Abstract
The phytochemical analysis of antioxidant and antibacterial activities of Erodium arborescens aerial part extracts constitute the focus of this research. The chemical composition of an acetone extract was investigated using LC-HESI-MS2, which revealed the presence of 70 compounds. The major identified components were tannin derivatives. Total polyphenol and total flavonoid contents were assessed in plant extracts (hexane, ethyl acetate, acetone and methanol). The results showed that the acetone extract exhibited the highest contents of polyphenols and flavonoids, 895.54 and 36.39 mg QE/g DE, respectively. Furthermore, when compared to other extracts, Erodium arborescens acetone extract was endowed with the highest antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and total antioxidant capacity (TAC) tests. In addition, the four extracts of Erodium arborescens showed variable degrees of antimicrobial activity against the tested strains, and the interesting activity was obtained with acetone and methanol extracts.
1. Introduction
Phytochemicals from plants are receiving ever a great investigation, which integrates chemistry, botany and the study of secondary metabolites. Secondary metabolites differ from primary metabolites in that they have a wide structural variety, a narrow taxonomic distribution and a narrow range of biological activity [1]. Polyphenols are important plant secondary metabolites with an extensive range of structural characteristics. These are required for various functions in plants, and are responsible for many of the organoleptic and nutritional aspects of plant-derived foods which have numerous applications [2]. Species constituting the Geraniaceae family are rich in polyphenolic compounds, including flavonoids and tannins and are involved in folk medicine as remedies with a large spectrum of indications [3]. This cultural heritage was passed down the centuries long before contemporary medical techniques and medication development strategies were formed. The first step toward understanding the preventive and therapeutic possibilities of using medicinal plants to treat diseases is to preserve this knowledge [4].
Erodium is a well-known genus belonging to the Geraniaceae family, and includes more than 70 species which can be found in many continents. The Mediterranean region is considered as the location of many Erodium species (more than 60 species). The species can be either annual or perennial and can reach from 2 to 40 cm high [5]. Their leaves are alternate or opposite, pinnate or pinnately lobed, sometimes undivided and the flowers are actinomorphic or slightly zygomorphic; sepals 5, imbricate; petals 5, alternating with glands; fertile stamens 5, antisepalous; staminodes 5, antipetalous; ovary of 5 carpels adnate to central column of flower [6]. Studies on the phytochemical profile of Erodium species have shown the presence of tannins and it was reported that ellagic acid was the main phenolic compound present in Erodium petraeum, Erodium botrys, and Erodium gruinum. However, brevifolin was the predominant compound in Erodium cicutarium and Erodium manescavi [7]. In addition, Erodium species showed interesting antioxidant activity and antimicrobial effect against pathogenic microorganisms [7].
To our knowledge, there is no previous works dealing with chemical and biological investigations of Erodium arborescens.
This work was designed to investigate, for the first time, the total phenolic and total flavonoid contents as well as the antioxidant and antimicrobial capabilities of extracts obtained from the aerial part of Erodium arborescens. The scavenging capacity of hexane, ethyl acetate, acetone and methanol extracts was examined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging potential, ferric reducing antioxidant power (FRAP) and total antioxidant capacity (CAT) tests. Moreover, the antimicrobial activity was evaluated using the diffusion and micro dilution methods. Finally, the chemical composition of acetone extract was examined using liquid chromatography coupled with linear ion trap mass spectrometry.
2. Results and Discussions
2.1. Extraction
The extraction yield of phenolic compounds was calculated for each solvent (hexane, ethyl acetate, acetone and methanol) and are shown in Table 1.

Table 1.
Yields (%) of Erodium arborescens aerial part extracts.
We found that the maximum extract yield was obtained with methanol extract while the lowest one was obtained with ethyl acetate extract. The variations between extract yields of the studied plant material could be attributed to the polarity similarity between the solvent and extractable material of plants [8].
2.2. Chemical Composition
Polyphenols are a class of natural chemicals that has recently gained a lot of scientific and medicinal attention [9]. The total phenolic contents (TPC) and the total flavonoid contents (TFC) were evaluated in the different extracts.
Table 2 shows that the phenolic contents in acetone extract was significantly the highest one (895.54 mg GAE/g DE) followed by in MeOH (382.42 mg GAE/g DE) then EtOAc (266.20 mg GAE/g DE) extracts; whereas the hexane extract had significantly the lowest phenolic content (205.92 mg GAE/g DE).

Table 2.
Total phenolic and total flavonoid contents in Erodium arborescens aerial part extracts.
The results showed that the TFC varied considerably from 13.03 to 36.39mg in terms of quercetin equivalents/g of dried extract. As was expected for TFC, the acetone extract presented the highest content with 36.39 mg QE/g DE.
2.3. Antioxidant Activity
Generally, the antioxidant activity of plant extracts cannot be evaluated using a single approach method due to divergence of phytochemicals, so it is critical to use widely validated assays to evaluate the antioxidant activity. To explain how antioxidants work, different assays such as 2,2-diphenyl-1-picrylhydrazyl test (DPPH), reducing power (FRAP) and total antioxidant capacity (TAC) are the most acknowledged ones and they were consequently utilized in this study to evaluate the antioxidant power of Erodium arborescens extracts [10].
2.3.1. Radical-Scavenging Activity (DPPH) Assay
The DPPH test, measured by the molar ratio of antioxidant to DPPH radical required for 50% reduction in DPPH radical concentration, is a reliable method that is widely used. Ascorbic acid was chosen as the antioxidant reference for this test. The degree of discoloration indicates the extract’s scavenging potential which can be quantitatively measured from the changes in absorbance [7,8].
In the present study, the IC50 value of each extract was calculated and presented in Table 3. Among all Erodium arborescens extracts, the acetone and MeOH ones displayed the most effective (p < 0.05) DPPH radical scavenging activity (0.03 mg·mL−1) comparable to the positive control (Vitamin C) followed by EtOAc extract (0.039 mg·mL−1). Their AAI values ranging between 1 and 2 indicated that they have a strong antioxidant activity. By contrast, the IC50 value related to the hexane extract (0.109 mg·mL−1) indicated its weak antioxidant activity.

Table 3.
Radical-scavenging activity (DPPH) assay of Erodium arborescens aerial part extracts compared to Vitamin C as reference.
2.3.2. Ferric Reducing Antioxidant Power (FRAP)
The ability of Erodium arborescens extracts to reduce ferric ions was determined by using the FRAP assay which evaluates the ability of the tested substances to reduce Fe3+ to Fe2+. Since the antioxidant property of a substance is based on its reducing capacity, the FRAP assay may therefore serve as a significant indicator of its antioxidant activity. The rapid evaluation of the total antioxidant capacity of plant extracts is frequently tested by this method [11].
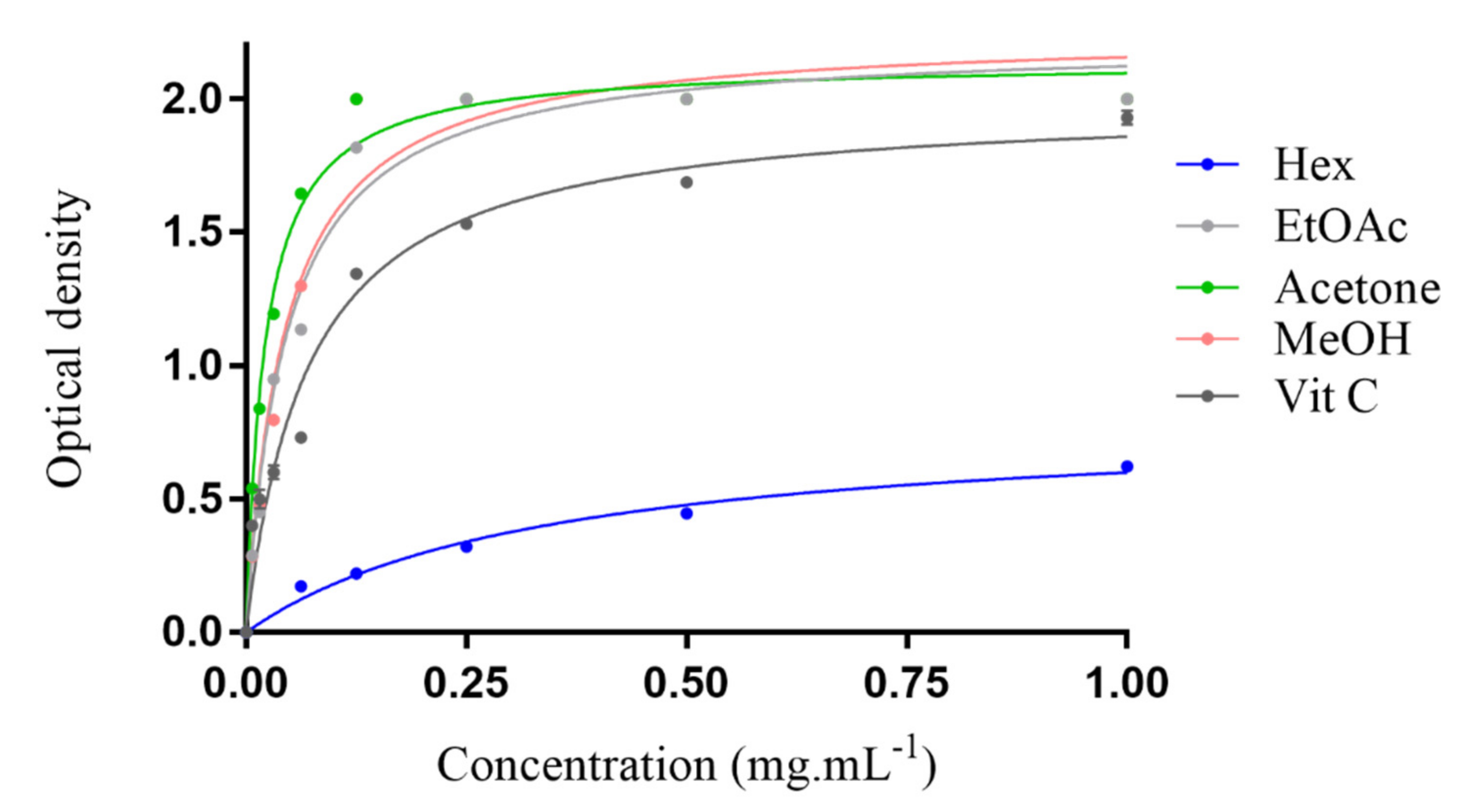
The ferric reducing antioxidant power of all tested extracts is shown in Figure 1. For all extracts, the reducing power increases with increasing concentration. The results clearly indicated that the MeOH, acetone and EtOAc extracts’ reducing powers were much higher than for Vitamin C. The lowest reducing property was observed for the hexane extract.
