Natural Dietary Compounds in the Treatment of Arsenic Toxicity
Abstract
1. Introduction
2. Main Cellular Targets Vulnerable to Arsenic Inhibition
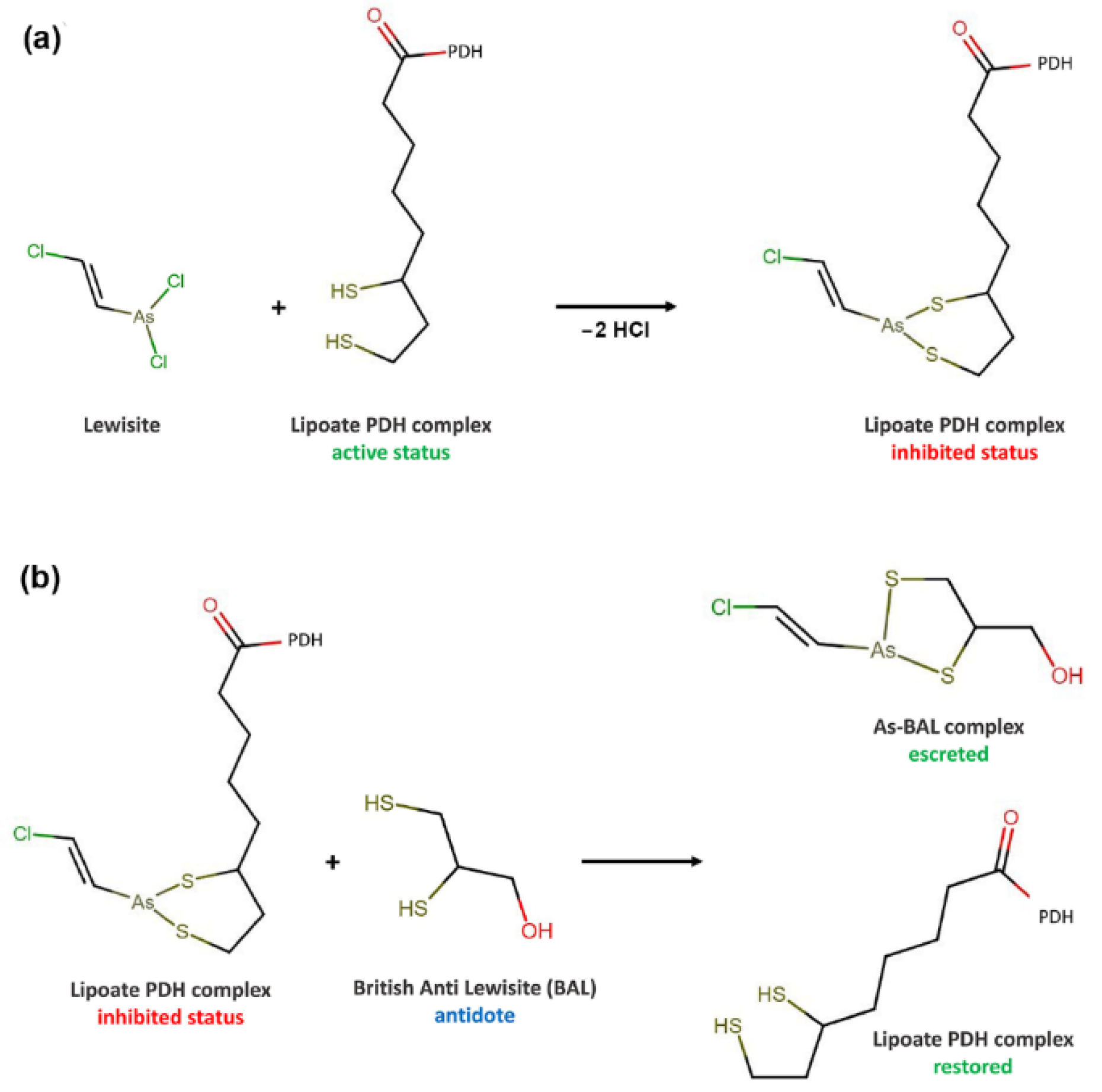
2.1. Pyruvate Dehydrogenase
2.2. Glutathione and Glutathione-Related Enzymes
2.3. Thioredoxin and Thioredoxin Reductase
2.4. Selenoproteins
2.5. Zinc-Finger Proteins
3. Approaches to the Treatment of Diseases Caused by Toxic Effects of Arsenic
3.1. Nutritional Interventions in Arsenic Toxicity/Poisoning
3.2. Natural Compounds with an Ameliorative Effect on Arsenic Toxicity
3.3. Selenium
3.4. Zinc
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| As | arsenic |
| BAL | British Anti-Lewisite |
| CAT | catalase |
| iAsIII | inorganic As(III) |
| MeAs(OH)2, MMAIII | monomethylarsonous acid |
| Me2AsOH, DMAIII | dimethylarsinous acid |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| GPX | glutathione peroxidases |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| GSTs | glutathione S-transferase |
| PARP | poly (ADP-ribose) polymerase |
| PDH | pyruvate dehydrogenase |
| SAM | S-adenosylmethionine |
| Se | Selenium |
| SOD | superoxide dismutase |
| Trx | thioredoxin |
| TrxR | thioredoxin reductase |
References
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Bjorklund, G.; Oliinyk, P.; Lysiuk, R.; Rahaman, M.S.; Antonyak, H.; Lozynska, I.; Lenchyk, L.; Peana, M. Arsenic intoxication: General aspects and chelating agents. Arch. Toxicol. 2020, 94, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Brinkel, J.; Khan, M.H.; Kraemer, A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int. J. Environ. Res. Public Health 2009, 6, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K. Arsenic contamination awareness among the rural residents in Bangladesh. Soc. Sci. Med. 2004, 59, 1741–1755. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Centeno, J.A.; Patlolla, A.K. Arsenic toxicity, mutagenesis, and carcinogenesis—A health risk assessment and management approach. Mol. Cell. Biochem. 2004, 255, 47–55. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Chatterjee, A.; Das, D.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K.; et al. Groundwater arsenic contamination and its health effects in India. Hydrogeol. J. 2017, 25, 1165–1181. [Google Scholar] [CrossRef]
- Chouhan, S.; Flora, S.J. Arsenic and fluoride: Two major ground water pollutants. Indian J. Exp. Biol. 2010, 48, 666–678. [Google Scholar]
- Schoof, R.A.; Yost, L.J.; Eickhoff, J.; Crecelius, E.A.; Cragin, D.W.; Meacher, D.M.; Menzel, D.B. A market basket survey of inorganic arsenic in food. Food Chem. Toxicol. 1999, 37, 839–846. [Google Scholar] [CrossRef]
- Samal, A.C.; Kar, S.; Bhattacharya, P.; Santra, S.C. Human exposure to arsenic through foodstuffs cultivated using arsenic contaminated groundwater in areas of West Bengal, India. J. Environ. Sci. Heal. Part A 2011, 46, 1259–1265. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Schuhmacher-Wolz, U.; Dieter, H.H.; Klein, D.; Schneider, K. Oral exposure to inorganic arsenic: Evaluation of its carcinogenic and non-carcinogenic effects. Crit. Rev. Toxicol. 2009, 39, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Moon, K.A.; Wang, S.L.; Silbergeld, E.; Navas-Acien, A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Barchowsky, A.; Wu, F. The global burden of disease for skin, lung, and bladder cancer caused by arsenic in food. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Vahidnia, A.; van der Voet, G.B.; de Wolff, F.A. Arsenic neurotoxicity—A review. Hum. Exp. Toxicol. 2007, 26, 823–832. [Google Scholar] [CrossRef]
- Guha Mazumder, D.N. 6—Health Effects Chronic Arsenic Toxicity. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2015; pp. 137–177. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef]
- Dey, T.K.; Banerjee, P.; Bakshi, M.; Kar, A.; Ghosh, S. Groundwater Arsenic Contamination in West Bengal: Current Scenario, Effects and Probable Ways of Mitigation. Int. Lett. Nat. Sci. 2014, 13, 45–58. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Khan, M.H.; Haque, M. Arsenic contamination in groundwater in Bangladesh: Implications and challenges for healthcare policy. Risk Manag. Healthc Policy 2018, 11, 251–261. [Google Scholar] [CrossRef]
- Bjorklund, G.; Mutter, J.; Aaseth, J. Metal chelators and neurotoxicity: Lead, mercury, and arsenic. Arch. Toxicol. 2017, 91, 3787–3797. [Google Scholar] [CrossRef]
