Development of the Volatile Fingerprint of Qu Aurantii Fructus by HS-GC-IMS
Abstract
:1. Introduction
2. Results
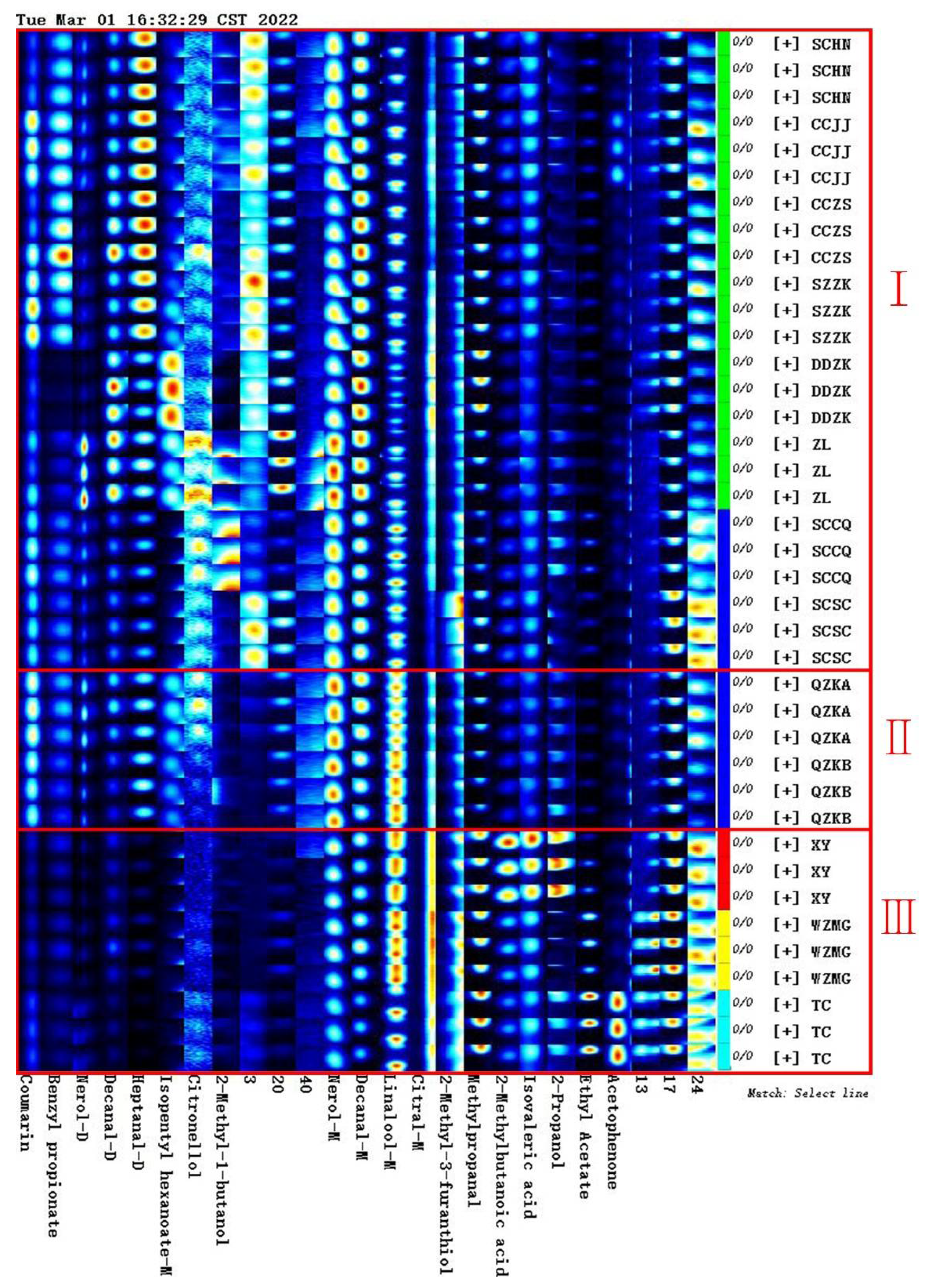
2.1. Volatile Compounds and Semi-Quantitative Analysis of Qu Aurantii Fructus
2.2. Comparative Analysis of Unique Volatile Compounds in Different Samples
2.2.1. Comparative Analysis of Volatile Compounds between Qu Aurantii Fructus and Aurantii Fructus
2.2.2. Comparative Analysis of Volatile Compounds of Qu Aurantii Fructus and the Common Adulterants
2.3. Stoichiometric Analysis
2.3.1. Principal Component Analysis (PCA)
2.3.2. Cluster Analysis (CA) for Qu Aurantii Fructus and Aurantii Fructus
2.3.3. Partial Least Square-Discriminant Analysis (PLS-DA)
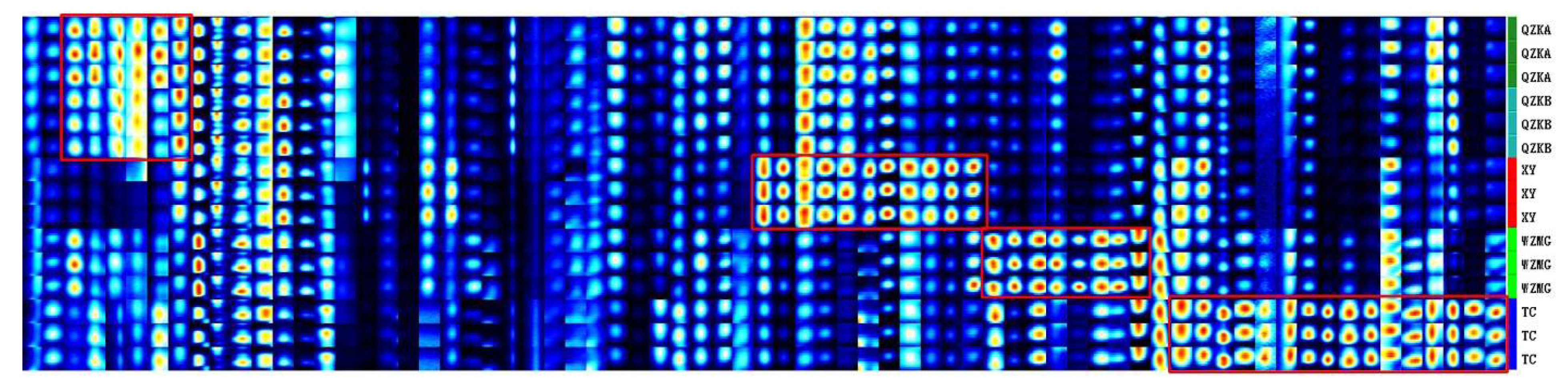
2.4. Establishment of Characteristic Fingerprint of Qu Aurantii Fructus
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. HS-GC-IMS Methods
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhejiang Food and Drug Administration. Standard for Processing of Traditional Chinese Medicine in Zhejiang Province; China Medical Science and Technology Press: Beijing, China, 2015; pp. 110–121. ISBN 978-7-5067-8456-6.
- Zhao, X.M.; Ye, X.Q.; Xi, Y.F.; Zhu, D.Y.; Jiang, S.H. Studies on Chemical Constitutents in the Peels of Citrus changshan-huyou (I). China J. Chin. Mater. Med. 2003, 28, 237–239. [Google Scholar]
- Zhao, X.M.; Ye, X.Q.; Xi, Y.F.; Zhu, D.Y.; Jiang, S.H. Flavonoids in peels of Citrus changshan huyou. Chin. Tradit. Herb. Drugs 2003, 34, 11–13. [Google Scholar]
- Zhao, X.M.; Ye, X.Q.; Zhu, D.Y. A novel compound isolated from the peels of Citrus changshan-huyou Y. B.Chang. Yaoxue Xuebao 2008, 43, 1208–1210. [Google Scholar]
- Zhao, X.M.; Ye, X.Q.; Zhu, D.Y. Isolation and identification of chemical constituents from peels of Citrus changshan-huyou Y.B. Chang. J. Peking Univ. Health Sci. 2009, 41, 575–577. [Google Scholar]
- Zhao, X.M.; Ye, X.Q.; Zhu, D.Y. Chemical constituents in peels of Citrus Changshan-huyou (III). Chin. Tradit. Herb. Drugs 2009, 40, 6–8. [Google Scholar]
- Liu, X.J.; Jiang, X.Q.; Fang, Y.J.; Xia, D.Z.; Wang, S.W.; Zhong, S.Y. Protective effect of total flavonoids from Fructus aurantii on lung injury of asthma mice infected with RSV through NF-kappaB signaling pathway. Chin. J. Nosocomiol. 2021, 31, 3376–3380. [Google Scholar]