Figure 1.
Ferric reducing antioxidant power (FRAP) of Erodium arborescens aerial part extracts and vitamin C.
The following findings confirm that the MeOH and acetone extracts possessed the strongest antioxidant activities.
2.3.3. Total Antioxidant Activity
Total antioxidant capacity (TAC) evaluates the cumulative activity of all antioxidants present in the plant extracts, providing an integrated parameter rather than a simple sum of measurable antioxidants [12]. The total antioxidant capacities of Erodium arborescens extracts shown in Table 4 are expressed as mg of gallic acid equivalents per g of dried extract (mg GAE/g DE).

Table 4.
Total antioxidant activity of Erodium arborescens aerial part extracts and of vitamin C.
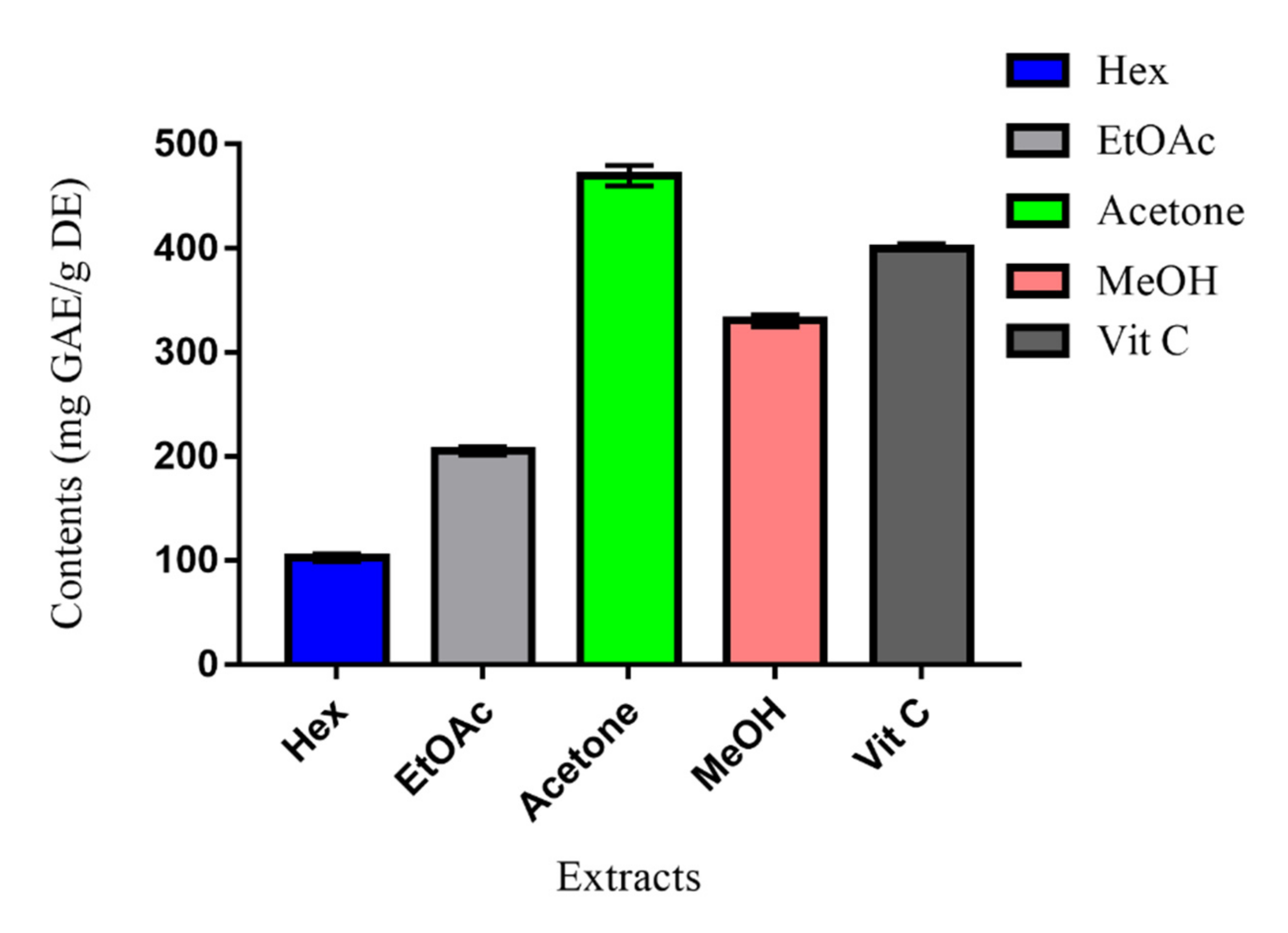
The results, summarized in Figure 2, indicated higher significant TAC of the MeOH and acetone extracts, respectively, at concentrations of 330 mg GAE/g DE and 470 mg GAE/g DE. Compared to vitamin C (400 mg GAE/g DE), the acetone extract presented the sharpest value. The antioxidant effect of these two extracts may be assigned to their phenolic and flavonoid contents. The order of antioxidant capacities of Erodium arborescens extracts was Hex < EtOAc < MeOH < Vit C < Acetone. This order is in good conformity with that of TPC, pointing to the fact that phenolic compounds may be the main antioxidant agents [13].
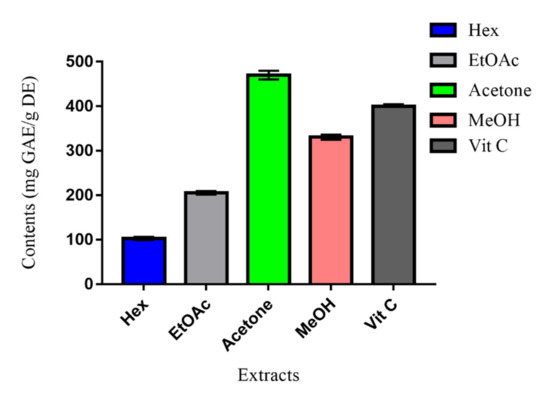
Figure 2.
Total antioxidant capacity (TAC) of Erodium arborescens aerial part extracts and Vit C.
2.4. Correlations between TFC, TPC and Antioxidant Activity
To explore the influence of phytochemical constituents on antioxidant capacity, the correlations between the phenolic contents and antioxidant activity of extracts of the aerial parts of Erodium arborescens were determined.
The results in Table 5 revealed important correlations for all the extracts between the TPC and DPPH, and the TFC and DPPH, with correlation coefficients R2 = 0.549 and R2 = 0.933, respectively. Similarly, an important linear correlation was established between the different phenolic contents of extracts and FRAP; R2 = 0.505 for TPC and R2 = 0.915 for TFC. For TAC and the different phenol contents, we noticed strong linear correlations with respective coefficients R2 = 0.922 (TAC-TPC) and R2 = 0.945 (TAC-TFC).

Table 5.
Correlation among phenolic compounds and assays *.
The statistical results obtained in this study indicated the presence of a significant correlation (p < 0.05) between FRAP and TAC (R2 =0.756). The DPPH test showed a good correlation with FRAP and TAC (R2 = 0.994 and R2 = 0.798, respectively).
2.5. Antimicrobial Activity
The four extracts (hexane, ethyl acetate, acetone and methanol) of Erodium arborescens were tested for their antimicrobial activity against six pathogenic microorganisms: two Gram-positive bacteria Listeria monocytogenes and Staphylococcus aureus; three Gram-negative bacteria Pseudomonas aeruginosa, Salmonella enterica typhimurium and Escherichia coli; and one fungus Candida albicans.
Listeria monocytogenes causes invasive diseases in humans, especially central nervous system infections [14]. Staphylococcus aureus is both a commensal and an extremely versatile pathogen in humans, causing several syndromes such as superficial lesions, deep-seated and systemic infections and toxemic syndromes [15]. Pseudomonas aeruginosa is an opportunistic pathogen affecting immunocompromised patients and it is known as the leading cause of morbidity and mortality in cystic fibrosis patients [16]. Salmonella enterica-typhimurium, the causative agent of typhoid fever, is a major public health threat and an estimated 27 million cases result in approximately 217,000 deaths annually worldwide due to this pathogenic bacterium [17]. In addition to being an important member of the normal intestinal microflora of humans and other mammals, E. coli contains many pathotypes that cause a variety of diseases [18]. Candida albicans, the most common human fungal pathogen, is an opportunistic pathogen that can threatenimmunologically weak and immunocompromised people and causes severe, life-threatening bloodstream infections in vulnerable patients [19].
