Tetrazine-Induced Bioorthogonal Activation of Vitamin E-Modified siRNA for Gene Silencing
Abstract
:1. Introduction
2. Results and Discussion
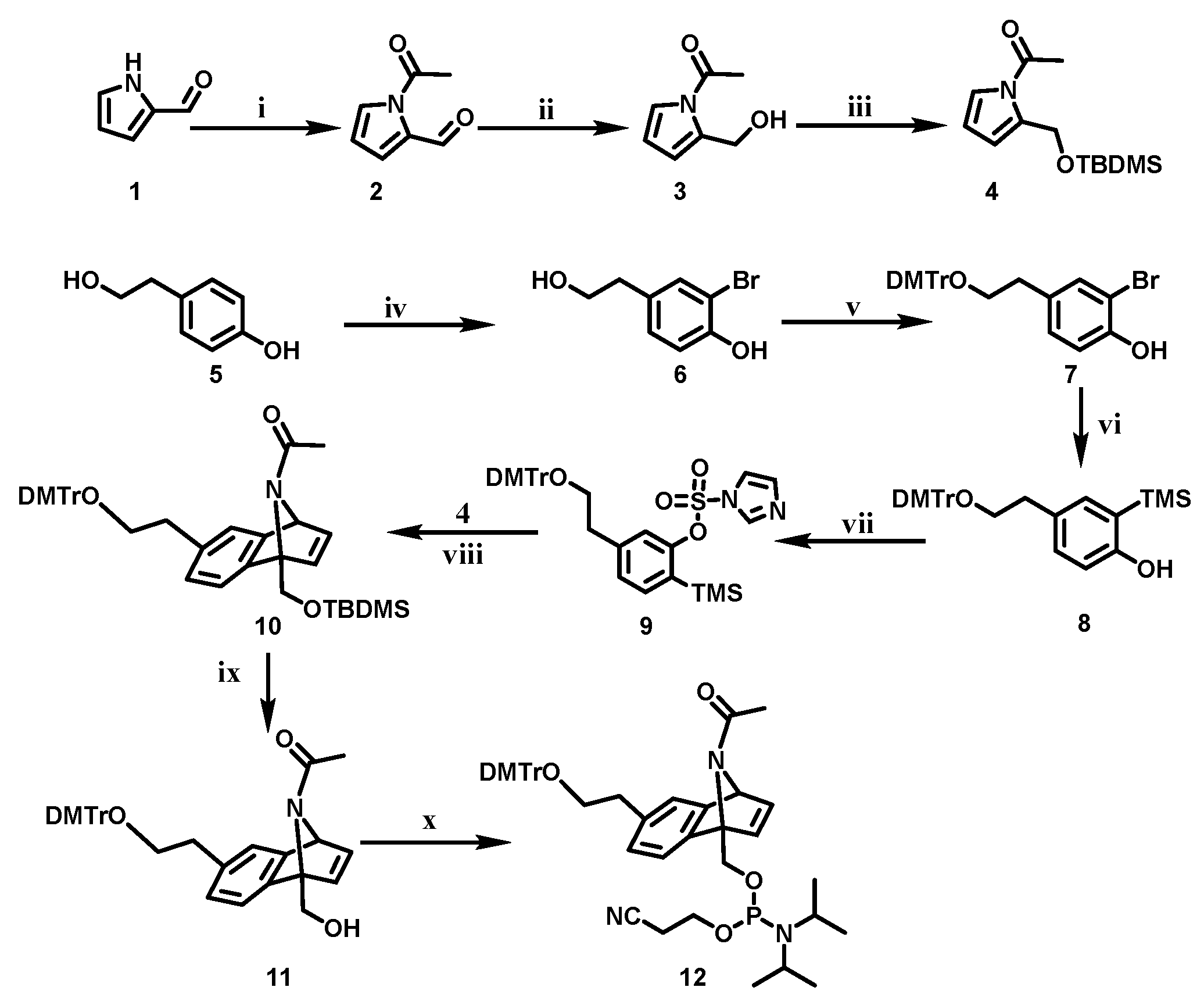
2.1. Rational Design and Synthesis of Benzonorbonadiene Linker and Vitamin E-Benzonorbonadiene-Caged siRNA
2.2. Activation of Vitamin E-Benzonorbonadiene-Caged siRNA for GFP Gene Silencing with Tetrazine
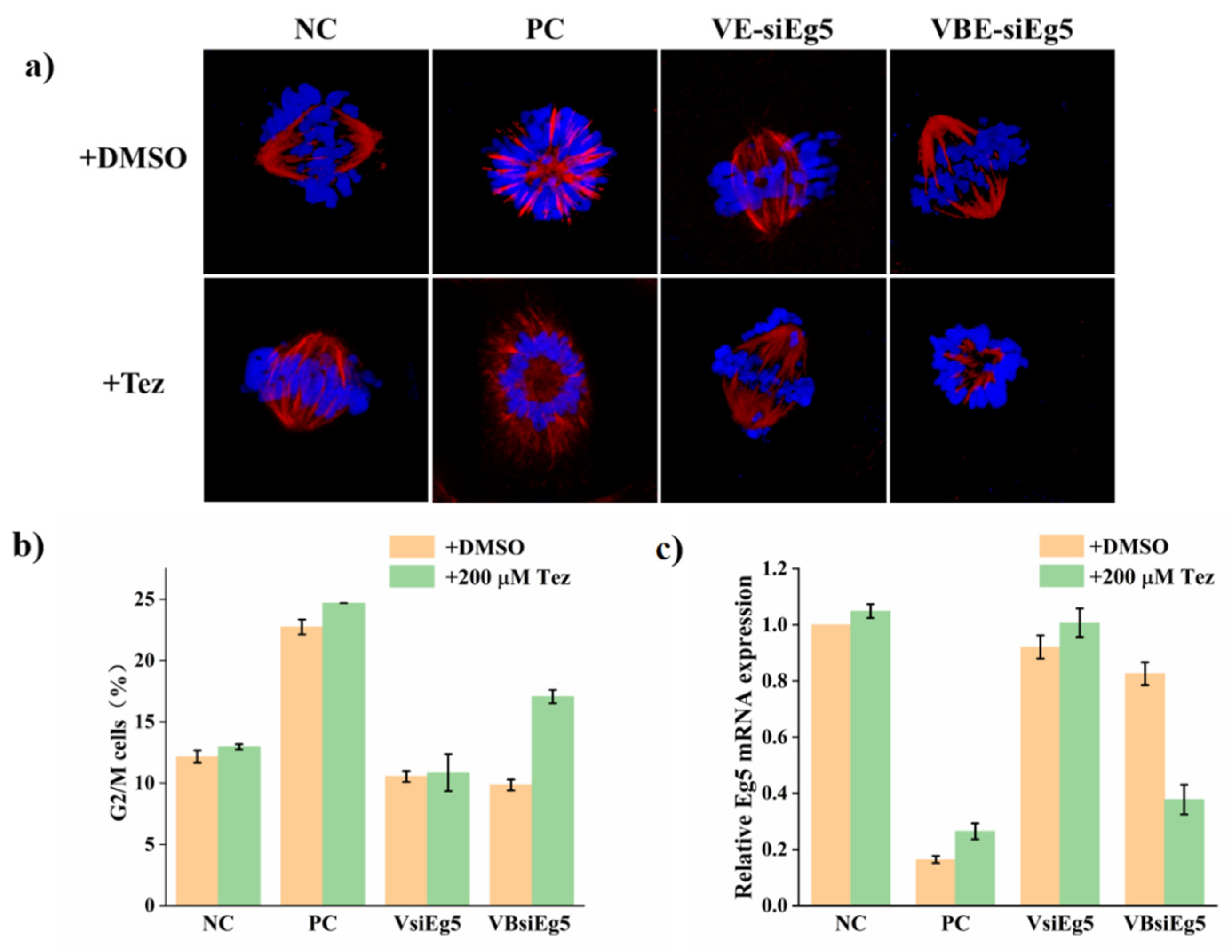
2.3. Activation of VBsiRNA for Eg5 Gene Silencing with Tetrazine
3. Materials and Methods
3.1. Bioorthogonal Reaction Kinetics between Tetrazine and Vitamin E-Benzonorbonadiene-Caged Oligonucleotide
3.2. GFP Gene Silencing Experiment
3.3. Cell Cycle Analysis in Eg5 Gene Silencing Experiment
3.4. qRT-PCR of Eg5 mRNA in Eg5 Gene Silencing Experiment
3.5. Cell Phenotypes in Eg5 Gene Silencing Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, M.K.G.; Idris, N.M.; Zhang, Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc. Natl. Acad. Sci. USA 2012, 109, 8483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruble, B.K.; Yeldell, S.B.; Dmochowski, I.J. Caged oligonucleotides for studying biological systems. J. Inorg. Biochem. 2015, 150, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Liu, F.; Liu, X.; Xing, B. NIR light controlled photorelease of siRNA and its targeted intracellular delivery based on upconversion nanoparticles. Nanoscale 2013, 5, 231–238. [Google Scholar] [CrossRef]
- Dovydenko, I.; Tarassov, I.; Venyaminova, A.; Entelis, N. Method of carrier-free delivery of therapeutic RNA importable into human mitochondria: Lipophilic conjugates with cleavable bonds. Biomaterials 2016, 76, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Meade, B.R.; Gogoi, K.; Hamil, A.S.; Palm-Apergi, C.; van den Berg, A.; Hagopian, J.C.; Springer, A.D.; Eguchi, A.; Kacsinta, A.D.; Dowdy, C.F.; et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 2014, 32, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, F.; Claveau, S.; Bertrand, J.-R.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Gymnotic delivery and gene silencing activity of reduction-responsive siRNAs bearing lipophilic disulfide-containing modifications at 2’-position. Bioorg. Med. Chem. 2018, 26, 4635–4643. [Google Scholar]
