Functionalized C3-Symmetric Building Blocks—The Chemistry of Triaminotrimesic Acid
Abstract
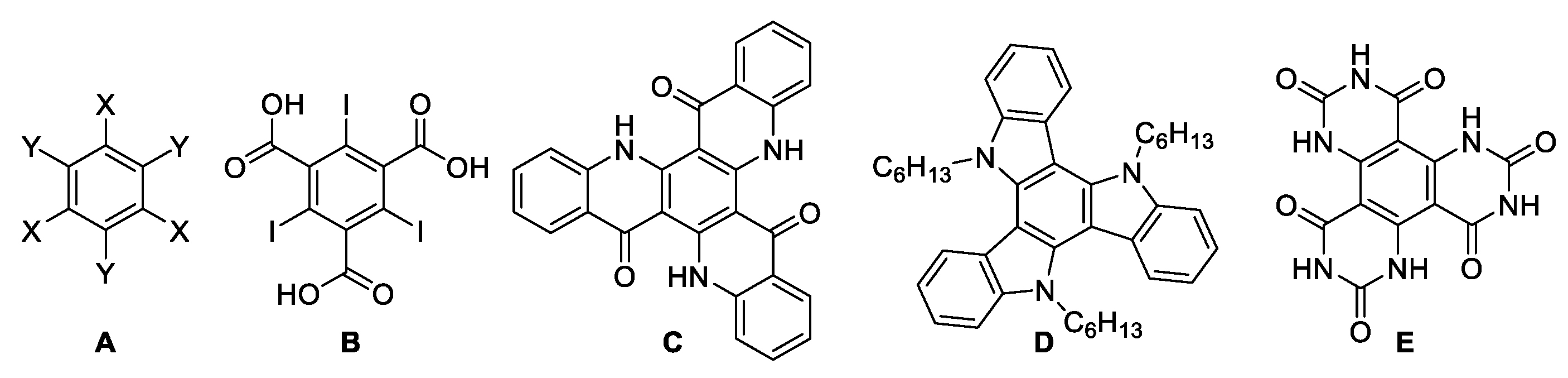
:1. Introduction
2. Results
2.1. Syntheses of C3-Symmetric Alkyl Triamino-Benzene-Tricarboxylates
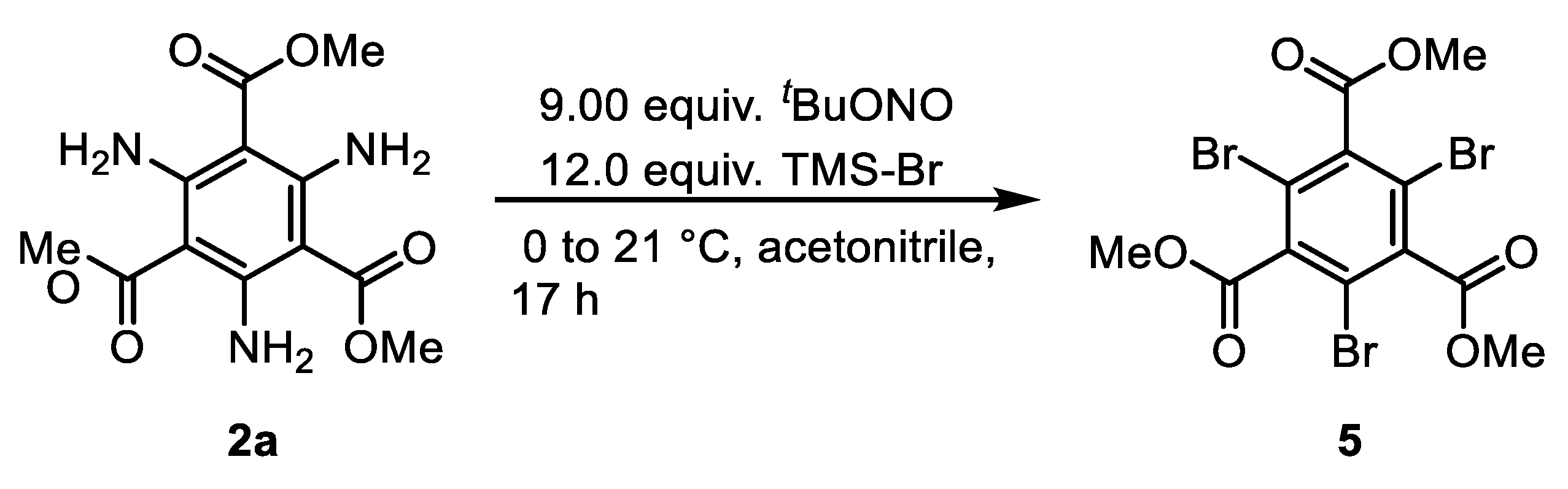
2.2. Diazotation and Azide Formation
2.3. Click Reactions
2.4. Alkylation
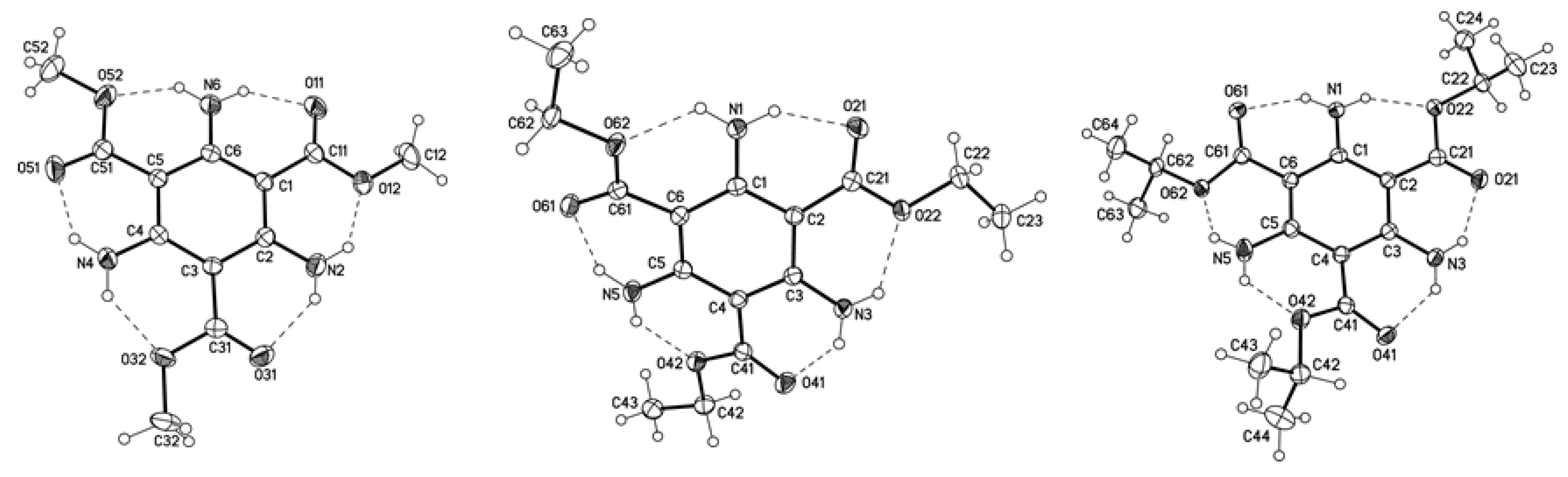
2.5. Molecular Structures
3. Materials and Methods
3.1. General Procedure for Cyclotrimerizations
3.2. General Procedure for the Syntheses of Azides
3.3. Typical Procedure for the Click Reactions
3.4. Crystal Structure Determination
3.5. NMR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References and Notes
- Through this manuscript, we refer to C3-symmetric building blocks: D3-symmetric ones are also included.
