Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Indices and Nutrients in WFV
2.2. Bioactive Ingredients in WFV
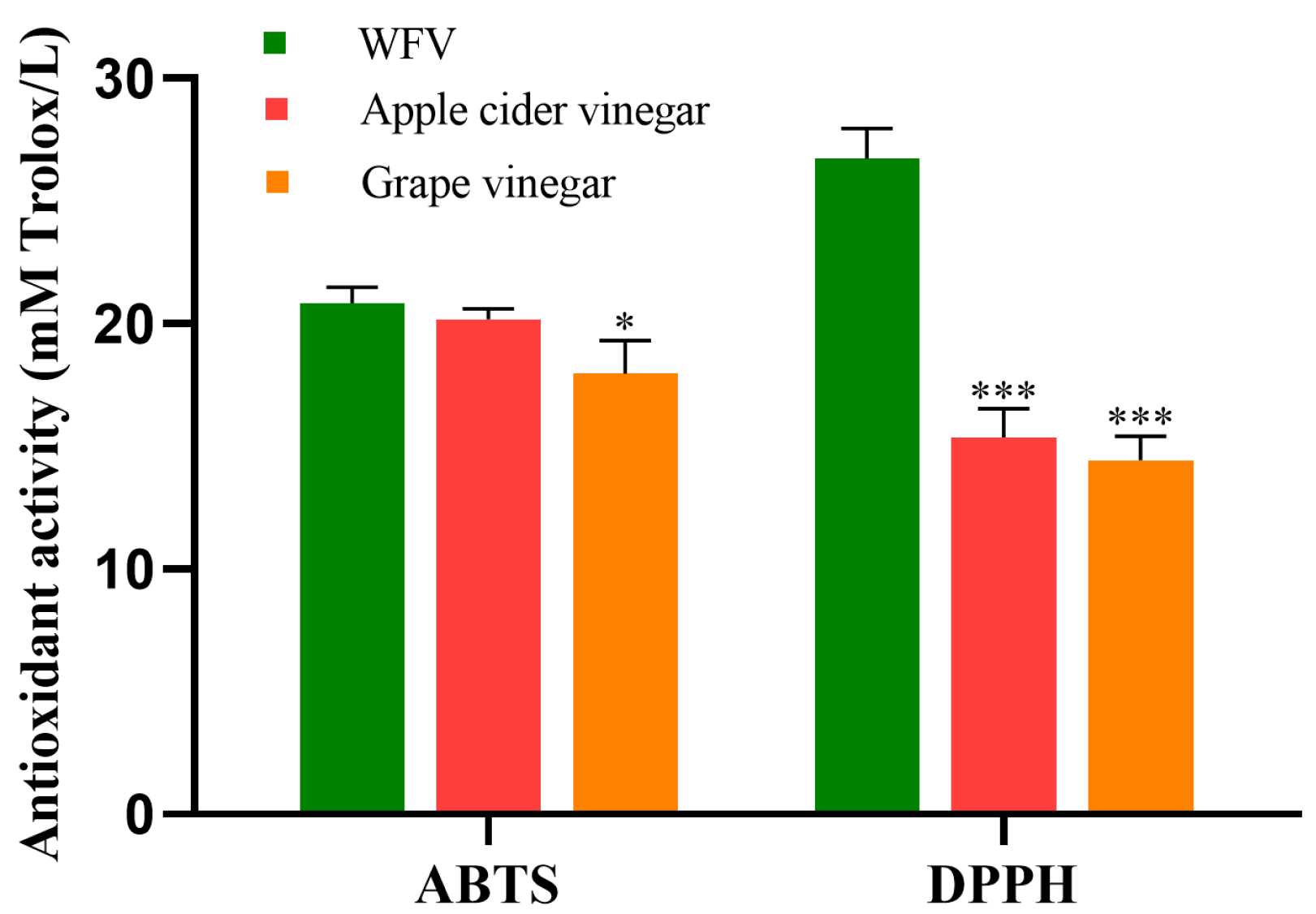
2.3. The Antioxidant Activity of WFV
2.4. Analysis of Polyphenols in WFV
2.5. Effect of WFV on the Damaged Liver in Mice
2.6. Effect of WFV on Biochemical Indexes in Mice with Oxidative Liver Injury
2.7. Effect of WFV on Oxidative and Antioxidative Levels in Mice
3. Materials and Methods
3.1. Chemical Reagents
3.2. Production of WFV
3.3. Determination of Nutrients and Physicochemical Indices in WFV
3.4. Determination of Active Ingredients in WFV
3.4.1. TPC and TFC
3.4.2. LBP
3.4.3. Carotenoids
3.4.4. Betaine
3.4.5. Polyphenols
3.5. Antioxidant Activity of WFV
3.6. Animals and Diets
3.7. Biochemical Analysis
3.8. Histopathological Examination
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Sample Availability
References
- Sivakrishnan, S.; Pharm, M. Liver disease overview. J. Pharm. Sci. 2019, 8, 1385–1395. [Google Scholar]
- Holt, M.P.; Ju, C. Mechanisms of drug-induced liver injury. AAPS J. 2006, 8, E48–E54. [Google Scholar] [CrossRef] [Green Version]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. Word J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 4. [Google Scholar]
- Wang, Y.J.; Liang, X.J.; Guo, S.J.; Li, Y.K.; Zhang, B.; Yin, Y.; An, W.; Cao, Y.L.; Zhao, J.H. Evaluation of nutrients and related environmental factors for wolfberry (Lycium barbarum) fruits grown in the different areas of China. Biochem. Syst. Ecol. 2019, 86, 103916. [Google Scholar]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.P.; Liu, K.; Zhao, Z.L.; Li, X.T. Study on identification method of fructus lycii origin by mineral elements. Adv. Mat. Res. 2014, 989, 1368–1371. [Google Scholar] [CrossRef]
- Hailu, S.; Admassu, S.; Jha, K. Vinegar production technology—An overview. Beverage Food World 2012, 2, 29–32. [Google Scholar]
- Xia, T.; Zhang, B.; Duan, W.H.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Samad, A.; Azlan, A.; Ismail, A. Therapeutic effects of vinegar: A review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Antioxidant properties of traditional balsamic vinegar and boiled must model systems. Eur. Food Res. Technol. 2008, 227, 835–843. [Google Scholar] [CrossRef]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Yusof, H.M.; Sharifuddin, S.A.; Jamaluddin, A.J.; Long, K.; Alitheen, N.B. Polyphenolic profiles and the in vivo antioxidant effect of nipa vinegar on paracetamol induced liver damage. RSC Adv. 2016, 6, 63304–63313. [Google Scholar] [CrossRef]