- Gauthier, F.; Malher, A.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Conjugation of Small Molecules to RNA Using a Reducible Disulfide Linker Attached at the 2′-OH Position through a Carbamate Function. Eur. J. Org. Chem. 2019, 2019, 5636–5645. [Google Scholar] [CrossRef]
- Kimura, Y.; Shu, Z.; Ito, M.; Abe, N.; Nakamoto, K.; Tomoike, F.; Shuto, S.; Ito, Y.; Abe, H. Intracellular build-up RNAi with single-strand circular RNAs as siRNA precursors. Chem. Commun. 2020, 56, 466–469. [Google Scholar] [CrossRef]
- Ochi, Y.; Nakagawa, O.; Sakaguchi, K.; Wada, S.-I.; Urata, H. A post-synthetic approach for the synthesis of 2′-O-methyldithiomethyl-modified oligonucleotides responsive to a reducing environment. Chem. Commun. 2013, 49, 7620–7622. [Google Scholar] [CrossRef]
- Khan, I.; Seebald, L.M.; Robertson, N.M.; Yigit, M.V.; Royzen, M. Controlled in-cell activation of RNA therapeutics using bond-cleaving bio-orthogonal chemistry. Chem. Sci. 2017, 8, 5705–5712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, P.R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 2016, 12, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, M.; Franzini, R.M. Dissociative Bioorthogonal Reactions. ChemBioChem 2019, 20, 1615–1627. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Zhao, J.; Wang, J.; Zheng, S.; Lin, S.; Chen, L.; Yang, M.; Jia, S.; Zhang, X.; et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 2014, 6, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Pan, Z.; Yu, B.; De La Cruz, L.K.; Zheng, Y.; Ke, B.; Wang, B. Click and release: Bioorthogonal approaches to “on-demand” activation of prodrugs. Chem. Soc. Rev. 2019, 48, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cisneros, B.T.; Cole, C.M.; Devaraj, N.K. Bioorthogonal tetrazine-mediated transfer reactions facilitate reaction turnover