- Xu, L.P.; Song, J.F.; Zhao, S.Q.; Yang, Y.; Yue, C.; Feng, J.Q.; Dai, D.X.; Mao, P.J.; Jin, J.; Wang, Y.; et al. Pharmacodynamic Comparison of Qi Regulating and Depression Dispersing between Citrus changshan-huyou and Aurantii Fructus From Different Sources. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 156–160. [Google Scholar]
- Jiang, J.P.; Yan, L.; Shi, Z.; Wang, L.X.; Shan, L.T.; Efferth, T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-kappa B and MAPKs. Phytomedicine 2019, 64, 1–9. [Google Scholar] [CrossRef]
- Wang, S.W.; Lan, T.; Zheng, F.; Lei, M.K.; Zhang, F. Effect of extract of Quzhou Aurantii Fructus on hepatic inflammation and NF-kappaB/NLRP3 inflammasome pathway in CCl4-induced liver fibrosis mice. China J. Chin. Mater. Med. 2021, 46, 1474–1479. [Google Scholar] [CrossRef]
- Fang, J.; Wu, X.N.; Jiang, J.P.; Mao, L. Related Analysis Between Antioxidant Activities and HPLC Fingerprint of Flavonoids in Citrus Changshan-huyou Y. B. Peels. Chin. J. Mod. Appl. Pharm. 2018, 35, 1489–1493. [Google Scholar]
- Zhang, J.; He, J.; Zhou, J.Y.; Lu, S. Alpha-Glucosidase Inhibitory Activity of Compounds from Citrus Changshan-huyou. J. Chin. Inst. Food Sci. Technol. 2013, 13, 79–82. [Google Scholar]
- Guo, J.J.; Gao, Z.P.; Li, G.Y.; Fu, F.H.; Liang, Z.G.N.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou Y. B. chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, pp. 257–258. ISBN 978-7-5214-1574-2. [Google Scholar]
- Xu, G.J.; Xu, L.S. South-China Edition, Species Systematization and Quality Evaluation of Commonly Used Chinese Traditional Drugs; Fujian Science and Technology Press: Fuzhou, China, 1994; pp. 502–535. ISBN 978-753-350-781-7. [Google Scholar]
- Fan, L.; Wu, C.Q.; Wang, W.Y.; Chen, W.P.; Mao, J.H.; Chen, Z.J.; Li, S.F. Study on the Quality Investigation and Fingerprint of Aurantii Fructus. Chin. J. Mod. Appl. Pharm. 2015, 32, 432–436. [Google Scholar]
- Huang, W.K.; Yue, C.; Song, J.F.; Ding, G.Q.; Zhang, W.T.; Zhao, W.L. Simultaneous Determination of Seven Constituents in Citrus Changshanhuyou, Y.B.Chang by HPLC. Chin. J. Mod. Appl. Pharm. 2018, 35, 404–407. [Google Scholar]
- Yue, C.; Zhang, W.P.; Zhao, W.L.; Liu, Z.; Wang, F. Quality Evaluation for Different Original Plant Species of Aurantii Fructus Based on HPLC Fingerprint with Chemical Pattern Analysis. Chin. J. Mod. Appl. Pharm. 2021, 38, 3002–3008. [Google Scholar]