- Kosnett, M.J. The role of chelation in the treatment of arsenic and mercury poisoning. J. Med. Toxicol. 2013, 9, 347–354. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Crespo-Alonso, M.; Zoroddu, M.A.; Peana, M. Kill or cure: Misuse of chelation therapy for human diseases. Coord. Chem. Rev. 2015, 284, 278–285. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjorklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Susan, A.; Rajendran, K.; Sathyasivam, K.; Krishnan, U.M. An overview of plant-based interventions to ameliorate arsenic toxicity. Biomed. Pharmacother. 2019, 109, 838–852. [Google Scholar] [CrossRef]
- Khandker, S.; Dey, R.K.; Islam, A.Z.M.M.; Ahmad, S.A.; Al-Mahmud, I. Arsenic-safe drinking water and antioxidants for the management of arsenicosis patients. Bangladesh J. Pharmacol. 2006, 1, 42–50. [Google Scholar] [CrossRef]
- Yu, H.; Liu, S.; Li, M.; Wu, B. Influence of diet, vitamin, tea, trace elements and exogenous antioxidants on arsenic metabolism and toxicity. Env. Geochem. Health 2016, 38, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm. Biol. 2017, 55, 349–354. [Google Scholar] [CrossRef]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef]
- Sharma, A.; Flora, S.J.S. Nutritional management can assist a significant role in alleviation of arsenicosis. J. Trace Elem. Med. Biol. 2018, 45, 11–20. [Google Scholar] [CrossRef]
- Deb, D.; Biswas, A.; Ghose, A.; Das, A.; Majumdar, K.K.; Guha Mazumder, D.N. Nutritional deficiency and arsenical manifestations: A perspective study in an arsenic-endemic region of West Bengal, India. Public Health Nutr. 2013, 16, 1644–1655. [Google Scholar] [CrossRef]
- Mehrandish, R.; Rahimian, A.; Shahriary, A. Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J. Herbmed Pharmacol. 2019, 8, 69–77. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhattacharyya, S.S.; Bhattacharjee, N.; Pathak, S.; Boujedaini, N.; Belon, P.; Khuda-Bukhsh, A.R. Ascorbic acid combats arsenic-induced oxidative stress in mice liver. Ecotoxicol. Environ. Saf. 2009, 72, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Lysiuk, R. Flavonoids as detoxifying and pro-survival agents: What’s new? Food Chem. Toxicol. 2017, 110, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Golembiovska, O.; Hudz, N.; Wieczorek, P.P. Phenolic compounds of herbal infusions obtained from some species of the family. Curr. Issues Pharm. Med. Sci. 2018, 31, 194–199. [Google Scholar] [CrossRef]
- Raihan, S.Z.; Chowdhury, A.K.; Rabbani, G.H.; Marni, F.; Ali, M.S.; Nahar, L.; Sarker, S.D. Effect of aqueous extracts of black and green teas in arsenic-induced toxicity in rabbits. Phytother. Res. 2009, 23, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.H.; Saha, S.K.; Akhtar, M.; Marni, F.; Mitra, A.K.; Ahmed, S.; Alauddin, M.; Bhattacharjee, M.; Sultana, S.; Chowdhury, A.K. Antioxidants in detoxification of arsenic-induced oxidative injury in rabbits: Preliminary results. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hossain, K.F.B.; Banik, S.; Sikder, M.T.; Akter, M.; Bondad, S.E.C.; Rahaman, M.S.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review. Ecotoxicol. Environ. Saf. 2019, 168, 146–163. [Google Scholar] [CrossRef]
- Zhou, X.; Speer, R.M.; Volk, L.; Hudson, L.G.; Liu, K.J. Arsenic co-carcinogenesis: Inhibition of DNA repair and interaction with zinc finger proteins. Semin. Cancer Biol. 2021, 76, 86–98. [Google Scholar] [CrossRef]
- Zhou, X.; Cooper, K.L.; Sun, X.; Liu, K.J.; Hudson, L.G. Selective Sensitization of Zinc Finger Protein Oxidation by Reactive Oxygen Species through Arsenic Binding. J. Biol. Chem. 2015, 290, 18361–18369. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, X.; Liu, W.; Sun, X.; Chen, C.; Hudson, L.G.; Jian Liu, K. Arsenite-induced ROS/RNS generation causes zinc loss and inhibits the activity of poly(ADP-ribose) polymerase-1. Free Radic. Biol. Med. 2013, 61, 249–256. [Google Scholar] [CrossRef]
- Saintilnord, W.N.; Fondufe-Mittendorf, Y. Arsenic-induced epigenetic changes in cancer development. Semin. Cancer Biol. 2021, 76, 195–205. [Google Scholar] [CrossRef]
- Wadgaonkar, P.; Chen, F. Connections between endoplasmic reticulum stress-associated unfolded protein response, mitochondria, and autophagy in arsenic-induced carcinogenesis. Semin. Cancer Biol. 2021, 76, 258–266. [Google Scholar] [CrossRef]