in nucleic acid-templated detection of microRNA. J. Am. Chem. Soc. 2014, 136, 17942–17945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Moreno, E.; Guo, Z.; Oliveira, B.L.; Albuquerque, I.S.; Kitowski, A.; Guerreiro, A.; Boutureira, O.; Rodrigues, T.; Jimenez-Oses, G.; Bernardes, G.J. Vinyl Ether/Tetrazine Pair for the Traceless Release of Alcohols in Cells. Angew. Chem. Int. Ed. 2017, 56, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Neumann, K.; Gambardella, A.; Bradley, M. The Emerging Role of Tetrazines in Drug-Activation Chemistries. ChemBioChem 2019, 20, 872. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Tu, J.; Franzini, R.M. Rapid and efficient tetrazine-induced drug release from highly stable benzonorbornadiene derivatives. Chem. Commun. 2017, 53, 6271–6274. [Google Scholar] [CrossRef]
- Nishina, K.; Unno, T.; Uno, Y.; Kubodera, T.; Kanouchi, T.; Mizusawa, H.; Yokota, T. Efficient In Vivo Delivery of siRNA to the Liver by Conjugation of α-Tocopherol. Mol. Ther. 2008, 16, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Nishina, T.; Numata, J.; Nishina, K.; Yoshida-Tanaka, K.; Nitta, K.; Piao, W.; Iwata, R.; Ito, S.; Kuwahara, H.; Wada, T.; et al. Chimeric Antisense Oligonucleotide Conjugated to α-Tocopherol. Mol. Ther. Nucleic Acids 2015, 4, e220. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Piao, W.; Miyata, K.; Nishina, K.; Mizusawa, H.; Yokota, T. High-Density Lipoprotein Facilitates In Vivo Delivery of α-Tocopherol–Conjugated Short-Interfering RNA to the Brain. Hum. Gene Ther. 2010, 22, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, B.T.; Kosbar, T.R.; Veedu, R.N. Novel Disulfide-Bridged Bioresponsive Antisense Oligonucleotide Induces Efficient Splice Modulation in Muscle Myotubes in Vitro. ACS Omega 2020, 5, 18035–18039. [Google Scholar] [CrossRef]