- Zhao, K.J.; Zheng, Y.Z.; Dong, T.X.; Tsim, W. Analysis of HPLC Fingerprints of Fructus Aurantii from Different Habitats and Contents of Naringin, Neo-hesperidin and Synephrine. Chin. Pharm. J. 2011, 46, 955–959. [Google Scholar]
- Li, Z.H.; Chen, H.F.; Luo, L.P.; Yang, B.; Wei, Y.; Yuan, J.B.; Gong, Q.F.; Yang, W.L. Determination of the active constituents in aurantii fructus from Jiangxi province at different harvest time by HPLC. J. Chin. Med. Mater. 2013, 36, 28–31. [Google Scholar]
- Liu, X.Q.; Sun, L.; Qiao, S.Y. Evaluation of the quality of commercial Fructus Aurantii Immaturus and Fructus Aurantii by HPLC fingerprint method. J. Int. Pharm. Res. 2014, 41, 244–248. [Google Scholar]
- Zhang, Q.H.; Jiang, Y.H.; Gong, Q.F.; Wang, Z.P. Analysis of Flavonoids from Fructus Aurantii of Zhang-band Processed Products. Lishizhen Med. Mater. Med. Res. 2010, 21, 2536–2537. [Google Scholar]
- He, Y.J.; Li, Z.k.; Wang, W.; Sooranna, S.R.; Shi, Y.T.; Chen, Y.; Wu, C.Q.; Zeng, J.G.; Tang, Q.; Xie, H.Q. Chemical Profiles and Simultaneous Quantification of Aurantii fructus by Use of HPLC-Q-TOF-MS Combined with GC-MS and HPLC Methods. Molecules 2018, 23, 2189. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Ma, L.k.; Song, J.F.; Zhang, W.T.; Zhao, W.L. Establishment of the HPLC Fingerprints of Citrus Changshan-huyou and Analysis of Its Characteristic Components. Chin. J. Mod. Appl. Pharm. 2018, 35, 1217–1220. [Google Scholar]
- Zheng, Y.; Wang, S.; Meng, X.S.; Bao, Y.R. Analysis of the orange essential oil by GC-MS and research on the prokinetic effect of it. Lishizhen Med. Mater. Med. Res. 2015, 26, 516–518. [Google Scholar]
- European Pharmacopoeia Commission. European Pharmacopoeia, 10th ed.; European Directorate for Quality Medicines: Strasbourg, France, 2019; pp. 1352–1353. ISBN 978-92-871-8912-7. [Google Scholar]
- Pharmaceuticals and Medical Devices Agency of Japan. The Japanese Pharmacopoeia, 18th ed.; the Ministry of Health, Labor and Welfare: Tokyo, Japan, 2021.
- Jiang, Y.H.; Yang, X.Y.; Zhang, Q.H.; Cao, M.M.; Gong, Q.F.; Shi, J.L. Analysis of volatile oil in Fructus Aurantii processed with Zhangband method by GC-MS. J. Chin. Med. Mater. 2010, 33, 1233–1236. [Google Scholar]