The emergence of these pathogenic microorganisms has led to renewed interest in exploring the potential of bioactive plant compounds for human medicine and food preservation.
As shown in Table 6, the four extracts of Erodium arborescens showed variable degrees of antimicrobial activity against the six tested microbial strains.

Table 6.
Antimicrobial activity of Erodium arborescens aerial part extracts against the six tested indicator microorganisms. The diameters of inhibition zones were reported in millimeter (mm).
The hexane extract presented moderate inhibitory activity against the Gram-positive pathogen L. monocytogenes and the three Gram-negative pathogenic bacteria P. aeruginosa, S. enterica-typhimurium and E. coli. Ethyl acetate and acetone extracts acted strongly against all tested bacteria and the fungus C. albicans. The effects of these two extracts were more pronounced against Gram-negative bacteria than against Gram-positive ones. The strongest activity was obtained against the Gram-negative pathogenic bacterium P. aeruginosa with an inhibitory zone of 28 and 30 mm for ethyl acetate and acetone extracts, respectively. Comparing the two, the acetone extract appeared to be more promising than the ethyl acetate extract. The methanol extract exhibited an important inhibitory activity against only pathogenic Gram-negative bacteria and the fungus C. albicans. For this extract, the highest activity was observed against P. aeruginosa, S. enterica-typhimurium and the fungus C. albicans with inhibitory zones of 26, 28 and 26 mm, respectively.
Pathogenic Gram-negative bacteria are a real threat to human health and a burden on the health and food industries. Gram-negative bacteria have a thin peptidoglycan layer and have an outer lipid membrane (OM) whereas Gram-positive bacteria have a thick peptidoglycan layer and no outer lipid membrane. The OM of Gram-negative bacteria is an asymmetric bilayer with an inner leaflet of phospholipids and an outer leaflet of lipopolysaccharide [20], which is selectively permeable and thus regulates access to the underlying structures [21]. Moreover, the OM contains proteins called the outer membrane proteins (OMPs) which allow the passage of small molecules such as amino acids and small saccharides. This outer membrane of Gram-negative bacteria constitutes a real and robust permeability barrier that prevents many antibiotics from reaching their intracellular targets [22]. To explain the inhibitory activity of the Erodium arborescens methanol extract against Gram-negative bacteria only, and not against Gram-positive ones, one hypothesis is to consider that bioactive constituents of the methanol extract act at the OM level and thus disturb its selectivity function.
Minimum inhibitory concentrations (MICs) of the four extracts (hexane, ethyl acetate, acetone and methanol) of Erodium arborescens were assessed by micro-dilution method against four indicator microorganisms: two Gram-positive bacteria L. monocytogenes and S. aureus, one Gram-negative bacterium S. enterica-typhimurium; and a fungus C. albicans. As shown in Table 7, the MICs values range from 3.9 to 62.5 µg·mL−1 against L. monocytogenes, and the lowest MIC value (3.9 µg·mL−1) was obtained with acetone extract, which was equal to that of ampicillin and three times lower than that of kanamycin (12.5 µg·mL−1). Against S. aureus, MICs values were 15.6 and 6.25 µg·mL−1 for ethyl acetate and acetone extracts, respectively, and the MIC value of the acetone extract was equal to that of kanamycin. The MICs values range from 3.9 to 62.5 µg·mL−1 against the Gram-negative bacterium S. enterica-typhimurium, and the lowest MIC was that of the methanol extract (3.9 µg·mL−1) equal to the MIC of ampicillin and three times lower than that of kanamycin (12.5 µg·mL−1). The MIC of the acetone extract against this Gram-negative bacterium was 6.25 µg·mL−1 and was two times lower than that of kanamycin. Concerning MICs against C. albicans, values ranged from 1.25 and 6.25 µg·mL−1. The lowest MIC was that of methanol extract (1.25 µg·mL−1), which was equal to the MIC of the standard fluconazole. The MIC of the acetone extract was 2.5 µg·mL−1, which was two times higher than that of fluconazole and five times lower than MIC of ethyl acetate extract (6.25 µg·mL−1).

Table 7.
Minimum Inhibitory Concentrations (MICs), expressed in µg·mL−1, of Erodium arborescens aerial part extracts compared to three standards (ampicillin, kanamycin and fluconazole) against L. monocytogenes, S. aureus, S. enterica-typhimurium and C. albicans.
According to previous research, it was reported that the antimicrobial properties of plants have been established to be due, mainly, to geraniin [23] with an MIC of 0.16 mg·mL−1 against C. albicans. Adding to that, corilagin also accounts for its antimicrobial properties validating its use in traditional medicine for treating infections [24]. In fact, the MICs of corilagin were 31.25 µg·mL−1 and 62.5 µg·mL−1 against S. aureus and C. albicans. Although both geraniin and corilagin are major in the acetone extract, the two together proved to be more active with MICs varying from 6.25 to 2.5 µg·mL−1 against testing strains. This can be explained by the additive and synergistic effects of each extract constituents to be more effective than the isolated constituents [13].
2.6. Identification of Polyphenols in Acetone Extract
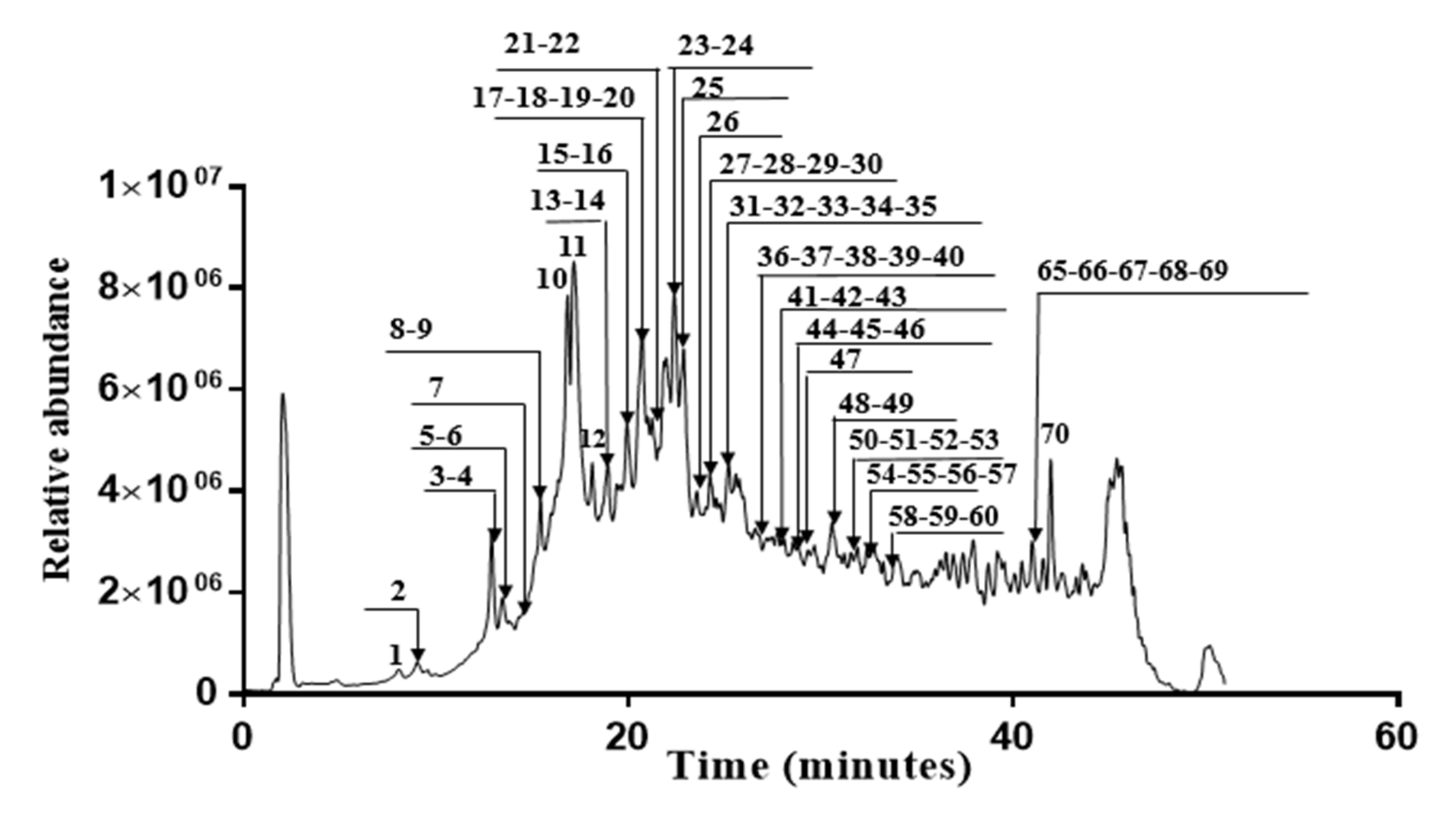
In this investigation, fifty polyphenols were identified in the Erodium arborescens acetone extract (Figure 3). Hydrolysable tannins, such as simple gallate esters, ellagic acid derivatives and glycosides and different ellagitannins were the main compounds in the acetone extract. HPLC coupled with hot electrospray ionization mass spectrometry was used to characterize all identified compounds listed in Table 8. For each peak, we have indicated the retention time, the relative intensity, the deprotonated mass and the fragment ions generated by MS2 operation.