- Peters, R.A.; Stocken, L.A.; Thompson, R.H. British anti-lewisite (BAL). Nature 1945, 156, 616–619. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Medici, S.; Cappai, R.; Nurchi, V.M.; Zoroddu, M.A. Metal Toxicity and Speciation: A Review. Curr. Med. Chem. 2021, 28, 7190–7208. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala-Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Chouchane, S.; Snow, E.T. In vitro effect of arsenical compounds on glutathione-related enzymes. Chem. Res. Toxicol. 2001, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Del Razo, L.M.; Limon-Pacheco, J.H.; Giordano, M.; Sanchez-Pena, L.C.; Uribe-Querol, E.; Gutierrez-Ospina, G.; Gonsebatt, M.E. Glutathione reductase inhibition and methylated arsenic distribution in Cd1 mice brain and liver. Toxicol. Sci. 2005, 84, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; White, C.C.; Cox, D.P.; Chan, J.Y.; Kavanagh, T.J.; Fausto, N.; Franklin, C.C. Distinct Nrf1/2-independent mechanisms mediate As 3+-induced glutamate-cysteine ligase subunit gene expression in murine hepatocytes. Free Radic. Biol. Med. 2009, 46, 1614–1625. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Tam, L.M.; Wang, Y. Arsenic Exposure and Compromised Protein Quality Control. Chem. Res. Toxicol. 2020, 33, 1594–1604. [Google Scholar] [CrossRef]
- Bjorklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Yan, H.; Lou, M.F.; Fernando, M.R.; Harding, J.J. Thioredoxin, thioredoxin reductase, and alpha-crystallin revive inactivated glyceraldehyde 3-phosphate dehydrogenase in human aged and cataract lens extracts. Mol. Vis. 2006, 12, 1153–1159. [Google Scholar] [PubMed]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, J.; Liu, Q.; Peng, H.; Popowich, A.; Wang, Z.; Li, X.F.; Le, X.C. p-Azidophenylarsenoxide: An Arsenical "Bait" for the In Situ Capture and Identification of Cellular Arsenic-Binding Proteins. Angew. Chem. Int. Ed. Engl. 2016, 55, 14051–14056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Santo, A.; Li, Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp. Biol. Med. 2012, 237, 143–149. [Google Scholar] [CrossRef]
- O'Leary, P.C.; Terrile, M.; Bajor, M.; Gaj, P.; Hennessy, B.T.; Mills, G.B.; Zagozdzon, A.; O’Connor, D.P.; Brennan, D.J.; Connor, K.; et al. Peroxiredoxin-1 protects estrogen receptor α from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014, 16, R79. [Google Scholar] [CrossRef] [PubMed]
- Pannala, V.R.; Dash, R.K. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Free Radic. Biol. Med. 2015, 78, 42–55. [Google Scholar] [CrossRef]
- Carballal, S.; Bartesaghi, S.; Radi, R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim. Biophys. Acta 2014, 1840, 768–780. [Google Scholar] [CrossRef]
- Hattori, F.; Murayama, N.; Noshita, T.; Oikawa, S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem. 2003, 86, 860–868. [Google Scholar] [CrossRef]
- Kim, S.U.; Jin, M.H.; Kim, Y.S.; Lee, S.H.; Cho, Y.S.; Cho, K.J.; Lee, K.S.; Kim, Y.I.; Kim, G.W.; Kim, J.M.; et al. Peroxiredoxin II preserves cognitive function against age-linked hippocampal oxidative damage. Neurobiol. Aging 2011, 32, 1054–1068. [Google Scholar] [CrossRef]
- O'Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chew, E.H.; Holmgren, A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-F.; Zheng, B.-L.; Bai, J.; Jiang, L.-P.; Zheng, Y.; Qi, B.-X.; Geng, C.-Y.; Zhong, L.-F.; Yang, G.; Chen, M.; et al. Low-level sodium arsenite induces apoptosis through inhibiting TrxR activity in pancreatic β-cells. Environ. Toxicol. Pharmacol. 2015, 40, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Raefsky, S.M.; Mattson, M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017, 102, 203–216. [Google Scholar] [CrossRef]
- Dikiy, A.; Novoselov, S.V.; Fomenko, D.E.; Sengupta, A.; Carlson, B.A.; Cerny, R.L.; Ginalski, K.; Grishin, N.V.; Hatfield, D.L.; Gladyshev, V.N. SelT, SelW, SelH, and Rdx12: Genomics and Molecular Insights into the Functions of Selenoproteins of a Novel Thioredoxin-like Family. Biochemistry 2007, 46, 6871–6882. [Google Scholar] [CrossRef]
- Jeong, D.-w.; Kim, T.S.; Chung, Y.W.; Lee, B.J.; Kim, I.Y. Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett. 2002, 517, 225–228. [Google Scholar] [CrossRef]
- Chung, Y.W.; Jeong, D.; Noh, O.J.; Park, Y.H.; Kang, S.I.; Lee, M.G.; Lee, T.H.; Yim, M.B.; Kim, I.Y. Antioxidative role of selenoprotein W in oxidant-induced mouse embryonic neuronal cell death. Mol. Cells 2009, 27, 609–613. [Google Scholar] [CrossRef]
- Gu, Q.P.; Sun, Y.; Ream, L.W.; Whanger, P.D. Selenoprotein W accumulates primarily in primate skeletal muscle, heart, brain and tongue. Mol. Cell. Biochem. 2000, 204, 49–56. [Google Scholar] [CrossRef]
- Christophersen, O. Coronary Artery Disease: 2011 Update. In Proceedings of the 9th International Congress on Coronary Artery Disease, Venice, Italy, 23–26 October 2011; Lewis, B.S., Flugelman, M.Y., Halon, D.A., Eds.; Medimond International Proceedings: Bologna, Italy, 2011; pp. 69–73. ISBN 978-88-7587-619-76. [Google Scholar]
