of skin wound healing-preliminary in vitro studies. Materials 2021, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Takeuchi, A.; Ozawa, M.; Li, X.; Saigo, K.; Kitazawa, K. C60 Fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 1993, 1784–1785. [Google Scholar] [CrossRef]
- Lizza, J.R.; Moura-Letts, G. Solvent-Directed Epoxide Opening with Primary Amines for the Synthesis of β-Amino Alcohols. Synthesis. 2017, 49, 1231–1242. [Google Scholar]
- CLSI M07. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Pitucha, M.; Woś, M.; Miazga-Karska, M.; Klimek, K.; Mirosław, B.; Pachuta-Stec, A.; Gładysz, A.; Ginalska, G. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016, 25, 1666–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Bacteria | Minimum Inhibitory Concentration (MIC) a μg/mL | ||
|---|---|---|---|
| G | 60F | 60FGG | |
| S. aureus ATCC 25923 | 0.125 | ND b | 0.125 |
| S. epidermidis ATCC 12228 | 0.25 | ND b | 0.25 |
| P. aeruginosa ATCC 27859 | 0.25 | ND b | 0.25 |
| E. coli ATCC 25922 | 1 | ND b | 1 |
| Bacteria | Minimum Bactericidal Concentration (MBC) a μg/mL | ||
|---|---|---|---|
| G | 60F | 60FGG | |
| S. aureus ATCC 25923 | 0.25 | NT b | 0.25 |
| S. epidermidis ATCC 12228 | 0.5 | NT b | 0.5 |
| P. aeruginosa ATCC 27859 | 0.25 | NT b | 0.25 |
| E. coli ATCC 25922 | 1 | NT b | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurzynska, A.; Piotrowski, P.; Klimek, K.; Król, J.; Kaim, A.; Ginalska, G. Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties. Molecules 2022, 27, 4366. https://doi.org/10.3390/molecules27144366
Nurzynska A, Piotrowski P, Klimek K, Król J, Kaim A, Ginalska G. Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties. Molecules. 2022; 27(14):4366. https://doi.org/10.3390/molecules27144366
Chicago/Turabian StyleNurzynska, Aleksandra, Piotr Piotrowski, Katarzyna Klimek, Julia Król, Andrzej Kaim, and Grazyna Ginalska. 2022. "Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties" Molecules 27, no. 14: 4366. https://doi.org/10.3390/molecules27144366
APA StyleNurzynska, A., Piotrowski, P., Klimek, K., Król, J., Kaim, A., & Ginalska, G. (2022). Novel C60 Fullerenol-Gentamicin Conjugate–Physicochemical Characterization and Evaluation of Antibacterial and Cytotoxic Properties. Molecules, 27(14), 4366. https://doi.org/10.3390/molecules27144366