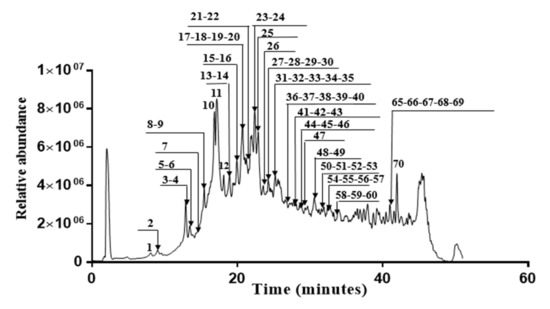
Figure 3.
Chromatogram of acetone extract from the aerial parts of Erodium arborescens obtained by LC-HESI-MS analysis into negative mode.
2.6.1. Characterization of Ellagitannins
Gallic acid and its derivatives are often found in Erodiums in ester form as hydrolysable tannins (HTs) [25]. HTs can be divided into three groups: galloyl glucoses (GGs) that are galloyl esters of glucose; gallotannins (GTs) that are galloyl glucoses where additional galloyl groups are attached with depside bonds; and ellagitannins (ETs) where two galloyl groups are attached to form hexahydroxydiphenoyl (HHDP) or further oxidized groups such as dehydrohexahydroxydiphenoyl (DHHDP) group [1].
- The precursor ion at m/z 495 of peak 4 detected at TR = 12.88 min was consistent with the presence of 3,5-di-O-galloylquinic acid [26]. The fragmentation of this precursor ion produced ions at m/z 343 due to the loss of galloyl moiety and at m/z 325 due to the elimination of a gallic acid molecule.
Geraniin is one of the most abundant compounds that was first isolated from this genus [27]. In this study, geraniin is the main compound in the acetone extract and co-occurs with an isomer named isogeraniin. In addition, several other ETs were identified from the aerial part such as corilagin, geraniinic acids and ascorgeraniin.
- Peak 1 gave the deprotonated molecule [M-H]−/z 169 at TR = 8.08 min. The major and only fragment was m/z 125 [M-CO2-H]− corresponding to the loss of a carbon dioxide moiety [28].
- Peak 11 mass spectrum shows an ion at m/z 951 [M-H]− at TR = 17.20 min and, on the basis of the fragmentation pattern and literature data, it was identified as Geraniin [29]. Its MS2 spectrum yielded fragments at m/z 933 due to dehydration [M-H-18], at m/z 479 due to a loss of HHDP-galloyl moiety and finally at m/z 301 corresponding to ellagic acid.
The hydrolysis products of geraniin are stable especially corilagin. Other compounds can be produced from geraniin such as brevifolin carboxylic acid. The literature about the phytochemical composition of Erodium genus gave an approach to characterize this class of secondary metabolites in Erodium arborescens.
- Corilagin at peak 10 has been already well characterized in previous studies [27]. This compound is a complex ellagitannin containing glucose, gallic acid and HHDP. Corilagin was detected in the TIC chromatogram at TR = 16.84 min. Its precursor [M-H]− was m/z 633 which gave two main fragments at m/z 463 and 301 due to consecutive losses of gallic acid and galloyl, supporting the identity of this compound.
- Peak 9 had the precursor ion m/z 247 at TR = 15.51 min and has been tentatively proposed as brevifolin carboxylate [30]. The product ion at m/z 219 may result from the cleavage of the ester bond and subsequent loss of the CO group from brevifolin carboxylate.
- Another peak 16 was proposed as methyl brevifolin carboxylate [27] with a molecular ion m/z 305 at TR = 19.93 min. Its MS2 spectrum showed a fragment ion at m/z 273, suggesting the loss of CH3OH.
- Peak 19 was observed with m/z 953 [M-H]− at TR = 20.36 min and the following fragmentations were: m/z 935 (dehydration), m/z 633 (loss of HHDP group), m/z 463 (combined losses of HHDP and galloyl groups) and m/z 301 (HHDP residue). It can be ascribed, however, to geraniinic acid A [31].
- Peak 26, generating a molecular ion [M-H]− at m/z 983, was eluted at TR = 23.80 min and gave one fragment at m/z 633, indicating the presence of a corilagin unit. It also showed fragments at m/z 939 due to the loss of CO2, and at m/z 769 due to the loss of gallic acid. Based on fragmentation data, this compound was tentatively identified as a corilagin derivative.
- Peak 36 at TR = 26.08 min can be identified as punicatannin A/B [32]. This identification was confirmed due to its pseudo molecular ion m/z 997. The MS2 spectrum of this compound product ions at m/z 633 and 301 supported the existence of a corilagin unit.
The DHHDP group is very reactive and its further oxidation yields ETs such as phyllanthusiins B and G. Those compounds were also identified in this extract.
- Peak 14 has a molecular ion [M H]− at m/z 925 at TR = 18.94 min, which is tentatively assigned to phyllanthusiin C [33], whereas the product fragment at m/z 907 is due to water loss, which produced the fragment at m/z 605 after the further loss of HDDP. The fragment at m/z 435 is the result of the removal of a galloyl group. The peak at m/z 301 shows the ionized HDDP unit.
- Another peak 15 corresponding to phyllanthusiin G [34] with a deprotonated ion [M-H]− at m/z 969 at TR = 19.37 min was detected. The MS2 spectra generated fragments at m/z 925 due to the loss of CO2 and at m/z 633 which indicated the presence of a corilagin unit. The aglycone fragment at m/z 301 confirms the presence of an ellagic acid. Typical losses during fragmentation are galloyl (152 amu), HHDP (302 amu), galloyl glucose (332 amu), HHDP glucose (482 amu) and galloyl-HHDP-glucose (634 amu) [35].
- A precursor ion m/z 987 (peak 39) at TR = 26.67 min was fragmented to give an intense fragment at m/z 955 by losing a CH3OH unit (−32 amu), and a fragment at m/z 653 by removing a HHDP unit. After sequential removal of gallic acid (−170 amu), the remaining fragment was HHDP glucose (m/z 483). Based on these fragmentations, we note that compound 39 has the same base molecule as ellagitannins and it was thus suggested as an ellagitannin derivative.
- Peak 35 at TR = 25.98 min exhibited an ion [M-H]− at m/z 965. Its MS2 spectrum shows produced fragments at m/z 933 and m/z 795 due to the loss of water and gallic acid moieties, respectively, and one major fragment at m/z 301 which is typical for castalagin [36]. Based on its MS2 spectrum, this compound was identified as a castalagin derivative.
2.6.2. Characterization of Flavonoids
A total of sixteen flavonoids were identified on the basis of their MS2 fragmentations. They were mostly with O and C-glycosides. Sugar moieties consist of hexoside, deoxyhexoside and pentoside as deduced from the losses of 162 amu, 146 amu and 132 amu, respectively. Moreover, we observed MS fragmentation patterns characteristic of C-glycosides flavonoids, including dehydration and cross ring cleavage of the glucose moiety that produce cross ring cleavage [M-H-120] and another cross ring cleavage [M-H-90].
Flavanones
Naringenin derivatives
- Peaks 33 and 55 have mono-charged molecular ion m/z 271 at TR = 25.74 and 32.76 min, respectively. Their MS2 gave fragments at m/z 151 and m/z 165 produced through retro Diels-Alder reactions by breaking two C-C bonds of the C-ring, which gave structurally informative ions of A-ring and B-ring. However, it should be noted that the two compounds exhibited significantly different retention times. According to these findings, it may be possible to attribute these two compounds to isomeric forms of naringenin [37].
Flavonols
Quercetin derivatives
- Peak 20 exhibited the [M-H]− ion at m/z 595 at TR = 20.69 min with its MS2 fragment at m/z 343 due to a loss of a pentose (−132 amu) and a part of hexose moiety (−120 amu), and another fragment at m/z 301 completing the loss of hexose, indicating that this compound is a quercetin diglycoside [30].
- Peak 54 at TR = 32.61 min, obtained with a molecular ion at m/z 301, corresponds to quercetin aglycone [37]. Its MS2 spectrum gave fragments at m/z 273 and 257, due to consecutive losses of CO and CO2, respectively. Fragments at m/z 179 and m/z 151 resulted from breaking two C-C of C-ring, retro cyclisation and the loss of CO, respectively.
Kaempferol derivatives
- Peak 31 was identified to kaempferol-O-glucoside [38]. Its MS2 spectrum gave fragments 327 [M-H-120]− and 285 [M-H-162]−.
- Peak 46 had a quasi-molecular ion m/z 489 at TR = 28.89 min giving a fragment at m/z 285, probably owing to the removal of acetylhexoside group (204 amu). Kaempferol acetylhexoside [39] might be identified with this molecule. Despite the loss of the acetylhexoside group, which was the most predominant fragment, a small fragment at m/z 327 was also discovered. In agreement with this hypothesis, we can conclude that the m/z 327 fragment can be produced as a result of the sugar cleavage. Furthermore, the presence of a fragment at m/z 285 indicates the presence of the aglycone kaempferol.
- Peak 25 gave [M-H]− at m/z 579 at TR = 22.89 min. In its MS2 spectrum, the ion at m/z 285 [M-H-(162 + 132)]− was the only one observed, suggesting the presence of hexose and pentose moiety. This compound was attributed to kaempferol-O-pentosyl-O-hexoside [40].