- Bouazza, A.; Bitam, A.; Amiali, M.; Bounihi, A.; Yargui, L.; Koceir, E.A. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm. Biol. 2016, 54, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Janchovska, E.; Janchovska, M.; Ristovski, B.; Bocevska, M. Antimicrobial and antioxidative activity of commercial versus traditional apple vinegar. In Proceedings of the International Conference on Sustainable Development, Belgrade, Serbia, 12–15 November 2015. [Google Scholar]
- Bekatorou, A. 10 Vinegar Production. In Advances in Vinegar Production, 2nd ed.; Tang, H., Song, J., Luo, L., Eds.; CRC Press: Boca Raton, FL, USA, 2019; Volume 1, p. 171. [Google Scholar]
- Chen, T.; Gui, Q.; Shi, J.J.; Zhang, X.Y.; Chen, F.S. Analysis of variation of main components during aging process of Shanxi aged vinegar. Acetic Acid Bact. 2013, 2, 31–38. [Google Scholar] [CrossRef]
- Tan, S.C. Vinegar Fermentation. LSU Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2005. [Google Scholar]
- Xu, W.; Huang, Z.Y.; Zhang, X.J.; Li, Q.; Lu, Z.M.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Monitoring the microbial community during solid-state acetic acid fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2011, 28, 1175–1181. [Google Scholar] [CrossRef]
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health Benefits and Applications of Goji Berries in Functional Food Products Development: A Review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant activities, phenolic profiles, and organic acid contents of fruit vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Yao, J.H.; Zhang, J.; Duan, W.H.; Zhang, B.; Xie, X.L.; Xia, M.L.; Song, J.; Zheng, Y.; Wang, M. Evaluation of nutritional compositions, bioactive compounds, and antioxidant activities of Shanxi aged vinegars during the aging process. J. Food Sci. 2018, 83, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, H.Y.; Dong, X.X.; Yang, S.; Ma, S.C.; Ni, J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, J.; Kamal, G.M.; Jiang, B.; Sun, P.; Zhang, X.; Liu, M.L. Characterization and comparison of commercial Chinese cereal and European grape vinegars using 1H NMR spectroscopy combined with multivariate analysis. Chin. J. Chem. 2016, 34, 1183–1193. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Rashid, M.T.; Wali, A.; Younas, S. Preliminary study to evaluate the phytochemicals and physiochemical properties in red and black date’s vinegar. Food Sci. Nutr. 2019, 7, 1976–1985. [Google Scholar] [CrossRef]
- Ye, X.Q.; Tian, J.H.; Zheng, Y.X.; Sun, W.X.; Yang, W.H. Traditional Goji Berry-Based Functional Food in Chinese History. In Phytochemicals in Goji Berries, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–13. [Google Scholar]
- Jiang, Y.Q.; Fang, Z.X.; Leonard, W.; Zhang, P.Z. Phenolic compounds in Lycium berry: Composition, health benefits and industrial applications. J. Funct. Foods. 2021, 77, 104340. [Google Scholar] [CrossRef]