- Yu, H.; Ning, X.X.; Chen, Q.; Xiong, S.S.; Gong, Q.F. Analysis of volatile oil in processed Aurantii Fructus from Jiangxi Province by GC-MS. Chin. Tradit. Pat. Med. 2015, 37, 592–598. [Google Scholar]
- Chen, Y.; Li, P.; Liao, L.Y.; Qin, Y.Y.; Jiang, L.W.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Yang, R.W.; Zhang, H.; Wang, S.L.; Chen, D.; Lin, S.Y. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Li, H.Y.; Wu, Q.; Liu, Q.N.; Jin, L.H.; Chen, B.; Li, C.; Xiao, J.B.; Shen, Y. Volatile Flavor Compounds of Pugionium cornutum (L.) Gaertn. Before and After Different Dehydration Treatments. Front. Nutr. 2022, 9, 884086. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yuan, Y.X.; Hong, B.; Zhao, X.; Gu, Z.Y. Characteristic Volatile Fingerprints of Four Chrysanthemum Teas Determined by HS-GC-IMS. Molecules 2021, 26, 7113. [Google Scholar] [CrossRef]
- Gu, S.A.; Zhang, J.; Wang, J.; Wang, X.Y.; Du, D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. Trac-Trends Anal. Chem. 2021, 144, 116435. [Google Scholar] [CrossRef]
- Cao, S.; Sun, J.Y.; Yuan, X.Y.; Deng, W.H.; Zhong, B.L.; Chun, J. Characterization of Volatile Organic Compounds of Healthy and Huanglongbing-Infected Navel Orange and Pomelo Leaves by HS-GC-IMS. Molecules 2020, 25, 4119. [Google Scholar] [CrossRef]
- He, J.; Ye, L.H.; Li, J.H.; Huang, W.K.; Huo, Y.J.; Gao, J.X.; Liu, L.; Zhang, W.T. Identification of Ophiopogonis Radix from different producing areas by headspace-gas chromatography-ion mobility spectrometry analysis. J. Food Biochem. 2021, 46, e13850. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhong, L.Y.; Ning, X.X.; Zhang, J.L.; Li, X.N.; Gong, Q.F. Analysis on Volatile Oil in Different Processed Products of Aurantii Fructus Immaturus from Jiangxi by GC-MS. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 12–18. [Google Scholar]
- Zhao, W.L.; Huang, Q.W.; Zhang, W.T.; Yue, C.; Song, J.F. Research for the Original Plant of Chinese Medicinal Materials Qu Aurantii Fructus. Chin. J. Mod. Appl. Pharm. 2019, 36, 1652–1655. [Google Scholar]
- Zhao, W.L.; Guo, Z.X.; Zhang, W.T.; Huang, Q.W.; Yi, Z.; Song, J.F. Study on original plant species and geographical distribution of Fructus Aurantii. China J. Chin. Mater. Med. 2018, 43, 4361–4364. [Google Scholar] [CrossRef]