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G.; Krohn, K.; Steingroever, K.; et al. Five Unique Compounds: Xyloketals from Mangrove Fungus Xylaria sp. from the South China Sea Coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Takezawa, H.; Fujita, M. A Double-Walled Knotted Cage for Guest-Adaptive Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 5504–5508. [Google Scholar] [CrossRef] [PubMed]
- Park, L.Y.; Hamilton, D.G.; McGehee, E.A.; McMenimen, K.A. Complementary C3-Symmetric Donor–Acceptor Components: Cocrystal Structure and Control of Mesophase Stability. J. Am. Chem. Soc. 2003, 125, 10586–10590. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J. Structures of benzene derivatives with symmetrical substitution by three donor and three acceptor groups. Adv. Strained Interesting Org. Mol. 1999, 7, 43–101. [Google Scholar] [CrossRef]
- Harada, Y.; Matsunaga, Y. New mesomorphic compounds: N,N′,N″-trialkanoyl-2,4,6-trimethyl-1,3,5-benzenetriamines. Bull. Chem. Soc. Jpn. 1988, 61, 2739–2741. [Google Scholar] [CrossRef] [Green Version]
- Omenat, A.; Barbera, J.; Serrano, J.L.; Houbrechts, S.; Persoons, A. Columnar liquid crystals with highly polar groups. Evaluation of the nonlinear optical properties. Adv. Mater. 1999, 11, 1292–1295. [Google Scholar] [CrossRef]
- Bushey, M.L.; Nguyen, T.-Q.; Nuckolls, C. Synthesis, Self-Assembly, and Switching of One-Dimensional Nanostructures from New Crowded Aromatics. J. Am. Chem. Soc. 2003, 125, 8264–8269. [Google Scholar] [CrossRef]
- Mamada, M.; Inada, K.; Komino, T.; Potscavage, W.J.; Nakanotani, H.; Adachi, C. Highly Efficient Thermally Activated Delayed Fluorescence from an Excited-State Intramolecular Proton Transfer System. ACS Cent. Sci. 2017, 3, 769–777. [Google Scholar] [CrossRef]
- Long, Y.; Mamada, M.; Li, C.; dos Santos, P.L.; Colella, M.; Danos, A.; Adachi, C.; Monkman, A. Excited State Dynamics of Thermally Activated Delayed Fluorescence from an Excited State Intramolecular Proton Transfer System. J. Phys. Chem. Lett. 2020, 11, 3305–3312. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Li, F.; Fan, J.; Song, Y.; Wang, C.-K.; Lin, L. Theoretical perspective for luminescent mechanism of thermally activated delayed fluorescence emitter with excited-state intramolecular proton transfer. J. Mater. Chem. C 2020, 8, 98–108. [Google Scholar] [CrossRef]
- Tang, L.M.; Wang, Y.J. Highly stable supramolecular hydrogels formed from 1,3,5-benzenetricarboxylic acid and hydroxyl pyridines. Chin. Chem. Lett. 2009, 20, 1259–1262. [Google Scholar] [CrossRef]