Isorhamnetin derivatives
- Peak 68 produced the deprotonated aglycone at m/z 315 at TR = 41.42 min. The characteristic product ions at m/z 300, 271, 255 and 227 led to its identification as isorhamnetin aglycone [41].
- Peak 29 produced a [M-H]− at m/z 623 at TR = 24.29 min. In its MS2 spectrum, a predominant fragment at m/z 315 [M-H-308]− was observed due to the loss of 308 amu (162 + 146) indicating that a hexose and a deoxyhexose are linked at the same position of the isorhamnetin aglycone. Isorhamnetin [42] as aglycone was confirmed by the presence of fragment at m/z 271.
- Isorhamnetin-O-glucuronide [43] was identified (peak 45) with a molecular ion m/z 491 at TR = 28.60 min. Its MS2 spectrum showed fragments at m/z 459 and 323, due to successive losses of CH3OH and glucuronide moiety, respctively. The loss of a glucuronic acid from the fragment at m/z 491 resulted in m/z 315 as the base peak.
- Peak 58 at TR = 33.28 min was proposed to be an isorhamnetin-O-pentosyl-hexoside [44] with m/z 609. Its MS2 showed a fragment at m/z 477 corresponding to the loss of pentoside [M-H-132]− and a fragment at m/z 315 which is the result of glucoside loss [M-H-132-162]−. The fragment at m/z 301 represents the loss of CH3.
Flavones
Apigenin derivatives
- According to the fragmentation of [M-H]− ion at m/z 563 and the retention times 18.83 and 20.28 min, peaks 13 and 18 were identified as 6-C-arabinosyl-8-C-glucosyl apigenin [45]. Their MS2 data showed fragments at m/z 473 [M-H-90]− and 443 [M-H-120]−, indicating the presence of a C-hexosyl unit. Another fragment was observed at m/z 503 [M-H-60]− corresponding to the fragmentation of pentose. The base peak at m/z 473 [M-H-90]− and the high abundance of the fragment at m/z 503 [M-H-60]− revealed the presence of a 6-C-pentosyl unit.
- Peak 34 at TR = 25.89 min was identified as apigenin-O-hexoside [46] showing an [M-H]− ion at m/z 431. Its MS2 spectrum gave a fragment at m/z 269 typical to apigenin aglycone, after sequential loss of hexose unit.
Luteolin derivatives
- Peak 61 had a molecular ion at m/z 285 with TR = 37.29 min. Its MS2 spectrum showed exhibition of neutral losses of CO (m/z 257) and CO2 (m/z 241) probably owing to the C ring. Another neutral loss concerns the C2H2O (m/z 199), in which cleavage occurs mainly on the C ring followed by a new cyclization implying the B ring. Moreover, the presence of a fragment at m/z 175 results from the losses of C3O2 then C2H2O, and supports the C-ring-localized cleavage for the C2H2O loss from the [M-H]− ion. This pattern of fragmentation is in concordance with that of luteolin [37].
- Peak 28 at TR= 24.23 min showing m/z 593 was identified as luteolin-O-rutinoside [47]. Its MS2 spectrum produced an ion at m/z 285, characteristic of the aglycone (luteolin) and due to a loss of hexoside and pentoside units.
- Peak 70 had a molecular ion at m/z 313 at TR = 41.95 min. Its MS2 spectrum gave a major ion at m/z 298, corresponding to luteolin genine. This compound was suggested as dimethoxyluteolin [48].
Acacetin derivatives
- Peak 49 showed [M-H]− at m/z 283 at TR = 30.60 min which yielded only a predominant fragment at m/z 239 due to a loss of CH3 and owing to the formation of a very stable anion radical structure. This phenolic compound was described previously and attributed to acacetin [41].
2.6.3. Characterization of Other Phenolic Compounds
- Peak 2 exhibited a molecular ion at m/z 325 and has been tentatively proposed as galloyl shikimic acid [49]. The MS2 spectrum of this compound generated a fragment at m/z 169, corresponding to the loss of shikimate moiety and the appearance of gallic acid.
- Peak 5 at TR = 13.42 min was identified as dihydroxyl glucosyl cyclohexane [50] with a molecular ion at m/z 293 and MS2 fragments at m/z: 173 [M-H-120]− (fragmentation in position 1–4 of the glycoside), 131 [M-H-C2H2O−] (the rest of sugar moiety) and 113 [M-H-18]− (dehydration), respectively.
- Peak 6 (TR = 13.69 min) assigned to a galloyl ester [51] generated a molecular ion at m/z 605. Its MS2 spectrum revealed a major fragment at m/z 453 [M-H-152]− (loss of galloyl) and a minor fragment at m/z 291 [M-H-162]− (loss of hexoside).
- Another peak 16 was proposed as methyl brevifolincarboxylate [27] at m/z 305. Its MS2 spectrum showed a fragment at m/z 273 suggesting the loss of CH3OH.
- Peaks 62 and 64 presented a molecular ion m/z 329 at TR = 39.54 and 39.83 min, respectively, and their MS2 fragment ions were m/z 311, 293, 229, 211, 171. Comparing with published data, these two compounds were identified as tricin. It is worth noting that these two compounds appeared at two different retention times, which allow us to suggest that they are two isomers of tricin [52].
It was reported that the main metabolites identified in the acetone extract of the aerial parts were tannins, which present more than 50% of the total identified compounds. The most abundant ellagitannin was geraniin and its hydrolyzed product corilagin.
In computational studies, the antioxidant activity of tannins increases when the number of galloyl groups and the molecular weight increase until the insolubility becomes a limiting factor.
Polyphenols get their biological activity from their phenolic hydroxy groups. The moderate acidity of the phenolic hydroxy group (pKa 8–12) is one of its most essential characteristics, as it allows it to easily donate hydrogen and produce negatively charged phenolate ions [53].

Table 8.
Identification of compounds from Erodium arborescens acetone extract by LC-HESI-MS2 (negative mode). The relative peak area indicates the contribution of each compound to all identified compounds in the extract, providing a measure of relative abundance.
Table 8.
Identification of compounds from Erodium arborescens acetone extract by LC-HESI-MS2 (negative mode). The relative peak area indicates the contribution of each compound to all identified compounds in the extract, providing a measure of relative abundance.
| Retention Time TR(Min) | Area (%) | [M-H]− | MS2 | Structure | Reference | |
|---|---|---|---|---|---|---|
| 1. | 8.08 | 0.04 | 169 | 125 | Gallic acid | [28] |
| 2. | 9.05 | 0.34 | 325 | 307/281/169(100)/125 | Galloyl shikimic acid I | [49] |
| 3. | 12.23 | 0.25 | 357 | 169(100)/125 | Unknown gallotannin | [54] |
| 4. | 12.88 | 1.51 | 495 | 343(100)/325 | 3,5-di-O-galloyl quinic acid | [26] |
| 5. | 13.42 | 1.66 | 293 | 131(100)/113/101 | Dihydroxyl glucosyl cyclohexane | [50] |
| 6. | 13.69 | 0.37 | 605 | 453(100)/435/393/291/273/247 | Galloyl ester | [51] |
| 7. | 14.55 | 0.51 | 631 | 613(100)/603/461 | Unidentified | |
| 8. | 15.47 | 2.19 | 291 | 247 | Brevifolin carboxylic acid | [31] |
| 9. | 15.51 | 2.181 | 247 | 247(100)/219 | Brevifolin carboxylate | |
| 10. | 16.84 | 10.28 | 633 | 463/301(100)/275 | Corilagin | [27] |
| 11. | 17.20 | 15.46 | 951 | 933(100) | Geraniin | [27,29] |
| 12. | 18.14 | 1.94 | 1109 | 1049(100)/973/935 | Ascorgeraniin | [1] |
| 13. | 18.83 | 1.96 | 563 | 545/503/473/443(100)/383/353 | 6-C-arabinosyl-8-C-glucosyl apigenin | [45] |
| 14. | 18.94 | 2.33 | 925 | 907/605/435/301(100) | Phyllanthusiin B/C | [33] |
| 15. | 19.37 | 1.13 | 969 | 951/925(100)/895/877/755/633/301 | Phyllanthusiin G | [1,34] |
| 16. | 19.93 | 3.10 | 305 | 273 | Methyl brevifolin carboxylate | [27] |
| 17. | 20.07 | 1.36 | 739 | 593 | Kaempferol-O-hexosyl-dirhamnoside | [40] |
| 18. | 20.28 | 0.62 | 563 | 545/503/473/443(100)/383/353 | 6-C-arabinosyl-8-C-glucosyl apigenin | [45] |
| 19. | 20.36 | 1.21 | 953 | 935(100)/909/801/651/633/463/301 | Geraniinic acid | [1,31] |
| 20. | 20.69 | 1.15 | 595 | 343/301(100) | Quercetin-O-arabinopyranosyl-galactopyranoside | [30] |
| 21. | 21.01 | 2.76 | 769 | 623 | Isorhamnetin-glycosyl-dirhamnoside | [55] |