| Compound | CAS | Formula | MW | RI | Dt (RIP Rel.) | Area Percentages (n = 8) | Range | Comment |
|---|---|---|---|---|---|---|---|---|
| limonene | 138-86-3 | C10H16 | 136.2 | 1025.2 | 1.68 | 6.93% | 5.93–8.29% | monomer |
| limonene | 138-86-3 | C10H16 | 136.2 | 1026.2 | 2.17 | dimer | ||
| α-farnesene | 502-61-4 | C15H24 | 204.4 | 1520.0 | 1.45 | 10.41% | 6.88–14.46% | monomer |
| α-farnesene | 502-61-4 | C15H24 | 204.4 | 1551.4 | 1.43 | dimer | ||
| γ-terpinene | 99-85-4 | C10H16 | 136.2 | 1066.9 | 1.21 | 6.24% | 5.62–7.07% | monomer |
| γ-terpinene | 99-85-4 | C10H16 | 136.2 | 1065.6 | 1.70 | dimer | ||
| linalool | 78-70-6 | C10H18O | 154.3 | 1118.7 | 1.222 | 5.51% | 4.20–8.20% | monomer |
| linalool | 78-70-6 | C10H18O | 154.3 | 1117.3 | 1.76 | dimer | ||
| linalool | 78-70-6 | C10H18O | 154.3 | 1118.7 | 2.24 | trimer | ||
| α-terpineol | 98-55-5 | C10H18O | 154.3 | 1209.5 | 1.22 | 5.07% | 4.30–5.84% | monomer |
| α-terpineol | 98-55-5 | C10H18O | 154.3 | 1211.2 | 1.78 | dimer | ||
| camphene | 79-92-5 | C10H16 | 136.2 | 959.8 | 1.64 | 4.50% | 3.65–5.37% | monomer |
| camphene | 79-92-5 | C10H16 | 136.2 | 959.1 | 2.19 | dimer | ||
| α-ocimene | 13877-91-3 | C10H16 | 136.2 | 1048.3 | 1.71 | 4.38% | 2.62–5.91% | monomer |
| β-ocimene | 13877-91-3 | C10H16 | 136.2 | 1049.7 | 2.14 | dimer | ||
| methyleugenol | 93-15-2 | C11H14O2 | 178.2 | 1436.2 | 1.47 | 4.19% | 3.38–6.08% | |
| linalool oxide | 60047-17-8 | C10H18O2 | 170.3 | 1081.1 | 1.26 | 2.92% | 1.56–3.70% | monomer |
| linalool oxide | 60047-17-8 | C10H18O2 | 170.3 | 1082.4 | 1.81 | dimer | ||
| α-thujene | 2867-05-2 | C10H16 | 136.2 | 916.0 | 1.67 | 2.80% | 2.23–3.20% | |
| nerol | 106-25-2 | C10H18O | 154.3 | 1239.4 | 1.31 | 2.33% | 1.62–3.85% | monomer |
| nerol | 106-25-2 | C10H18O | 154.3 | 1238.4 | 1.75 | dimer | ||
| β-pinene | 127-91-3 | C10H16 | 136.2 | 979.8 | 1.72 | 2.15% | 1.93–2.33% | monomer |
| β-pinene | 127-91-3 | C10H16 | 136.2 | 982.1 | 2.17 | dimer | ||
| linalyl acetate | 115-95-7 | C12H20O2 | 196.3 | 1337.0 | 1.22 | 2.09% | 1.53–3.41% | monomer |
| linalyl acetate | 115-95-7 | C12H20O2 | 196.3 | 1337.4 | 1.69 | dimer | ||
| linalyl acetate | 115-95-7 | C12H20O2 | 196.3 | 1338.2 | 1.89 | trimer | ||
| tricyclene | 508-32-7 | C10H16 | 136.2 | 905.0 | 1.66 | 2.01% | 1.01–2.55% | |