- Nishina, K.; Piao, W.; Yoshida-Tanaka, K.; Sujino, Y.; Nishina, T.; Yamamoto, T.; Nitta, K.; Yoshioka, K.; Kuwahara, H.; Yasuhara, H.; et al. DNA/RNA heteroduplex oligonucleotide for highly efficient gene silencing. Nat. Commun. 2015, 6, 7969. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Yang, J.; Wu, L.; Yu, L.; Tang, X. Photochemical Regulation of Gene Expression Using Caged siRNAs with Single Terminal Vitamin E Modification. Angew. Chem. Int. Ed. 2016, 55, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Galindo-Murillo, R.; Cheatham, T.E.; Franzini, R.M. Dissociative reactions of benzonorbornadienes with tetrazines: Scope of leaving groups and mechanistic insights. Org. Biomol. Chem. 2017, 15, 9855–9865. [Google Scholar] [CrossRef]
- Yu, L.; Liang, D.; Chen, C.; Tang, X. Caged siRNAs with Single cRGD Modification for Photoregulation of Exogenous and Endogenous Gene Expression in Cells and Mice. Biomacromolecules 2018, 19, 2526–2534. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef] [Green Version]
- Weil, D.; Garçon, L.; Harper, M.; Duménil, D.; Dautry, F.; Kress, M. Targeting the Kinesin Eg5 to Monitor siRNA Transfection in Mammalian Cells. BioTechniques 2002, 33, 1244–1248. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Gubu, A.; Xu, J.; Yan, N.; Su, W.; Feng, D.; Wang, Q.; Tang, X. Tetrazine-Induced Bioorthogonal Activation of Vitamin E-Modified siRNA for Gene Silencing. Molecules 2022, 27, 4377. https://doi.org/10.3390/molecules27144377
Zhang X, Gubu A, Xu J, Yan N, Su W, Feng D, Wang Q, Tang X. Tetrazine-Induced Bioorthogonal Activation of Vitamin E-Modified siRNA for Gene Silencing. Molecules. 2022; 27(14):4377. https://doi.org/10.3390/molecules27144377
Chicago/Turabian StyleZhang, Xueli, Amu Gubu, Jianfei Xu, Ning Yan, Wenbo Su, Di Feng, Qian Wang, and Xinjing Tang. 2022. "Tetrazine-Induced Bioorthogonal Activation of Vitamin E-Modified siRNA for Gene Silencing" Molecules 27, no. 14: 4377. https://doi.org/10.3390/molecules27144377
APA StyleZhang, X., Gubu, A., Xu, J., Yan, N., Su, W., Feng, D., Wang, Q., & Tang, X. (2022). Tetrazine-Induced Bioorthogonal Activation of Vitamin E-Modified siRNA for Gene Silencing. Molecules, 27(14), 4377. https://doi.org/10.3390/molecules27144377