| 22. | 21.22 | 1.71 | 965 | 933(100)/613/301 | Castalagin derivative | [36] |
| 23. | 22.37 | 14.99 | 991 | 973/933(100)/907/825/689/519/353/301 | Castalagin derivative | |
| 24. | 22.70 | 3.22 | 907 | 755/737/633(100)/587/435/291 | HDDP-decarboxy-valoneoyl-glucoside | [31] |
| 25. | 22.89 | 5.59 | 579 | 285 | Kaempferol-O-pentosyl-O-hexoside | [40] |
| 26. | 23.80 | 0.29 | 983 | 965/939(100)/911/769/681/633/493/467/301 | Corilagin derivative | |
| 27. | 24.03 | 0.43 | 963 | 945/878/811(100)/793/605/435/291 | Unidentified | |
| 28. | 24.23 | 1.23 | 593 | 285 | Luteolin-O-rutinoside | [56] |
| 29. | 24.29 | 0.77 | 623 | 357/315(100)/300/271 | Isorhamnetin-O-rutinoside | [57] |
| 30. | 24,59 | 0.44 | 917 | 873/721/445/301(100) | Unidentified | |
| 31. | 25.23 | 1.78 | 447 | 327/285(100)/255 | Kaempferol-O-glucoside | [38] |
| 32. | 25.59 | 1.32 | 951 | 907/737/649/587/479/435/335/301(100) | Geraniinic acid B/C (HHDP-valoneoyl-glucoside isomer) | [31] |
| 33. | 25.74 | 0.65 | 271 | 177/165/151(100) | Naringenin | [37,58] |
| 34. | 25.89 | 0.75 | 431 | 269 | Apigenin-O-hexoside | [46] |
| 35. | 25.98 | 0.36 | 965 | 933/795(100)/301 | Castalagin derivatives | [36] |
| 36. | 26.08 | 0.16 | 997 | 633(100)/363/301 | Punicatannin A/B | [32] |
| 37. | 26.33 | 0.38 | 909 | 877/739/615/437/301(100) | Unidentified | |
| 38. | 26.59 | 0.32 | 553 | 509/401(100) | 2,3-dihydro biapigenin methyl ether | [59] |
| 39. | 26.67 | 0.24 | 987 | 955(100)/653/483/301 | Ellagitannin derivative | |
| 40. | 26.84 | 0.47 | 923 | 879/825/621/577/451/407/353/301(100) | Unidentified | |
| 41. | 27.48 | 0.43 | 521 | 331(100)/271 | Unidentified | |
| 42. | 27.75 | 0.35 | 965 | 921/795(100)/493/301 | Unidentified | |
| 43. | 27.99 | 0.14 | 539 | 377(100)/307/275 | Oleuropein | [60] |
| 44. | 28.18 | 0.25 | 1005 | 973/915/301(100) | Unidentified | |
| 45. | 28.60 | 0.54 | 491 | 459/323/315(100) | Isorhamnetin-O-glucuronide | [43] |
| 46. | 28.89 | 0.22 | 489 | 327/285 (100)/255 | Kaempferol acetyl-hexoside | [39] |
| 47. | 29.69 | 0.67 | 523 | 523(100)/361/313//271/169 | Unidentified | |
| 48. | 30.44 | 0.16 | 987 | 943/685/641/515/301(100) | Unidentified | |
| 49. | 30.60 | 0.66 | 283 | 283(100)/239 | Acacetin | [41] |
| 50. | 31.24 | 0.07 | 679 | 517(100)/355 | Polysaccharide: Glc1 → 4Hex1 → 6Hex1 → 6Hex | [61] |
| 51. | 31.55 | 0.26 | 299 | 299/284(100)/271/255/240 | Rhamnocitrin | [58] |
| 52. | 31.73 | 0.24 | 965 | 795/301(100) | Unidentified | |
| 53. | 31.92 | 0.70 | 565 | 550/337/193(100)/175 | Unidentified | |
| 54. | 32.61 | 0.43 | 301 | 301(100)/273/257/179/151 | Quercetin dehydrate | [37] |
| 55. | 32.76 | 0.17 | 271 | 177/151(100) | Naringenin | [37] |
| 56. | 32.85 | 0.20 | 473 | 455/379/269(100) | Unidentified | |
| 57. | 32.95 | 0.12 | 461 | 446/315(100) | Dimethyl ellagic acid pentoside | [39] |
| 58. | 33.28 | 0.24 | 609 | 477/315(100)/301 | Isorhamnetin-O-pentosyl-hexoside | [44] |
| 59. | 33.80 | 0.47 | 605 | 452(100)/329/271 | Unidentified | |
| 60. | 33.99 | 0.21 | 563 | 548/518/337/235/193(100)/175 | Unidentified | |
| 61. | 37.29 | 0.29 | 285 | 257/241(100)/199/189/175 | Luteolin | [37] |
| 62. | 39.54 | 0.04 | 329 | 311/293/229(100)/211/171 | Tricin | [52] |
| 63. | 39.69 | 0.10 | 301 | 301/283/257/191/151/137(100) | Ellagic acid | [48]] |
| 64. | 39.83 | 0.15 | 329 | 311/293/229(100)/211/171 | Tricin | [52] |
| 65. | 40.46 | 0.85 | 357 | 357/339/285/151/109 | Unidentified | |
| 66. | 41.00 | 0.79 | 282 | No fragmentation | Unidentified | |
| 67. | 41.28 | 0.18 | 441 | 371(100)/369 | Unidentified | |
| 68. | 41.42 | 0.14 | 315 | 315/300(100)/271 | Isorhamnetin | [41,48] |
| 69. | 41.59 | 0.69 | 299 | No fragmentation | Unidentified | |
| 70. | 41.95 | 2.42 | 313 | 313/298(100) | Dimethoxyluteolin | [48] |
3. Materials and Methods
3.1. Collection of Plant Material
The aerial part (stems and leaves) of Erodium arborescens were collected in February 2020 from southeastern Tunisia, Sfax; 34°44′52.249″ N 10°45′58.187″ E. This plant was authenticated by Dr. Zouhair Bouallagi, Department of Biology, Faculty of Sciences of Sfax, and its voucher specimen (LCSN 153) was deposited in the Herbarium of the Laboratory of Organic Chemistry (LC17ES08), Department of Chemistry, Faculty of Sciences of Sfax; University of Sfax, Tunisia.
3.2. Extraction
The plant was harvested and then placed in shadow at 25 °C until it was completely dry. Exactly 1 kg of E. arborescens aerial part was milled and extracted three times by maceration during 24 h using different solvents of increasing polarity: hexane, ethyl acetate, acetone and methanol. The extraction yields were then determined using Equation (1):
Extraction Yield (%) = (Dry extract weight/dry starting material weight) × 100
3.3. Chemical Composition
All assays were carried out in triplicate and the results are expressed as mean values ± standard deviations. Higher absorbance indicates higher reducing power.
3.3.1. Determination of Total Phenolic Contents (TPC)
The Folin–Ciocalteu colorimetric method was used to determine total phenolic contents with slight modifications [62] Gallic acid was used as a standard phenolic compound by dissolving gallic acid (0.2 mg) in ethanol (1 mL) then diluting to prepare different concentrations. Briefly, 50 μL of each extract (1 mg·mL−1 in ethanol) were added to 2.5 mL of Folin–Ciocalteu reagent (10 times diluted). After 5 min, 2 mL of saturated sodium carbonate solution (75 g·L−1) were added. The mixtures were incubated at 30 °C for 1.5 h and the absorbance of the resulting solution was measured at 765 nm. The amounts of phenolic compounds in plant extracts were expressed as milligrams gallic acid equivalents per gram of dry extract (mg GAE/g DE).
3.3.2. Determination of Total Flavonoid Contents (TF)
Total flavonoid contents were determined by aluminum chloride colorimetric assay based on the formation of a complex flavonoid-aluminum with some modifications [63]. Quercetin solution (1 mg·mL−1) and its different concentrations were used to make the calibration curve. An amount of 0.25 mL of each extract was mixed with 75 µL of 5% NaNO2 for 6 min. After that, 75 µL of 10% AlCl3 was added to react for another 6 min. The reaction was stopped by addition of 500 µL of 4% NaOH and the total volume was topped up to 2.5 mL with 60% ethanol. The absorbance was measured after 15 min at 510 nm. Total flavonoid contents were expressed as milligrams quercetin equivalents per gram dry extract (mg QE/g DE).
3.4. Antioxidant Activity
The assays were carried out in triplicate and the results are expressed as mean values ± standard deviations. Higher absorbance indicates higher reducing power.
3.4.1. DPPH Antiradical Activity
This method was adapted as reported by Mhalla et al. [64]. Briefly, 50 μL of various concentrations (0.0625; 0.125; 0.25; 0.5 and 1 mg·mL−1) of each extract was added to 2 mL of a DPPH solution (0.04 g·L−1 in ethanol) followed by 30 min incubation in the dark at room temperature. Ascorbic acid was used as a positive control. The absorbance was measured at 517 nm using a spectrophotometer against the corresponding blank containing ethanol without DPPH solution. The percentage of inhibition (PI %) of DPPH radicals was calculated using the Equation (2):
IC50 was determined by extrapolation from linear regression analysis as the antioxidant concentration reducing 50% of DPPH free radicals.
The results were expressed as the antioxidant activity index (AAI) using the following Equation (3):
According to the AAI values, we considered:
- AAI < 0.5: poor antioxidant activity
- 0.5 ≤ AAI ≤ 1: moderate antioxidant activity
- 1.0 ≤ AAI ≤ 2.0: strong antioxidant activity
- AAI > 2.0: very strong antioxidant activity
- DPPH and IC50 are expressed in µg·mL−1.