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.B.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, S.; Tan, K.; Pandey, H.; Diwakara, S.D.; McCandless, G.T.; Grinffiel, D.I.; Durand-Silva, A.; Thonhauser, T.; Smaldone, R.A. 2D-Covalent Organic Frameworks with Interlayer Hydrogen Bonding Oriented through Designed Nonplanarity. J. Am. Chem. Soc. 2020, 142, 12987–12994. [Google Scholar] [CrossRef]
- Wei, H.; Ning, J.; Cao, X.; Li, X.; Hao, L. Benzotrithiophene-Based Covalent Organic Frameworks: Construction and Structure Transformation under Ionothermal Condition. J. Am. Chem. Soc. 2018, 140, 11618–11622. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ontoria, A.; Zimmermann, I.; Garcia-Benito, I.; Gratia, P.; Roldan-Carmona, C.; Aghazada, S.; Graetzel, M.; Nazeeruddin, M.K.; Martin, N. Benzotrithiophene-Based Hole-Transporting Materials for 18.2% Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 6270–6274. [Google Scholar] [CrossRef] [Green Version]
- Wolff, J.J.; Siegler, F.; Matschiner, R.; Wortmann, R. Optimized two-dimensional NLO chromophores with a threefold symmetry axis. Angew. Chem. Int. Ed. 2000, 39, 1436–1439. [Google Scholar] [CrossRef]
- Ledoux, I.; Zyss, J.; Siegel, J.S.; Brienne, J.; Lehn, J.M. Second-harmonic generation from nondipolar noncentrosymmetric aromatic charge-transfer molecules. Chem. Phys. Lett. 1990, 172, 440–444. [Google Scholar] [CrossRef]
- Cho, B.R.; Lee, S.J.; Lee, S.H.; Son, K.H.; Kim, Y.H.; Doo, J.-Y.; Lee, G.J.; Kang, T.I.; Lee, Y.K.; Cho, M.; et al. Octupolar Crystals for Nonlinear Optics: 1,3,5-Trinitro-2,4,6-tris(styryl)benzene Derivatives. Chem. Mater. 2001, 13, 1438–1440. [Google Scholar] [CrossRef]
- Sukul, P.K.; Das, P.; Dhakar, G.L.; Das, L.; Malik, S. Effect of Tricarboxylic Acids on the Formation of Hydrogels with Melem or Melamine: Morphological, Structural and Rheological Investigations. Gels 2022, 8, 51. [Google Scholar] [CrossRef]
- Sukul, P.K.; Malik, S. Removal of toxic dyes from aqueous medium using adenine based bicomponent hydrogel. RSC Adv. 2013, 3, 1902–1915. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, B.; Cui, P.; Kilina, S.; Sun, W. Multinuclear 2-(Quinolin-2-yl)quinoxaline-Coordinated Iridium(III) Complexes Tethered by Carbazole Derivatives: Synthesis and Photophysics. Inorg. Chem. 2020, 59, 17096–17108. [Google Scholar] [CrossRef]
- Huang, C.; Fu, W.; Li, C.-Z.; Zhang, Z.; Qiu, W.; Shi, M.; Heremans, P.; Jen, A.K.Y.; Chen, H. Dopant-Free Hole-Transporting Material with a C3h Symmetrical Truxene Core for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Truxene after Trujillo Coke. Truxillense (Trujillo or Peruvian leaf) which is the variety used to flavor beverages such as Coca Cola. The names stems from “Turris Iulia”, the old name fro Trujillo.