- Brigelius-Flohe, R. The evolving versatility of selenium in biology. Antioxid. Redox Signal. 2015, 23, 757–760. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Fomenko, D.E.; Labunskyy, V.M.; Lachke, S.A.; Qiu, W.; Moncaster, J.A.; Zhang, J.; Wojnarowicz, M.W., Jr.; Natarajan, S.K.; Malinouski, M.; et al. Roles of the 15-kDa selenoprotein (Sep15) in redox homeostasis and cataract development revealed by the analysis of Sep 15 knockout mice. J. Biol. Chem. 2011, 286, 33203–33212. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Ntoupa, P.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschella, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Kandias, N.G.; Chasapis, C.T.; Bentrop, D.; Episkopou, V.; Spyroulias, G.A. High yield expression and NMR characterization of Arkadia E3 ubiquitin ligase RING-H2 finger domain. Biochem. Biophys. Res. Commun. 2009, 378, 498–502. [Google Scholar] [CrossRef]
- Hartwig, A.; Pelzer, A.; Asmuss, M.; Bürkle, A. Very low concentrations of arsenite suppress poly(ADP-ribosyl)ation in mammalian cells. Int. J. Cancer 2003, 104, 1–6. [Google Scholar] [CrossRef]
- Tam, L.M.; Price, N.E.; Wang, Y. Molecular Mechanisms of Arsenic-Induced Disruption of DNA Repair. Chem. Res. Toxicol. 2020, 33, 709–726. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, X.; Cooper, K.L.; Wang, F.; Liu, K.J.; Hudson, L.G. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J. Biol. Chem. 2011, 286, 22855–22863. [Google Scholar] [CrossRef]
- Yager, J.W.; Wiencke, J.K. Inhibition of poly(ADP-ribose) polymerase by arsenite. Mutat. Res. Rev. Mutat. Res. 1997, 386, 345–351. [Google Scholar] [CrossRef]
- Borszekova Pulzova, L.; Ward, T.A.; Chovanec, M. XPA: DNA Repair Protein of Significant Clinical Importance. Int. J. Mol. Sci. 2020, 21, 2182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Paramasivam, M.; Cai, Q.; Dai, X.; Wang, P.; Lin, K.; Song, J.; Seidman, M.M.; Wang, Y. Arsenite binds to the RING finger domains of RNF20-RNF40 histone E3 ubiquitin ligase and inhibits DNA double-strand break repair. J. Am. Chem. Soc. 2014, 136, 12884–12887. [Google Scholar] [CrossRef] [PubMed]
- Birkou, M.; Chasapis, C.T.; Marousis, K.D.; Loutsidou, A.K.; Bentrop, D.; Lelli, M.; Herrmann, T.; Carthy, J.M.; Episkopou, V.; Spyroulias, G.A. A Residue Specific Insight into the Arkadia E3 Ubiquitin Ligase Activity and Conformational Plasticity. J. Mol. Biol. 2017, 429, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bellani, M.; Li, L.; Wang, P.; Seidman, M.M.; Wang, Y. Arsenite Binds to the RING Finger Domain of FANCL E3 Ubiquitin Ligase and Inhibits DNA Interstrand Crosslink Repair. ACS Chem. Biol. 2017, 12, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Medina, S.; Bolt, A.M.; Zhang, H.; Wan, G.; Xu, H.; Lauer, F.T.; Wang, S.C.; Burchiel, S.W.; Liu, K.J. Inhibition of red blood cell development by arsenic-induced disruption of GATA-1. Sci. Rep. 2020, 10, 19055. [Google Scholar] [CrossRef] [PubMed]
- Bondesson, M.; Hao, R.; Lin, C.Y.; Williams, C.; Gustafsson, J.A. Estrogen receptor signaling during vertebrate development. Biochim. Biophys. Acta 2015, 1849, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kitchin, K.T.; Wallace, K. Arsenite binding to synthetic peptides based on the Zn finger region and the estrogen binding region of the human estrogen receptor-alpha. Toxicol. Appl. Pharmacol. 2005, 206, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Hasan, Z.; Shahidullah, S.M.; Sharmin, S.; Jakariya, M.D.; Rahman, M.; Dear, K.; Smith, W. Association between nutritional status and arsenicosis due to chronic arsenic exposure in Bangladesh. Int. J. Environ. Health Res. 2004, 14, 99–108. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Manna, S.K.; Roy, S.K.; Chakraborty, M.; Das, S.; Naskar, J.P. Plasma-aminothiols status and inverse correlation of total homocysteine with B-vitamins in arsenic exposed population of West Bengal, India. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2016, 51, 962–973. [Google Scholar] [CrossRef]
- Vahter, M. Health effects of early life exposure to arsenic. Basic Clin. Pharmacol. Toxicol. 2008, 102, 204–211. [Google Scholar] [CrossRef]
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef]
- Depner, C.M.; Torres-Gonzalez, M.; Tripathy, S.; Milne, G.; Jump, D.B. Menhaden oil decreases high-fat diet-induced markers of hepatic damage, steatosis, inflammation, and fibrosis in obese Ldlr-/- mice. J. Nutr. 2012, 142, 1495–1503. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Saravanan, R. Protective effects of azelaic acid against high-fat diet-induced oxidative stress in liver, kidney and heart of C57BL/6J mice. Mol. Cell. Biochem. 2013, 377, 23–33. [Google Scholar] [CrossRef]
- Dutta, M.; Ghosh, D.; Ghosh, A.K.; Bose, G.; Chattopadhyay, A.; Rudra, S.; Dey, M.; Bandyopadhyay, A.; Pattari, S.K.; Mallick, S.; et al. High fat diet aggravates arsenic induced oxidative stress in rat heart and liver. Food Chem. Toxicol. 2014, 66, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 897484. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, D.; Raisuddin, S.; Sahu, A.P. Genotoxic effects of arsenic: Prevention by functional food-jaggery. Cancer Lett. 2008, 268, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009, 235, 338–350. [Google Scholar] [CrossRef]