- Tian, X.J.; Liang, T.S.; Liu, Y.L.; Ding, G.T.; Zhang, F.M.; Ma, Z.R. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Kelebek, H.; Kadiroğlu, P.; Demircan, N.B.; Selli, S. Screening of bioactive components in grape and apple vinegars: Antioxidant and antimicrobial potential. J. Inst. Brew. 2017, 123, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Recent trends in the analysis of amino acids in fruits and derived foodstuffs. Anal. Bioanal. Chem. 2013, 405, 7941–7956. [Google Scholar] [CrossRef]
- Ousaaid, D.; Mechchate, H.; Laaroussi, H.; Hano, C.; Bakour, M.; El Ghouizi, A.; Conte, R.; Lyoussi, B.; El Arabi, I. Fruits vinegar: Quality characteristics, phytochemistry, and functionality. Molecules 2022, 27, 222. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Piórecki, N. Bioactive compounds in cornelian cherry vinegars. Molecules 2018, 23, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Troncoso, A.M.; Morales, M.L. Employment of different processes for the production of strawberry vinegars: Effects on antioxidant activity, total phenols and monomeric anthocyanins. LWT Food Sci. Technol. 2013, 52, 139–145. [Google Scholar] [CrossRef]
- Ubeda, C.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Evaluation of antioxidant activity and total phenols index in persimmon vinegars produced by different processes. LWT Food Sci. Technol. 2011, 44, 1591–1596. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavor. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Abbas, C.A. Production of antioxidants, aromas, colours, flavours, and vitamins by yeasts. In Yeasts in Food and Beverages, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 285–334. [Google Scholar]
- Ohue-Kitano, R.; Taira, S.; Watanabe, K.; Masujima, Y.; Kuboshima, T.; Miyamoto, J.; Nishitani, Y.; Kawakami, H.; Kuwahara, H.; Kimura, I. 3-(4-hydroxy-3-methoxyphenyl) propionic acid produced from 4-hydroxy-3-methoxycinnamic acid by gut microbiota improves host metabolic condition in diet-induced obese mice. Nutrients 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, A.M.; Castro, R.; Rodrıguez, M.C.; Guillen, D.A.; Barroso, C.G. Study of the antioxidant power of brandies and vinegars derived from Sherry wines and correlation with their content in polyphenols. Food Res. Int. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Nakamura, K.; Ogasawara, Y.; Endou, K.; Fujimori, S.; Koyama, M.; Akano, H. Phenolic compounds responsible for the superoxide dismutase-like activity in high-Brix apple vinegar. J. Agric. Food Chem. 2010, 58, 10124–10132. [Google Scholar] [CrossRef]
- Xing, X.; Liu, F.; Xiao, J.; So, K.F. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med. 2016, 18, 253–263. [Google Scholar] [CrossRef]
- Xiao, J.; Tipoe, G.L. The antioxidant, anti-inflammatory, and antiapoptotic effects of wolfberry in fatty liver disease. In Lycium Barbarum and Human Health, 2nd ed.; Springer: Dordrech, The Netherlands, 2015; pp. 45–63. [Google Scholar]