- Wang, J.-Y.; Yan, J.; Ding, L.; Ma, Y.; Pei, J. One-dimensional microwires formed by the co-assembly of complementary aromatic donors and acceptors. Adv. Funct. Mater. 2009, 19, 1746–1752. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Y.; Niu, Z.-Q.; Zhou, Q.-F.; Ma, Y.; Pei, J. Three-dimensional architectures for highly stable pure blue emission. J. Am. Chem. Soc. 2007, 129, 11314–11315. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, T.; Rominger, F.; Mastalerz, M. An Isosteric Triaza Analogue of a Polycyclic Aromatic Hydrocarbon Monkey Saddle. Chem. A Eur. J. 2020, 26, 14560–14564. [Google Scholar] [CrossRef]
- Ramos, F.J.; Rakstys, K.; Kazim, S.; Grätzel, M.; Nazeeruddin, M.K.; Ahmad, S. Rational design of triazatruxene-based hole conductors for perovskite solar cells. RSC Adv. 2015, 5, 53426–53432. [Google Scholar] [CrossRef]
- Connell, A.; Wang, Z.; Lin, Y.-H.; Greenwood, P.C.; Wiles, A.A.; Jones, E.W.; Furnell, L.; Anthony, R.; Kershaw, C.P.; Cooke, G.; et al. Low cost triazatruxene hole transporting material for >20% efficiency perovskite solar cells. J. Mater. Chem. C 2019, 7, 5235–5243. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.H.; Kim, S.W.; Lee, H.; Ko, I.J.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors. Nat. Photonics 2019, 13, 540–546. [Google Scholar] [CrossRef]
- Lai, W.-Y.; He, Q.-Y.; Zhu, R.; Chen, Q.-Q.; Huang, W. Kinked star-shaped fluorene/triazatruxene co-oligomer hybrids with enhanced functional properties for high-performance, solution-processed, blue organic light-emitting diodes. Adv. Funct. Mater. 2008, 18, 265–276. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Jiang, D. Conjugated Microporous Polymers as Molecular Sensing Devices: Microporous Architecture Enables Rapid Response and Enhances Sensitivity in Fluorescence-On and Fluorescence-Off Sensing. J. Am. Chem. Soc. 2012, 134, 8738–8741. [Google Scholar] [CrossRef]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Gratzel, M.; et al. Triazatruxene-Based Hole Transporting Materials for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef] [PubMed]
- Reynaerts, R.; Minoia, A.; Gali, S.M.; Daukiya, L.; Van Velthoven, N.; De Vos, D.; Lazzaroni, R.; Mali, K.S.; De Feyter, S. Coplanar versus Noncoplanar Carboxyl Groups: The Influence of Sterically Enforced Noncoplanarity on the 2D Mixing Behavior of Benzene Tricarboxylic Acids. J. Phys. Chem. C 2020, 124, 24874–24882. [Google Scholar] [CrossRef]
- Oakdale, J.S.; Sit, R.K.; Fokin, V.V. Ruthenium-Catalyzed Cycloadditions of 1-Haloalkynes with Nitrile Oxides and Organic Azides: Synthesis of 4-Haloisoxazoles and 5-Halotriazoles. Chem. Eur. J. 2014, 20, 11101–11110. [Google Scholar] [CrossRef] [PubMed]