- Sinha, D.; Roy, S.; Roy, M. Antioxidant potential of tea reduces arsenite induced oxidative stress in Swiss albino mice. Food Chem. Toxicol. 2010, 48, 1032–1039. [Google Scholar] [CrossRef]
- Herrera, A.; Pineda, J.; Antonio, M.T. Toxic effects of perinatal arsenic exposure on the brain of developing rats and the beneficial role of natural antioxidants. Environ. Toxicol. Pharmacol. 2013, 36, 73–79. [Google Scholar] [CrossRef]
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Das, B.; Chaudhuri, K. Amelioration of sodium arsenite induced toxicity by diallyl disulfide, a bioactive component of garlic: The involvement of antioxidants and the chelate effect. RSC Adv. 2014, 4, 20964–20973. [Google Scholar] [CrossRef]
- Vithanage, M.; Dabrowska, B.B.; Mukherjee, A.B.; Sandhi, A.; Bhattacharya, P. Arsenic uptake by plants and possible phytoremediation applications: A brief overview. Environ. Chem. Lett. 2012, 10, 217–224. [Google Scholar] [CrossRef]
- Ogra, Y.; Awaya, Y.; Anan, Y. Comparison of accumulation of four metalloids in Allium sativum. Bull. Environ. Contam. Toxicol. 2015, 94, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: Uptake, human health risk and remedial effects of biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef] [PubMed]
- Adegboyega, A.; Odunola, O. The modulatory effects of aqueous extracts of Viscum album and garlic on sodium arsenite induced toxicity in Wistar albino rat. J. Chem. Pharm. Res. 2012, 4, 4698–4701. [Google Scholar]
- Sheikh, A.; Yeasmin, F.; Agarwal, S.; Rahman, M.; Islam, K.; Hossain, E.; Hossain, S.; Karim, M.R.; Nikkon, F.; Saud, Z.A.; et al. Protective effects of Moringa oleifera Lam. leaves against arsenic-induced toxicity in mice. Asian Pac. J. Trop. Biomed. 2014, 4, S353–S358. [Google Scholar] [CrossRef] [PubMed]
- Barai, M.; Ahsan, N.; Paul, N.; Hossain, K.; Abdur Rashid, M.; Kato, M.; Ohgami, N.; Azim Akhand, A. Amelioration of arsenic-induced toxic effects in mice by dietary supplementation of Syzygium cumini leaf extract. Nagoya J. Med. Sci. 2017, 79, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.; Ahsan, N.; Kato, M.; Ohgami, N.; Rashid, A.; Akhand, A.A. Protective effects of Phyllanthus emblica leaf extract on sodium arsenite-mediated adverse effects in mice. Nagoya J. Med. Sci. 2015, 77, 145–153. [Google Scholar]
- Dua, T.K.; Dewanjee, S.; Gangopadhyay, M.; Khanra, R.; Zia-Ul-Haq, M.; De Feo, V. Ameliorative effect of water spinach, Ipomea aquatica (Convolvulaceae), against experimentally induced arsenic toxicity. J. Transl. Med. 2015, 13, 81. [Google Scholar] [CrossRef]
- Sharmila Banu, G.; Kumar, G.; Murugesan, A.G. Effects of leaves extract of Ocimum sanctum L. on arsenic-induced toxicity in Wistar albino rats. Food Chem. Toxicol. 2009, 47, 490–495. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, M.K.; Kumar, M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of Swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2007, 100, 249–257. [Google Scholar] [CrossRef]
- Shanaida, M. Antioxidant activity of essential oils obtained from aerial part of some Lamiaceae species. Int. J. Green Pharm. (IJGP) 2018, 12, 200–204. [Google Scholar]
- Acharyya, N.; Chattopadhyay, S.; Maiti, S. Chemoprevention against arsenic-induced mutagenic DNA breakage and apoptotic liver damage in rat via antioxidant and SOD1 upregulation by green tea (Camellia sinensis) which recovers broken DNA resulted from arsenic-H2O2 related in vitro oxidant stress. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 338–361. [Google Scholar] [CrossRef]
- Zagayko, A.; Senuyk, I.; Lenchyk, L.; Galimullin, R. Study of antioxidant activity of the extract from plum ordinary leaves. Ukr. Biopharm. J. 2014, 1, 25–28. [Google Scholar]
- Eliaz, I.; Hotchkiss, A.T.; Fishman, M.L.; Rode, D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother. Res. 2006, 20, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, V.P.; Girija, S.; Soumya, R.S.; Raghu, K.G. Polyphenol-rich apple (Malus domestica L.) peel extract attenuates arsenic trioxide induced cardiotoxicity in H9c2 cells via its antioxidant activity. Food Funct. 2014, 5, 502–511. [Google Scholar] [CrossRef]
- Khatun, S.; Maity, M.; Perveen, H.; Dash, M.; Chattopadhyay, S. Spirulina platensis ameliorates arsenic-mediated uterine damage and ovarian steroidogenic disorder. FACETS 2018, 3, 736–753. [Google Scholar] [CrossRef]