3.4.2. Reducing Power Assay
The reducing power was determined according to the method of Ferreira et al. [65]. Testing solutions of each extract were prepared by mixing 2.5 mL of its different concentrations with 2.5 mL of 200 mmol·L−1 sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50 °C for 20 min, 2.5 mL of 10% trichloroacetic acid (w/v) were added followed by centrifugation at 650 rpm for 10 min. Next, 1 mL deionised water and 0.2 mL of 0.1% of ferric chloride were mixed with 1 mL of the upper layer. The absorbance was measured at 700 nm against a blank prepared with the same solution in which the extract was replaced with distilled water. Ascorbic acid was used as a positive control.
3.4.3. Total Antioxidant Capacity
Total antioxidant capacity was carried out according to Prieto et al. [66,67]. An aliquot of 0.1 mL of sample solution containing each extract was combined with 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The testing solution was incubated in a water bath at 95 °C for 90 min. After cooling at room temperature, the absorbance was measured at 695 nm against a blank containing 1 mL of reagent solution in which the extract had been replaced with the appropriate volume of the same solvent used for the sample. Gallic acid was used as standard in the assay.
3.5. Analysis of Individual Phenolic Compounds by Analytical LC–HESI–MS
Thermo Scientific LTQ XL MS was used to explore the composition of the aerial part acetone extract. The LC system was equipped with an electrospray ionization source (ESI). Spectra were recorded in negative ion mode, monitored and processed using Thermo Xcalibur Roadmap software. The VC-P10-A pump system, the VC-A12-A auto sampler and the VC-C10-A column were the main elements of the LC system. For analysis, Surveyor HPLC was provided with a C18 (2 µm, 150 mm × 2.1 mm) reversed phase Acclaim column (ThermoFisher) at 30 °C. The solvents were A (0.1% formic acid in water–ACN, 95–5, v/v) and B (0.1% formic acid in acetonitrile, v/v). The elution gradient established was from 0 to 40% of solvent B during 40 min, from 40 to 100% B over 2 min and maintained for 3 min before returning to initial conditions. The flow rate of the mobile phase was 0.2 mL·min−1, and the injection volume was 20 µL. High-purity nitrogen served as the nebulizer and auxiliary gas for the HESI source, and the capillary temperature was calibrated at 300 °C. The ion spray voltage was set at 3.5 V. The sheath and auxiliary gas were set at 50 and 5 psi, respectively. The acquisition range was from 50 to 1200 m/z. The method combined full scans and MS2 experiments using a collision energy ranging from 10 to 35 eV, depending on the molecular mass of compounds.
3.6. Antimicrobial Activity
3.6.1. Microorganisms, Media and Growth Conditions
Antimicrobial activity was determined against six indicator microorganisms which are: two Gram-positive bacteria Listeria monocytogenes (L. monocytogenes) ATCC 19117 and Staphylococcus aureus (S. aureus) ATCC6538; three Gram-negative bacteria Pseudomonas aeruginosa (P. aeruginosa) ATCC 49189, Salmonella enterica typhimurium (S. enterica-typhimurium) ATCC 14028 and Escherichia coli (E. coli) ATCC 8739; and the fungus Candida albicans (C. albicans) ATCC 10231. All these indicator cells were obtained from International Culture Collections (ATCC) and local culture collection of the Laboratory of Microbial Biotechnology and Enzyme Engineering of the Center of Biotechnology of Sfax-Tunisia.
The microorganisms were grown overnight in Luria Bertani (LB) medium (g·L−1: peptone 10; yeast extract 5 and NaCl 5, pH 7.2) under aerobic condition and constant agitation (200 rpm) at 30 °C for L. monocytogenes and S. enterica-typhimurium, at 37 °C for E. coli, S. aureus and P. aeruginosa. Then, they were diluted 1:100 in LB media and incubated for 5 h under constant agitation at the appropriate temperature. In addition, C. albicans was cultured at 30 °C on Sabouraud medium (g·L−1: dextrose 40, peptone 10, pH 5.6) under aerobic condition and constant agitation, then diluted 1:50 in the same medium and incubated for 5 h under agitation.
3.6.2. Agar Well Diffusion Method
For the determination of the antimicrobial activity of the extracts, the agar well diffusion method was employed according to Guven et al. (2006) [68]. Briefly, 15 mL of molten agar (45 °C) were poured into sterile Petri dishes (Ø 90 mm). A volume of 50 μL of 5 h-old culture of each tested bacteria and 100 μL of 5 h-old culture of the fungus C. albicans were evenly spread onto the surface of the agar plates of LB, using agar medium for bacteria and Sabouraud agar medium for C. albicans. Once the plates had been aseptically dried, 5 mm wells were punched into the agar with a sterile cork borer. Each extract was dissolved in dimethyl sulfoxide (DMSO–water, 1–9; v/v) to a final concentration of 1 mg·mL−1, filtered through 0.22 μm pore-size black polycarbonate filters (Millipore, Fontenay sous Bois, France), and finally 100 μL of each obtained extract solution was placed into the corresponding well. After staying at 4 °C for 2 h, the plates were incubated at the appropriate temperature during 24 h for bacterial strains, and during 48 h for C. albicans. The antimicrobial activity was assayed by measuring in mm the diameter of the inhibition zone formed around the wells.
3.6.3. Minimum Inhibitory Concentrations (MICs)
MICs of the extract solutions and the standards ampicillin, kanamycin and fluconazole (stock solutions at 20 mg·mL−1) were determined against four microorganisms: two Gram-positive bacteria L. monocytogenes and S. aureus, one Gram-negative bacterium S. enterica-typhimurium and one fungus C. albicans, following Sellem et al. [69]. The test was performed in sterile 96-well microplates with a final volume in microplate well of 100 μL. Extract solutions and standards were serially diluted with dimethyl sulfoxide (DMSO). To each test well, cell suspension was added to a final inoculum concentration of 106 colony forming unit (CFU)/mL of the studied indicator microorganism. The plates were then incubated at appropriate growth conditions of the corresponding indicator microorganism. The MIC was defined as the lowest concentration of the extract solution and standard at which the microorganism does not demonstrate visible growth after incubation. A volume of 25 μL of thiazolyl blue tetrazolium bromide (MTT) at 0.5 mg.mL−1 were added to the wells and incubated at room temperature for 30 min. The colorless tetrazolium salt acts as an electron acceptor and was reduced to a red-colored formazan product by the indicator microorganisms. When microbial growth was inhibited, the solution in the well remained clear after incubation with MTT.
For the antimicrobial activity determination (inhibition zones and CMIs), each experiment was carried out simultaneously three times under the same conditions. The obtained diameters of inhibition zones reported in mm of the three tests were quite similar and the reported results are the average of the three experiments. Concerning MICs values reported in µg·mL−1, the three analyses were identical.
4. Statistical Analysis
Replicate errors were in all cases < 10% (n = 3). The differences were analyzed using Duncan and Tukey’s post hoc tests for multiple comparisons with p < 0.05. The Statistical Product and Service Solutions program (SPSS) version 20 was used to analyze the differences and calculate the correlation coefficients R2 in order to highlight on the one hand, the correlation between the different phenolic contents of all extracts and their antioxidant activity, and the correlation between the different antioxidant activity tests, on the other.
5. Conclusions
This research presents an examination of the polyphenol composition of Erodium arborescens aerial part acetone extracts, and demonstrates the identification of fifty one compounds grouped into flavonoids, derivatives of phenolic acids and ellagitannins.
In addition, the study revealed that the antioxidant activity of the acetone and methanol extracts is in agreement with the highest amounts of phenolic and flavonoid contents, which exhibited the greatest antioxidant activity in the scavenging of DPPH free radical, ferric reducing antioxidant power (FRAP) and total antioxidant capacity (TAC) tests.
The obtained results yielded high antimicrobial activity, since most of the microbial strains selected for this study have an effective sensitivity to the tested extracts. Acetone and methanol extracts were demonstrated as inhibitors of L. monocytogenes, S. aureus, S. enterica typhimurium and C. albicans strains. The antimicrobial potential of the acetone extract may be associated with its richness of phenolic constituents, including corilagin and geraniin, according to LC/MS-MS analysis.
Finally, future studies should focus on the isolation of active compounds and the investigation of more pharmacological activities.