- Rieser, J.; Ismail, N.; Abou-Elenien, G.; Wallenfels, K. Mono-, bis- and trishydrazo, and -azo compounds in the tricyanobenzene series. I. Preparation and properties of the title compounds. Liebigs Ann. Chem. 1981, 12, 1586–1597. [Google Scholar] [CrossRef]
- Stoessel, P.; Buesing, A.; Breuning, E.; Pflumm, C.; Parham, A.H.; Eberle, T.; Mujica-Fernaud, T. Heteroaromatic Compounds for Organic Electroluminescent Devices. WO 2012095143A1, 19 July 2012. [Google Scholar]
- Desimoni, G.; Gamba Invernizzi, A.; Quadrelli, P.; Righetti, P.P. Copper(II) in organic synthesis. IX. The copper(II)-catalyzed Michael reaction as a route to polysubstituted benzene derivatives. Gazz. Chim. Ital. 1991, 121, 483–485. [Google Scholar]
- Gill, J.E.; MacGillivray, R.; Munro, J. The preparation of symmetrical aromatic triamines and triisocyanates. J. Chem. Soc. 1949, 374, 1753–1754. [Google Scholar] [CrossRef]
- Chapyshev, S.V.; Misochko, E.Y.; Akimov, A.V.; Dorokhov, V.G.; Neuhaus, P.; Grote, D.; Sander, W. Molecular Structure and Magnetic Parameters of Septet 2,4,6-Trinitrenotoluene. J. Org. Chem. 2009, 74, 7238–7244. [Google Scholar] [CrossRef]
- Breslow, D.S.; Marcantonio, A.F. Polyazide Crosslinking Agents. US 3297674, 10 January 1967. [Google Scholar]
- Dorofeeva, O.V.; Ryzhova, O.N.; Suntsova, M.A. Accurate Prediction of Enthalpies of Formation of Organic Azides by Combining G4 Theory Calculations with an Isodesmic Reaction Scheme. J. Phys. Chem. A 2013, 117, 6835–6845. [Google Scholar] [CrossRef]
- Du, H.; Xu, X.; Liu, Y.; Liu, H.; Wang, F.; Zhang, J.; Gong, X. Theoretical studies on the nitro and azido derivatives of benzene. Huaxue Xuebao 2011, 69, 269–276. [Google Scholar]
- Hoogboom, J.; Juricek, M.; Lauko, J.; Rehak, J.; Woltinge, T.; van de Rult, K.; Flipse, K.F.J.; Rowan, A.E. Click chemistry approach to graphitic structures. PMSE Prepr. 2007, 97, 110–111. [Google Scholar]
- Kim, H.J.; Lee, W.S. Anion Receptor Comprising Aromatic Amines Substituted with Electron Withdrawing Groups and Electrolyte Using the Same for Alkali Metal Batteries. WO 2007126262A1, 8 November 2007. [Google Scholar]
- Kugler, T. Organic Electroluminescent Diode. GB2462433A, 10 February 2010. [Google Scholar]
- Liu, X.-F.; Xu, W.-G.; Lu, S.-X. DFT theoretical study on nitrogen-rich compounds C6H6−n(N3)n(n = 1–6). Gaodeng Xuexiao Huaxue Xuebao 2009, 30, 1406–1409. [Google Scholar]
- Liu, X.-J.; He, W.-P.; Huang, J. QSPR study on thermodynamic properties of the nitro and azido derivatives of benzene. Yuanzi Yu Fenzi Wuli Xuebao 2015, 32, 754–762. [Google Scholar]