- Muthumani, M.; Prabu, S.M. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol. Mech. Methods 2012, 22, 277–288. [Google Scholar] [CrossRef]
- Ghosh, S.; Mishra, R.; Biswas, S.; Bhadra, R.K.; Mukhopadhyay, P.K. alpha-Lipoic Acid Mitigates Arsenic-Induced Hematological Abnormalities in Adult Male Rats. Iran. J. Med. Sci. 2017, 42, 242–250. [Google Scholar]
- Dwivedi, N.; Flora, G.; Kushwaha, P.; Flora, S.J. Alpha-lipoic acid protects oxidative stress, changes in cholinergic system and tissue histopathology during co-exposure to arsenic-dichlorvos in rats. Environ. Toxicol. Pharmacol. 2014, 37, 7–23. [Google Scholar] [CrossRef]
- Mershiba, S.D.; Dassprakash, M.V.; Saraswathy, S.D. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol. Biol. Rep. 2013, 40, 3681–3691. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Rajani, C.V.; Sivaram, M.; Selvaraju, S. Protective role of epigallocatechin-3-gallate on arsenic induced testicular toxicity in Swiss albino mice. Biomed. Pharmacother. 2017, 96, 685–694. [Google Scholar] [CrossRef]
- Yu, N.H.; Pei, H.; Huang, Y.P.; Li, Y.F. (-)-Epigallocatechin-3-Gallate Inhibits Arsenic-Induced Inflammation and Apoptosis through Suppression of Oxidative Stress in Mice. Cell. Physiol. Biochem. 2017, 41, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Liu, Z.; Qi, Z.J.; Huang, Y.P.; Gao, X.Q.; Zhang, Y.Y. (-)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem. Toxicol. 2016, 93, 102–110. [Google Scholar] [CrossRef]
- Shinkai, Y.; Sumi, D.; Fukami, I.; Ishii, T.; Kumagai, Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006, 580, 1771–1774. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, S.; Lian, F.; Chau, B.T.; Chen, J.; Sun, G.; Fang, D.; Lantz, R.C.; Zhang, D.D. Sulforaphane prevents pulmonary damage in response to inhaled arsenic by activating the Nrf2-defense response. Toxicol. Appl. Pharmacol. 2012, 265, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Thangapandiyan, S.; Ramesh, M.; Hema, T.; Miltonprabu, S.; Uddin, M.S.; Nandhini, V.; Bavithra Jothi, G. Sulforaphane Potentially Ameliorates Arsenic Induced Hepatotoxicity in Albino Wistar Rats: Implication of PI3K/Akt/Nrf2 Signaling Pathway. Cell. Physiol. Biochem. 2019, 52, 1203–1222. [Google Scholar] [CrossRef]
- Yang, D.; Lv, Z.; Zhang, H.; Liu, B.; Jiang, H.; Tan, X.; Lu, J.; Baiyun, R.; Zhang, Z. Activation of the Nrf2 Signaling Pathway Involving KLF9 Plays a Critical Role in Allicin Resisting Against Arsenic Trioxide-Induced Hepatotoxicity in Rats. Biol. Trace Elem. Res. 2017, 176, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Meng, X.; Wang, F.; Bao, Y.; Huo, J. Eriodictyol attenuates arsenic trioxide-induced liver injury by activation of Nrf2. Oncotarget 2017, 8, 68668–68674. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Prakash, C.; Dabur, R.; Kumar, V. Protective Effect of Hydroxytyrosol Against Oxidative Stress Mediated by Arsenic-Induced Neurotoxicity in Rats. Appl. Biochem. Biotechnol. 2018, 186, 27–39. [Google Scholar] [CrossRef]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Liu, J.M.; Guo, S.X. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-induced Oxidative Reproductive Toxicity in Male Mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar] [CrossRef]
- Ogun, M.; Ozcan, A.; Karaman, M.; Merhan, O.; Ozen, H.; Kukurt, A.; Karapehlivan, M. Oleuropein ameliorates arsenic induced oxidative stress in mice. J. Trace Elem. Med. Biol. 2016, 36, 1–6. [Google Scholar] [CrossRef]
- Keshtzar, E.; Khodayar, M.J.; Javadipour, M.; Ghaffari, M.A.; Bolduc, D.L.; Rezaei, M. Ellagic acid protects against arsenic toxicity in isolated rat mitochondria possibly through the maintaining of complex II. Hum. Exp. Toxicol. 2016, 35, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Telang, A.G.; Ramya, K.; Vijayakaran, K.; Kesavan, M.; Sarkar, S.N. Protective action of curcumin and nano-curcumin against arsenic-induced genotoxicity in rats in vivo. Mol. Biol. Rep. 2014, 41, 7413–7422. [Google Scholar] [CrossRef]
- Jalaludeen, A.M.; Ha, W.T.; Lee, R.; Kim, J.H.; Do, J.T.; Park, C.; Heo, Y.T.; Lee, W.Y.; Song, H. Biochanin A Ameliorates Arsenic-Induced Hepato- and Hematotoxicity in Rats. Molecules 2016, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Li, G.Y.; Liu, Y.; Chai, L.M.; Chen, J.X.; Zhang, Y.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br. J. Pharmacol. 2008, 154, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, L.; Cheng, Y.; Jiang, J.; Chen, Y.; Jiang, H.; Yu, H.; Shan, A.; Cheng, B. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. BioMed Res. Int. 2014, 2014, 617202. [Google Scholar] [CrossRef]