- Omar, N.A.A.; Allithy, A.N.E.A.; Faleh, F.M.; Mariah, R.A.; Ayat, M.M.A.; Shafik, S.R.; Elshweikh, S.A.; Baghdadi, H.; Sayed, S.M.E. Apple cider vinegar (a prophetic medicine remedy) protects against nicotine hepatotoxicity: A histopathological and biochemical report. Am. J. Cancer Prev. 2015, 3, 122–127. [Google Scholar]
- Campo, G.M.; Avenoso, A.; Campo, S.; Nastasi, G.; Traina, P.; Dascola, A.; Rugolo, C.A.; Calatroni, A. The antioxidant activity of chondroitin-4-sulphate, in carbon tetrachloride-induced acute hepatitis in mice, involves NF-κB and caspase activation. Br. J. Pharmacol. 2008, 155, 945–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solnica, B.; Sygitowicz, G.; Sitkiewicz, D.; Cybulska, B.; Jozwiak, J.; Odrowąz-Sypniewska, G.; Banach, M. 2020 Guidelines of the Polish Society of Laboratory Diagnostics (PSLD) and the Polish Lipid Association (PoLA) on laboratory diagnostics of lipid metabolism disorders. Arch. Med. Sci. 2020, 16, 237. [Google Scholar] [CrossRef]
- Rasool, M.; Iqbal, J.; Malik, A.; Ramzan, H.S.; Qureshi, M.S.; Asif, M.; Qazi, M.H.; Kamal, M.A.; Chaudhary, A.G.A.; Al-Qahtani, M.H.; et al. Hepatoprotective effects of Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) in combination: A possible synergy. Evid. Based Complementary Altern. Med. 2014, 2014, 641597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Zentella, M.L.; Hernandez-Munoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxid. Med. Cell Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhou, X.; Huang, S.W.; Yang, J.; Liu, R.J.; Liu, C.H. Lycium Barbarum polysaccharides and wolfberry juice prevent DEHP-induced hepatotoxicity via PXR-regulated detoxification pathway. Molecules 2021, 26, 859. [Google Scholar] [CrossRef]
- Yang, J.Y.; Li, Y.; Wang, F.; Wu, C.F. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Rees, M.D.; Kennett, E.C.; Whitelock, J.M.; Davies, M.J. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic. Biol. Med. 2008, 44, 1973–2001. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TRAC Trend Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern. Med. 2012, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.N.; Long, X.Y.; Yi, R.K.; Zhao, X. Polyphenols in Liubao tea can prevent CCl4-induced hepatic damage in mice through its antioxidant capacities. Nutrients 2018, 10, 1280. [Google Scholar] [CrossRef] [Green Version]
- Zeng, B.; Su, M.H.; Chen, Q.X.; Chang, Q.; Wang, W.; Li, H.H. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride-induced acute liver injury in mice. J. Ethnopharmacol. 2017, 200, 124–135. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.L.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters (Units) | Contents | |
|---|---|---|