- Boiko, V.N.; Gogoman, I.V.; Shchupak, G.M.; Yagupol’skii, L.M. Nucleophilic substitution in aromatic compounds with fluorine-containing substituents. X. Reactions of 2,4,6-trichloro- and 2,6-dichloro-3,5-bis[(trifluoromethyl)sulfonyl]nitrobenzenes with nucleophiles. Zh. Org. Khim. 1987, 23, 2586–2591. [Google Scholar]
- Chapyshev, S.V. Synthesis of 2,4,6-triazidotoluene for Use as Photoaffinic and Crosslinking Agent. RU 2430080C2, 27 September 2011. [Google Scholar]
- Chapyshev, S.V.; Chernyak, A.V. Triazidation of 2,4,6-trifluorobenzenes. J. Fluorine Chem. 2013, 156, 303–306. [Google Scholar] [CrossRef]
- Misochko, E.Y.; Masitov, A.A.; Akimov, A.V.; Korchagin, D.V.; Chapyshev, S.V. Heavy Atom Effect on Magnetic Anisotropy of Matrix-Isolated Monobromine Substituted Septet Trinitrene. J. Phys. Chem. A 2015, 119, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, J.; Fan, G.; Ma, Q.; Qu, Y. Synthesis Method of Energetic Compound 1-(3,5-dinitro-2,4,6-triaminobenzene)-1,2,4-triazole and Its Derivatives. CN 106588792A, 26 April 2017. [Google Scholar]
- Yang, W.; Lu, H.; Liao, L.; Fan, G.; Ma, Q.; Huang, J. Synthesis, and single crystal structure of fully-substituted polynitrobenzene derivatives for high-energy materials. RSC Adv. 2018, 8, 2203–2208. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lin, X.; Yang, L.; Zhang, T. A novel method to synthesize stable nitrogen-rich polynitrobenzenes with π-stacking for high-energy-density energetic materials. Chem. Commun. 2018, 54, 10296–10299. [Google Scholar] [CrossRef]
- Brase, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides. An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Keicher, T.; Loebbecke, S. Lab-Scale Synthesis of Azido Compounds: Safety Measures and Analysis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 3–27. [Google Scholar]
- Chapyshev, S.V.; Korchagin, D.V.; Grote, D.; Sander, W. EPR spectroscopy of multicomponent, multispin molecular system obtained by the photolysis of 2,4,6-triazido-3-cyano-5-fluoropyridine in solid argon. Magn. Reson. Chem. 2019, 57, 472–478. [Google Scholar] [CrossRef]
- Chapyshev, S.V.; Mendez-Vega, E.; Sander, W. Molecular Magnets: The Synthesis and Characterization of High-Spin Nitrenes. Chem.-Eur. J. 2021, 27, 1258–1269. [Google Scholar] [CrossRef]
- Mendez-Vega, E.; Mieres-Perez, J.; Chapyshev, S.V.; Sander, W. Persistent Organic High-Spin Trinitrenes. Angew. Chem. Int. Ed. 2019, 58, 12994–12998. [Google Scholar] [CrossRef] [PubMed]
- Stoessel, P.; Buesing, A.; Voges, F.; Pflumm, C.; Parham, A.H.; Eberle, T.; Mujica-Fernaud, T. Benzene Derivatives with Nitrogen-Containing Substituents for Organic Electronic Devices and Their Preparation and the Devices. WO 2012143079A1, 26 October 2012. [Google Scholar]