- Das, R.; Das, A.; Roy, A.; Kumari, U.; Bhattacharya, S.; Haldar, P.K. beta-Carotene ameliorates arsenic-induced toxicity in albino mice. Biol. Trace Elem. Res. 2015, 164, 226–233. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, C.; Zhang, Y.; Hang, P.; Liu, Y.; Pan, Z.; Wang, N.; Du, Z. Genistein ameliorates adverse cardiac effects induced by arsenic trioxide through preventing cardiomyocytes apoptosis. Cell. Physiol. Biochem. 2013, 31, 80–91. [Google Scholar] [CrossRef]
- Jahan, S.; Iftikhar, N.; Ullah, H.; Rukh, G.; Hussain, I. Alleviative effect of quercetin on rat testis against arsenic: A histological and biochemical study. Syst. Biol. Reprod. Med. 2015, 61, 89–95. [Google Scholar] [CrossRef]
- Sarkozi, K.; Papp, A.; Mate, Z.; Horvath, E.; Paulik, E.; Szabo, A. Rutin, a flavonoid phytochemical, ameliorates certain behavioral and electrophysiological alterations and general toxicity of oral arsenic in rats. Acta Biol. Hung. 2015, 66, 14–26. [Google Scholar] [CrossRef]
- Mittal, M.; Flora, S.J. Vitamin E supplementation protects oxidative stress during arsenic and fluoride antagonism in male mice. Drug Chem. Toxicol. 2007, 30, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, B.; Suresh, R.; Venugopal, R. Modulatory effects of ascorbic acid and α-tocopherol on arsenic induced micronuclei formation. IJP Int. J. Pharmacol. 2010, 6, 676–680. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Investigating the protective actions of D-pinitol against arsenic-induced toxicity in PC12 cells and the underlying mechanism. Environ. Toxicol. Pharmacol. 2020, 74, 103302. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.V.; Liu, X.; Slavkovich, V.; Pilsner, J.R.; Ilievski, V.; Factor-Litvak, P.; Levy, D.; Alam, S.; Islam, M.; Parvez, F.; et al. Folic acid supplementation lowers blood arsenic. Am. J. Clin. Nutr. 2007, 86, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Muthumani, M.; Miltonprabu, S. Ameliorative efficacy of tetrahydrocurcumin against arsenic induced oxidative damage, dyslipidemia and hepatic mitochondrial toxicity in rats. Chem. Biol. Interact. 2015, 235, 95–105. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Arsenic-induced oxidative myocardial injury: Protective role of arjunolic acid. Arch. Toxicol. 2008, 82, 137–149. [Google Scholar] [CrossRef]
- Lin, A.M.; Fang, S.F.; Chao, P.L.; Yang, C.H. Melatonin attenuates arsenite-induced apoptosis in rat brain: Involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J. Pineal Res. 2007, 43, 163–171. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Misbahuddin, M.; Bashar, T.; Hossain, M.A. Effectiveness of garlic oil in the treatment of arsenical palmar keratosis. Bangladesh J. Pharmacol. 2013, 8, 22–27. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.N.; Pickering, I.J.; Prince, R.C.; Ringwald, S.C.; Pemberton, J.E.; Glass, R.S.; Younis, H.S.; DeYoung, D.W.; Aposhian, H.V. A Metabolic Link between Arsenite and Selenite: The Seleno-bis(S-glutathionyl) Arsinium Ion. J. Am. Chem. Soc. 2000, 122, 4637–4639. [Google Scholar] [CrossRef]
- Krohn, R.M.; Raqib, R.; Akhtar, E.; Vandenberg, A.; Smits, J.E. A high-selenium lentil dietary intervention in Bangladesh to counteract arsenic toxicity: Study protocol for a randomized controlled trial. Trials 2016, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Subramanian, R.B.; Madamwar, D.; Flora, S.J. Protective effects of selenium, calcium, and magnesium against arsenic-induced oxidative stress in male rats. Arch. Ind. Hyg. Toxicol. 2010, 61, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Vandenberg, A.; Smits, J. Treating chronic arsenic toxicity with high selenium lentil diets. Toxicol. Appl. Pharmacol. 2013, 272, 256–262. [Google Scholar] [CrossRef]
- Smits, J.E.; Krohn, R.M.; Akhtar, E.; Hore, S.K.; Yunus, M.; Vandenberg, A.; Raqib, R. Food as medicine: Selenium enriched lentils offer relief against chronic arsenic poisoning in Bangladesh. Environ. Res. 2019, 176, 108561. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Liontiris, M.I. Commentary: Health Concerns of Brazil Nut Consumption. J. Altern. Complement. Med. 2018, 24, 3–6. [Google Scholar] [CrossRef]
- Lima, L.W.; Stonehouse, G.C.; Walters, C.; Mehdawi, A.F.E.; Fakra, S.C.; Pilon-Smits, E.A.H. Selenium Accumulation, Speciation and Localization in Brazil Nuts (Bertholletia excelsa H.B.K.). Plants 2019, 8, 289. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Monzani, A. Mercury and Selenium Content in Selected Seafood. J. Food Compos. Anal. 2001, 14, 461–467. [Google Scholar] [CrossRef]