| Physicochemical indices | pH value | 3.38 ± 0.08 |
| Total acid (g/100 mL) | 6.72 ± 0.12 | |
| Non-volatile acid (g/100 mL) | 0.84 ± 0.11 | |
| Soluble solids (%) | 8.66 ± 0.32 | |
| Amino nitrogen (g/100 mL) | 0.07 ± 0.01 | |
| Reducing sugar (g/100 mL) | 1.42 ± 0.14 | |
| Alcohol (% vol) | 0.06 ± 0.01 | |
| Nutrients | Total sugar (g/100 mL) | 2.46 ± 0.14 |
| Protein (g/100 mL) | 0.27 ± 0.01 | |
| Fat (g/100 mL) | 0.12 ± 0.02 |
| Indexes | TPC (mg GAE/mL) | TFC (mg RE/mL) | LBP (mg/mL) | Betaine (mg/mL) | Carotenoids (mg/mL) |
|---|---|---|---|---|---|
| WFV | 2.42 ± 0.05 | 1.67 ± 0.03 | 8.94 ± 0.27 | 2.88 ± 0.22 | 0.42 ± 0.02 |
| Polyphenols | Retention Time (min) | Content (mg/mL) | |
|---|---|---|---|
| WFV | |||
| 1 | p-hydroxybenzaldehyde | 14.602 | 0.022 ± 0.001 |
| 2 | salicylic acid | 18.345 | 0.059 ± 0.002 |
| 3 | 2-(4-hydroxyphenyl) ethanol | 19.817 | 0.216 ± 0.024 |
| 4 | p-hydroxybenzoic acid | 21.146 | 0.317 ± 0.011 |
| 5 | p-hloroglucinol | 21.691 | 0.105 ± 0.004 |
| 6 | 4-hydroxyphenylacetic acid | 24.220 | 0.028 ± 0.001 |
| 7 | vanillic acid | 24.360 | 0.097 ± 0.003 |
| 8 | m-hydroxycinnamic acid | 24.880 | 0.311 ± 0.011 |
| 9 | protocatechuic acid | 25.665 | 0.022 ± 0.001 |
| 10 | 3-(4-hydroxy-3-methoxyphenyl) propanoic acid | 27.190 | 0.151 ± 0.005 |
| 11 | 3-(4-hydroxyphenyl)-2-hydroxypropionic acid | 27.450 | 0.038 ± 0.001 |
| 12 | 3-hydroxy-4-methoxycinnamic acid | 27.754 | 0.062 ± 0.002 |
| 13 | 4-hydroxycinnamic acid | 28.060 | 0.131 ± 0.005 |
| 14 | 3,4-diphenylpropionic acid | 28.315 | 0.027 ± 0.001 |
| 15 | 3-(4-hydroxy-3-methoxyphenyl) propionic acid | 30.625 | 0.222 ± 0.008 |
| 16 | ferulic acid | 31.130 | 0.134 ± 0.005 |
| 17 | caffeic acid | 32.025 | 0.143 ± 0.005 |
| 18 | chlorogenic acid | 47.890 | 0.222 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Xia, T.; Qiang, X.; Zhao, Y.; Li, S.; Wang, Y.; Zheng, Y.; Yu, J.; Wang, J.; Wang, M. Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry. Molecules 2022, 27, 4422. https://doi.org/10.3390/molecules27144422
Tian Y, Xia T, Qiang X, Zhao Y, Li S, Wang Y, Zheng Y, Yu J, Wang J, Wang M. Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry. Molecules. 2022; 27(14):4422. https://doi.org/10.3390/molecules27144422
Chicago/Turabian StyleTian, Yinglei, Ting Xia, Xiao Qiang, Yuxuan Zhao, Shaopeng Li, Yiming Wang, Yu Zheng, Junwei Yu, Jianxin Wang, and Min Wang. 2022. "Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry" Molecules 27, no. 14: 4422. https://doi.org/10.3390/molecules27144422
APA StyleTian, Y., Xia, T., Qiang, X., Zhao, Y., Li, S., Wang, Y., Zheng, Y., Yu, J., Wang, J., & Wang, M. (2022). Nutrition, Bioactive Components, and Hepatoprotective Activity of Fruit Vinegar Produced from Ningxia Wolfberry. Molecules, 27(14), 4422. https://doi.org/10.3390/molecules27144422