Author Contributions
S.S. and A.A. performed the plants extractions, the chemical experiments and the antioxidant activity. R.M.-J. designed the chemical experiments. N.A. helped in experimental work. S.S., M.T. and N.T. designed and performed the LC-HESI-MS experiments. L.M. and M.F. performed the experiments and wrote about the antimicrobial activity. The data was examined and the paper was verified by all of the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial support provided by the Tunisian Ministry of Higher Education and Scientific Research to the Laboratory of Organic Chemistry LR17ES08, University of Sfax, Tunisia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuominen, A. Tannins and other polyphenols in Geranium sylvaticum: Identification, intraplant distribution and biological activity. In Turun Yliopiston Julkaisuja—Annales Universitatis Turkuensi; Yliopisto: Turku, Finland, 2017; Volume 569, ISBN 978-951-29-7050-6. ISSN 2343-3175. [Google Scholar]
- Cheynier, V.; Tomas-Barberan, F.A.; Yoshida, K. Polyphenols: From plants to a variety of food and nonfood uses. J. Agric. Food Chem. 2015, 63, 7589–7594. [Google Scholar] [CrossRef]
- Nikitina, V.S.; Kuz, L.Y.; Melent, A.I.; Shendel, G.V. Antibacterial activity of polyphenolic compounds isolated from plants of Geraniaceae and Rosaceae families. Appl. Biochem. Microbiol. 2007, 43, 629–634. [Google Scholar] [CrossRef]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naggar, S.M.E. Numerical taxonomy of the genus Erodium L’Hérit. (Geraniaceae) in Egypt. Feddes Repert. 1991, 7–8, 535–540. [Google Scholar] [CrossRef]
- Hadidi, M.E.; Fayed, A.A.; Naggar, S.E. Systematic revision of Erodium (Geraniaceae) in Egypt. Plant Syst. Evol. 1984, 144, 307–314. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Alcántara, C.; Collado, M.C.; Garcia-Perez, J.V.; Saraiva, J.A.; Lopes, R.P.; Barbae, F.J.; Silva, L.d.P.; Sant’Ana, A.S.; Fierro, E.M.; et al. Ethnopharmacology, phytochemistry and biological activity of Erodium species: A review. Food Res. Int. 2019, 126, 108659. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Singh, S.; Kumar, S.; Arora, S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem. Toxicol. 2007, 45, 1216–1223. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Mustafa, Ö.; Mustafa, B.; Kubilay, G.; Reşat, A. Antioxidant/antiradical properties of microwave-assisted extracts of three wild edible mushrooms. Food Chem. 2014, 157, 323–331. [Google Scholar] [CrossRef]
- Bouzayani, B.; Koubaa, I.; Frikha, D.; Samet, S.; Ben Younes, A.; Chawech, R.; Maalej, S.; Allouche, N.; Mezghani Jarraya, R. Spectrometric analysis, phytoconstituents isolation and evaluation of in vitro antioxidant and antimicrobial activities of Tunisian Cistanche violacea (Desf). Chem. Pap. 2022, 76, 3031–3050. [Google Scholar] [CrossRef]
- Goebel, W.; Gonza, B.; Domi, G.; De Patoge, G. The objectives of the business intelligence project inetSoft webinar clin. Microbiol. Rev. 2013, 14, 584–640. [Google Scholar] [CrossRef]
- Jarraud, S. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- John, E.D.M.; Crump, A.; Luby, S.P. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Kim, J.; Sudbery, P. Candida albicans, a major human fungal pathogen. J. Microbiol. 2011, 49, 171–177. [Google Scholar] [CrossRef]
- Doerrler, W.T. Lipid trafficking to the outer membrane of Gram-negative bacteria. Mol. Microbiol. 2006, 60, 542–552. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Ahmad, A.F.; Ramis, E.S. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem. 2014, 7, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef]
- Adesina, S.K.; Idowu, O.; Ogundaini, A.O.; Oladimeji, H.; Olugbade, T.A.; Onawunmi, G.O.; Pais, M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Acalypha hispida. Phytother. Res. 2000, 14, 371–374. [Google Scholar] [CrossRef]
- Li, N.; Luo, M.; Fu, Y.; Zu, Y.; Wang, W.; Zhang, L.; Yao, L.P.; Zhao, C.J.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phytother. Res. 2012, 27, 1517–1523. [Google Scholar] [CrossRef]
- Haddock, E.A.; Gupta, R.K.; AL-ShafI, S.M.K.; Layden, K.; Haslam, E. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 2.1 Esters of (S)-hexahydroxydiphenic acid with D-glucopyranose (4C1). J. Chem. Soc. Perkin Trans. 1982, 21, 2525–2534. [Google Scholar] [CrossRef]
- Maldini, M.; Montoro, P.; Pizza, C. Phenolic compounds from Byrsonima crassifolia L. bark: Phytochemical investigation and quantitative analysis by LC-ESI MS/MS. J. Pharm. Biomed. Anal. 2011, 56, 1–6. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, P.; Bajpai, V.; Singh, A.; Srivastava, M.; Mishra, D.K.; Kumar, B. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind. Crops Prod. 2015, 69, 143–152. [Google Scholar] [CrossRef]
- Sprenger, R.; Da, F.; Cass, Q.B. Characterization of four Phyllanthus species using liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2013, 1291, 97–103. [Google Scholar] [CrossRef]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- Roxo, M.; Peixoto, H.; Wetterauer, P.; Lima, E.; Wink, M.; Soković, M. Piquiá Shells (Caryocar villosum): A fruit by-product with antioxidant and antiaging properties in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2020, 2020, 7590707. [Google Scholar] [CrossRef]
- Liu, Y.; Seeram, N.P. Liquid chromatography coupled with time-of-flight tandem mass spectrometry for comprehensive phenolic characterization of pomegranate fruit and flower extracts used as ingredients in botanical dietary supplements. J. Sep. Sci. 2018, 41, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Flavonoids and ellagitannins characterization, antioxidant and cytotoxic activities of Phyllanthus acuminatus Vahl. Plants 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kähkönen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.P.; Heinonen, M. Antioxidant activity of isolated ellagitannins from red raspberries and cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Dincheva, I.; Badjakov, I.; Kondakova, V.; Dobson, P.; McDougall, G.; Stewart, D. Identification of the phenolic components in bulgarian raspberry cultivars by LC-MS. Int. J. Agric. Sci. Res. 2013, 3, 127–138. [Google Scholar]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; De Hoffmann, E.J.Q.-L. Determination of flavone, flavonol and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.M.; El-Halawany, A.M.; Saleh, D.O.; Naggar, E.M.B.E.; El-Shabrawy, A.E.R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. Farm. 2015, 25, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Bubba, M.D.; Checchini, L.; Chiuminatto, U. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef]
- Lorenz, P.; Bunse, M.; Klaiber, I.; Conrad, J.; Laumann-Lipp, T.; Stintzing, F.C.; Kammerer, D.R. Comprehensive phytochemical characterization of herbal parts from kidney vetch (Anthyllis vulneraria L.) by LC/MSn and GC/MS. Chem. Biodivers. 2020, 17, e2000485. [Google Scholar] [CrossRef]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef]
- Li, J.; Kuang, G.; Chen, X.; Zeng, R. Identification of chemical composition of leaves and flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules 2016, 21, 947. [Google Scholar] [CrossRef] [Green Version]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, antimicrobial, antioxidant properties and effects on cell migration of phenolic compounds of selected transylvanian medicinal plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Dou, J.; Lee, V.S.Y.; Tzen, J.T.C.; Lee, M.R. Identification and comparison of phenolic compounds in the preparation of Oolong tea manufactured by semifermentation and drying processes. J. Agric. Food Chem. 2007, 55, 7462–7468. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography−negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar] [CrossRef] [Green Version]
- Abu-reidah, I.M.; Ali-shtayeh, M.S. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Chua, L.S.; Yap, K.C.; Jaganath, I.B. Comparison of total phenolic content, scavenging activity and HPLC-ESI-MS/MS profiles of both young and mature leaves and stems of Andrographis paniculata. Nat. Prod. Commun. 2013, 8, 1725–1729. [Google Scholar] [CrossRef] [Green Version]
- Fraternale, D.; Ricci, D.; Verardo, G.; Gorassini, A.; Stocchi, V.; Sestili, P. Activity of Vitis vinifera tendrils extract against phytopathogenic fungi. Nat. Prod. Commun. 2015, 10, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Agalar, H.G.; Çiftçi, A.G.; Göger, F.; Kırımer, N. Activity guided fractionation of Arum italicum miller tubers and the LC/MS-MS profiles. Rec. Nat. Prod. 2018, 12, 64–75. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Direct identification of phenolic constituents in Boldo Folium (Peumus boldus Mol.) infusions by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 443–449. [Google Scholar] [CrossRef]
- Parejo, I.; Jáuregui, O.; Viladomat, F.; Bastida, J.; Codina, C. Characterization of acylated flavonoid-O-glycosides and methoxylated flavonoids from Tagetes maxima by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2801–2810. [Google Scholar] [CrossRef]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.M.; Falcão, S.I.; Peres, A.M.; Domingues, M.R.M. Oleuropein/ligstroside isomers and their derivatives in Portuguese olive mill wastewaters. Food Chem. 2011, 129, 291–296. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, L.; Liang, J.; Yang, B.; Kuang, H. Chromatography and mass spectrometry-based approaches for perception of polysaccharides in wild and cultured fruit bodies of Auricularia auricular-judae. Int. J. Biol. Macromol. 2019, 137, 1232–1244. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Li, J.E.; Fan, S.T.; Qiu, Z.H.; Li, C.; Nie, S.P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT Food Sci. Technol. 2015, 64, 1022–1027. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouassida, K.Z.; Chawech, R.; Bouaziz, A.; Makni, S.; Jlail, L.; Tounsi, S.; Mezghani Jarraya, R.; Trigui, M. Antioxidant, hepatoprotective and antidepression effects of Rumex tingitanus extracts and identification of a novel bioactive compound. Biomed. Res. Int. 2018, 2018, 7295848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Affes, S.; Ben Younes, A.; Frikha, D.; Allouche, N.; Treilhou, M.; Tene, N.; Mezghani-Jarraya, R. ESI-MS/MS analysis of phenolic compounds from Aeonium arboreum leaf extracts and evaluation of their antioxidant and antimicrobial activities. Molecules 2021, 26, 4338. [Google Scholar] [CrossRef]
- Güven, K.; Yücel, E.; Cetintaş, F. Antimicrobial activities of fruits of Crataegus and Pyrus species. Pharm. Biol. 2006, 44, 79–83. [Google Scholar] [CrossRef]
- Sellem, I.; Kaaniche, F.; Mtibaa, A.C.; Mellouli, L. Anti-oxidant, antimicrobial and anti-acetylcholinesterase activities of organic extracts from aerial parts of three tunisian plants and correlation with polyphenols and flavonoids contents. Bangladesh J. Pharmacol. 2016, 11, 531–544. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).