- Bugel, S.; Sandstrom, B.; Skibsted, L.H. Pork meat: A good source of selenium? J. Trace Elem. Med. Biol. 2004, 17, 307–311. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Li, J.J.; Chen, C.; Zhang, P.C.; Dong, L.; Chen, J.H.; Chen, Q.; Zhang, X.T.; Wang, Z.L. Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem. Toxicol. 2013, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ahsan, H.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Associations of plasma selenium with arsenic and genomic methylation of leukocyte DNA in Bangladesh. Environ. Health Perspect. 2011, 119, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Wadaa, M.A.; Farooq, M.; Daghestani, M.H.; Sami, A.S. Effectiveness of zinc in modulating perinatal effects of arsenic on the teratological effects in mice offspring. Biol. Res. 2013, 46, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ganger, R.; Garla, R.; Mohanty, B.P.; Bansal, M.P.; Garg, M.L. Protective Effects of Zinc Against Acute Arsenic Toxicity by Regulating Antioxidant Defense System and Cumulative Metallothionein Expression. Biol. Trace Elem. Res. 2016, 169, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Nasiry Zarrin Ghabaee, D.; Talebpour Amiri, F.; Esmaeelnejad Moghaddam, A.; Khalatbary, A.R.; Zargari, M. Administration of zinc against arsenic-induced nephrotoxicity during gestation and lactation in rat model. J. Nephropathol. 2017, 6, 74–80. [Google Scholar] [CrossRef]
- Lenchyk, V.L. Complex pharmacognostic study of Rosaceae plant raw materials and development of phytomedicins on their base. Ph.D. Thesis, Zaporizhzhia State Medical University, Zaporizhia, Ukraine, 2017. [Google Scholar]

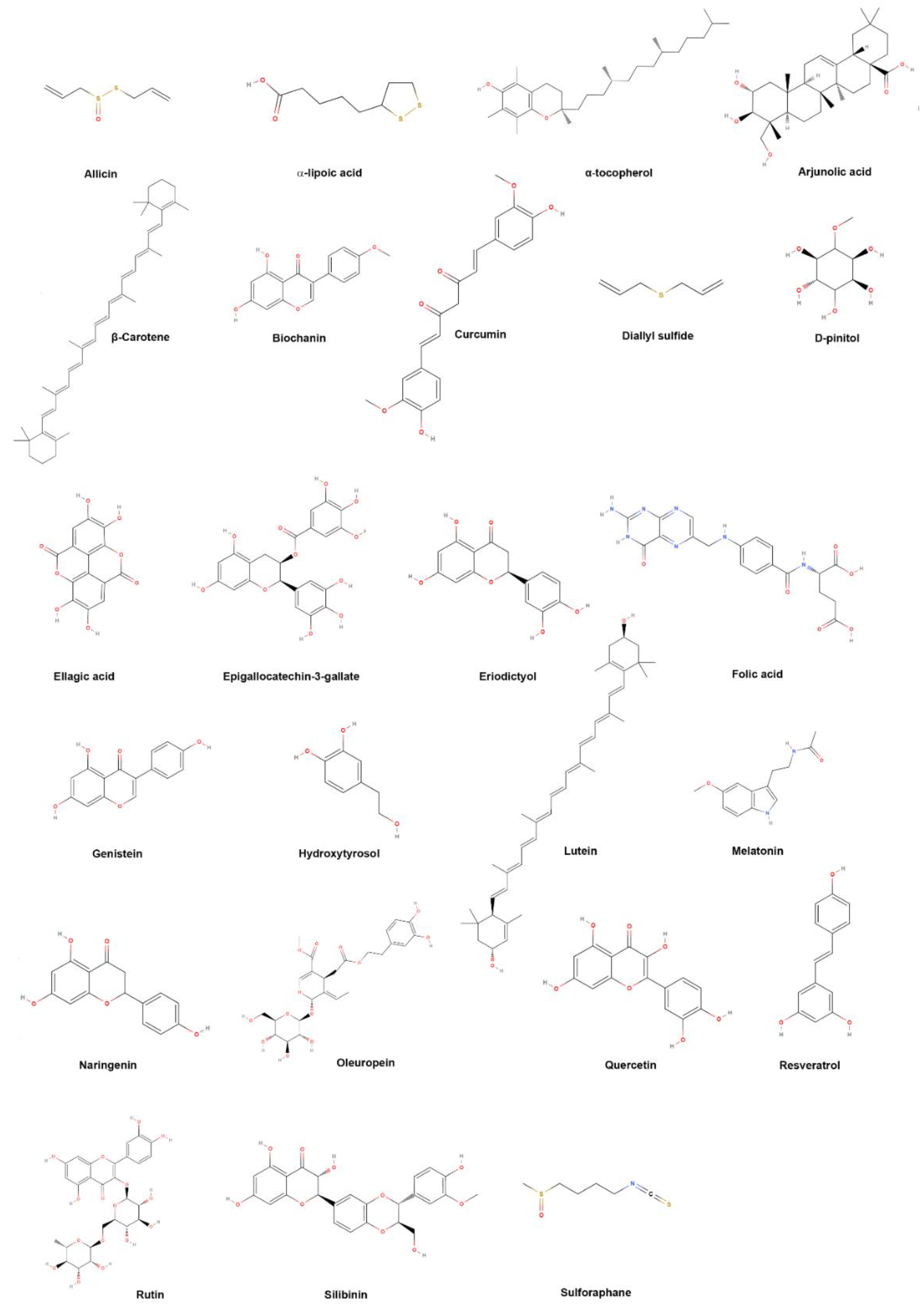
| Common Name | IUPAC Name |
|---|---|
| Allicin | 3-prop-2-enylsulfinylsulfanylprop-1-ene |
| α-Lipoic acid | 5-(dithiolan-3-yl)pentanoic acid |
| α-Tocopherol | (2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol |
| Arjunolic acid | (4aS,6aR,6aS,6bR,8aR,9R,10R,11R,12aR,14bS)-10,11-dihydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
| Biochanin | 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one |
| β-Carotene | 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene |
| Curcumin | (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione |
| Diallyl sulfide | 3-prop-2-enylsulfanylprop-1-ene |
| D-pinitol | (1S,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol |
| Ellagic acid | 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.04,16.011,15]hexadeca-1(15),4,6,8(16),11,13-hexaene-3,10-dione |
| Epigallocatechin-3-gallate | [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |
| Eriodictyol | (2S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one |
| Folic acid | (2S)-2-[[4-[(2-amino-4-oxo-3H-pteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid |
| Genistein | 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one |
| Hydroxytyrosol | 4-(2-hydroxyethyl)benzene-1,2-diol |
| Lutein | (1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4R)-4-hydroxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-3,5,5-trimethylcyclohex-3-en-1-ol |
| Melatonin | N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide |
| Naringenin | 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one |
| Oleuropein | methyl (4S,5E,6S)-4-[2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl]-5-ethylidene-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4H-pyran-3-carboxylate |
| Quercetin | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one |
| Resveratrol | 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol |
| Rutin | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| Sibilin | (2R,3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2,3-dihydrochromen-4-one |
| Sulforaphane | 1-isothiocyanato-4-methylsulfinylbutane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørklund, G.; Rahaman, M.S.; Shanaida, M.; Lysiuk, R.; Oliynyk, P.; Lenchyk, L.; Chirumbolo, S.; Chasapis, C.T.; Peana, M. Natural Dietary Compounds in the Treatment of Arsenic Toxicity. Molecules 2022, 27, 4871. https://doi.org/10.3390/molecules27154871
Bjørklund G, Rahaman MS, Shanaida M, Lysiuk R, Oliynyk P, Lenchyk L, Chirumbolo S, Chasapis CT, Peana M. Natural Dietary Compounds in the Treatment of Arsenic Toxicity. Molecules. 2022; 27(15):4871. https://doi.org/10.3390/molecules27154871
Chicago/Turabian StyleBjørklund, Geir, Md. Shiblur Rahaman, Mariia Shanaida, Roman Lysiuk, Petro Oliynyk, Larysa Lenchyk, Salvatore Chirumbolo, Christos T. Chasapis, and Massimiliano Peana. 2022. "Natural Dietary Compounds in the Treatment of Arsenic Toxicity" Molecules 27, no. 15: 4871. https://doi.org/10.3390/molecules27154871
APA StyleBjørklund, G., Rahaman, M. S., Shanaida, M., Lysiuk, R., Oliynyk, P., Lenchyk, L., Chirumbolo, S., Chasapis, C. T., & Peana, M. (2022). Natural Dietary Compounds in the Treatment of Arsenic Toxicity. Molecules, 27(15), 4871. https://doi.org/10.3390/molecules27154871