- Lu, P.; Wu, Y.; Kang, H.; Wei, H.; Liu, H.; Fang, M. What can pKa and NBO charges of the ligands tell us about the water and thermal stability of metal organic frameworks? J. Mater. Chem. A 2014, 2, 16250–16267. [Google Scholar] [CrossRef]
- Effenberger, F.; Niess, R. Aminobenzole, I.V. N-Persubstituierte 3.5-Diamino-phenole und 1.3.5-Triamino-benzole. Chem. Ber. 1968, 101, 3787–3793. [Google Scholar] [CrossRef]
- Beller, M.; Breindl, C.; Riermeier, T.H.; Tillack, A. Synthesis of 2,3-Dihydroindoles, Indoles, and Anilines by Transition Metal-Free Amination of Aryl Chlorides. J. Org. Chem. 2001, 66, 1403–1412. [Google Scholar] [CrossRef]
- Witulski, B.; Senft, S.; Thum, A. Palladium-Catalyzed Multiple Aryl Aminations of Polybromobenzenes. Synlett 1998, 1998, 504–506. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

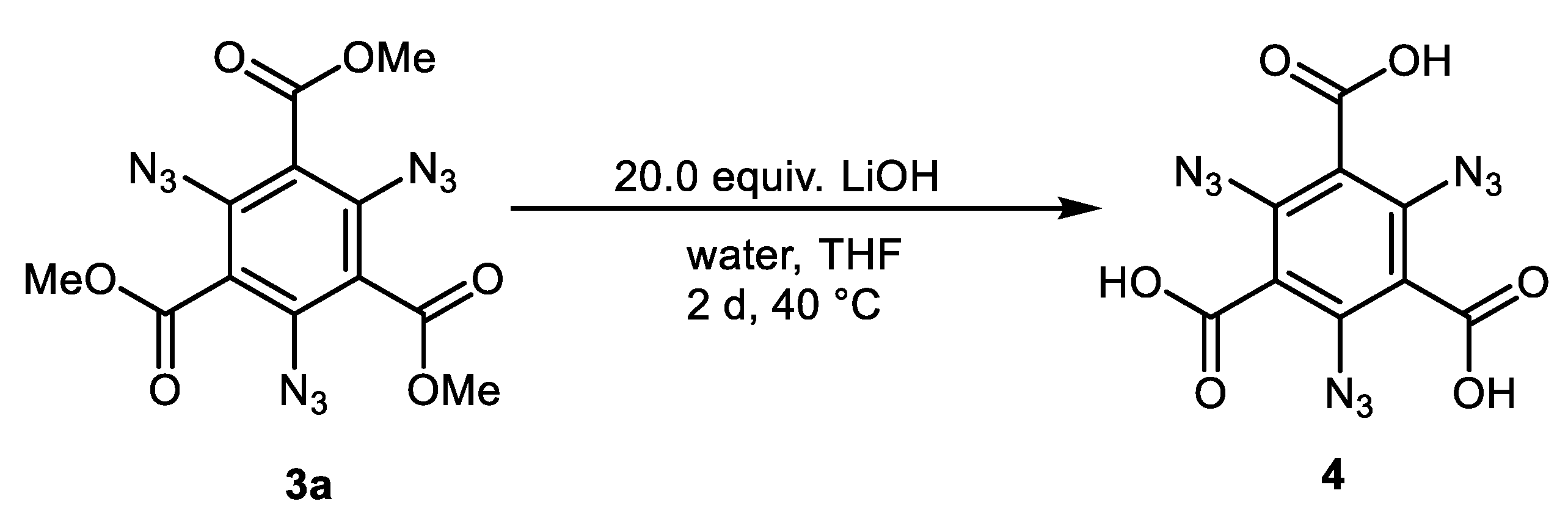

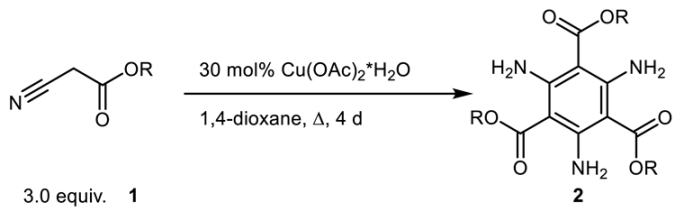 | ||||
|---|---|---|---|---|
| Starting Material | R | Product | Temp. [°C] | Yields [%] |
| 1a | Me | 2a | 130 | 35 a |
| 1b | Et | 2b | 130 | 28 |
| 1c | i Pr | 2c | 130 | 18 |
| 1d | t Bu | 2d | 100 | 15 |
| 1e | i Bu | 2e | 100 | 12 |
| 1f | n Pent | 2f | 100 | 22 |
| 1g | Bn | 2g | 130 | 23 |
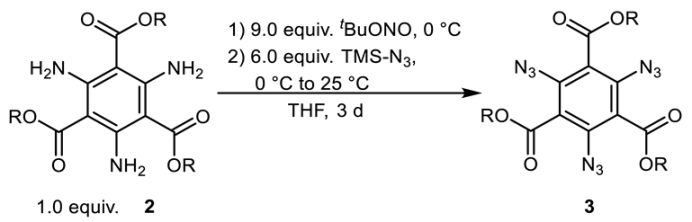 | |||
|---|---|---|---|
| Starting Material | R | Product | Yield [%] |
| 2a | Me | 3a | 60 |
| 2b | Et | 3b | 47 |
| 2c | i Pr | 3c | 50 |
| 2g | Bn | 3g | 36 |
 | ||||
|---|---|---|---|---|
| Starting Material | R1 | Product | R2 | Yield |
| 3a | Me | 6a-Ph | Ph | 19 |
| 3a | Me | 6a-C6H4Br | C6H4Br | 18 |
| 3b | Et | 6b-Ph | Ph | 27 |
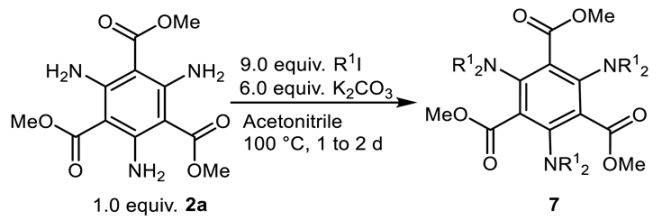 | ||
|---|---|---|
| Product | R | Yield [%] |
| 7a | Me | 48 |
| 7b | Et | 38 |
| 7c | Bn | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, L.; Wagner, D.; Nieger, M.; Bräse, S. Functionalized C3-Symmetric Building Blocks—The Chemistry of Triaminotrimesic Acid. Molecules 2022, 27, 4369. https://doi.org/10.3390/molecules27144369
Schmidt L, Wagner D, Nieger M, Bräse S. Functionalized C3-Symmetric Building Blocks—The Chemistry of Triaminotrimesic Acid. Molecules. 2022; 27(14):4369. https://doi.org/10.3390/molecules27144369
Chicago/Turabian StyleSchmidt, Lisa, Danny Wagner, Martin Nieger, and Stefan Bräse. 2022. "Functionalized C3-Symmetric Building Blocks—The Chemistry of Triaminotrimesic Acid" Molecules 27, no. 14: 4369. https://doi.org/10.3390/molecules27144369
APA StyleSchmidt, L., Wagner, D., Nieger, M., & Bräse, S. (2022). Functionalized C3-Symmetric Building Blocks—The Chemistry of Triaminotrimesic Acid. Molecules, 27(14), 4369. https://doi.org/10.3390/molecules27144369