| α-terpinene | 99-86-5 | C10H16 | 136.2 | 1006.7 | 1.22 | 1.97% | 1.22–2.55% | monomer |
| α-terpinene | 99-86-5 | C10H16 | 136.2 | 1009.3 | 1.72 | dimer | ||
| terpinen-4-ol | 562-74-3 | C10H18O | 154.3 | 1163.6 | 1.22 | 1.61% | 1.12–2.49% | monomer |
| terpinen-4-ol | 562-74-3 | C10H18O | 154.3 | 1164.2 | 1.72 | dimer | ||
| (Z)-β-farnesene | 28973-97-9 | C15H24 | 204.4 | 1489.7 | 1.45 | 1.50% | 0.76–2.18% | |
| coumarin | 91-64-5 | C9H6O2 | 146.1 | 1520.6 | 1.22 | 1.46% | 0.79–3.05% | |
| 2-methoxy-4-methylphenol | 93-51-6 | C8H10O2 | 138.2 | 1163.6 | 1.19 | 1.09% | 0.53–1.94% | |
| geraniol | 106-24-1 | C10H18O | 154.3 | 1267.9 | 1.22 | 1.08% | 0.75–1.48% | |
| decanal | 112-31-2 | C10H20O | 156.3 | 1261.4 | 1.55 | 1.06% | 0.55–1.98% | monomer |
| decanal | 112-31-2 | C10H20O | 156.3 | 1260.6 | 2.06 | dimer | ||
| propan-2-one | 67-64-1 | C3H6O | 58.1 | 485.7 | 1.12 | 0.96% | 0.42–1.82% | |
| trans-p-menth-2-en-1-ol | 29803-81-4 | C10H18O | 154.3 | 1137.0 | 1.70 | 0.95% | 0.54–2.07% | |
| myrcene | 123-35-3 | C10H16 | 136.2 | 994.9 | 1.68 | 0.83% | 0.16–1.32% | |
| γ-octalactone | 104-50-7 | C8H14O2 | 142.2 | 1298.5 | 1.31 | 0.80% | 0.31–2.08% | monomer |
| γ-octalactone | 104-50-7 | C8H14O2 | 142.2 | 1299.3 | 1.80 | dimer | ||
| acetic acid | 64-19-7 | C2H4O2 | 60.1 | 576.3 | 1.16 | 0.64% | 0.39–0.98% | |
| α-pinene | 80-56-8 | C10H16 | 136.2 | 931.1 | 1.21 | 0.63% | 0.18–0.96% | |
| 2-methylprop-2-enal | 78-85-3 | C4H6O | 70.1 | 581.9 | 1.21 | 0.57% | 0.42–0.92% | |
| borneol | 507-70-0 | C10H18O | 154.3 | 1184.3 | 1.90 | 0.57% | 0.44–0.76% | |
| citral | 5392-40-5 | C10H16O | 152.2 | 1309.3 | 1.05 | 0.55% | 0.42–0.83% | monomer |
| citral | 5392-40-5 | C10H16O | 152.2 | 1310.1 | 1.61 | dimer | ||
| benzothiazole | 95-16-9 | C7H5NS | 135.2 | 1229.2 | 1.16 | 0.49% | 0.25–0.77% | |
| 1-(furan-2-yl)ethanone | 1192-62-7 | C6H6O2 | 110.1 | 893.5 | 1.12 | 0.49% | 0.21–0.69% | monomer |
| 1-(furan-2-yl)ethanone | 1192-62-7 | C6H6O2 | 110.1 | 893.5 | 1.44 | dimer | ||
| 3-methylbut-2-enal | 107-86-8 | C5H8O | 84.1 | 766.6 | 1.36 | 0.49% | 0.32–0.65% | |
| 3-methylbutyl hexanoate | 2198-61-0 | C11H22O2 | 186.3 | 1281.4 | 1.53 | 0.46% | 0.20–1.10% | monomer |
| 3-methylbutyl hexanoate | 2198-61-0 | C11H22O2 | 186.3 | 1280.5 | 2.15 | dimer | ||
| ethyl octanoate | 106-32-1 | C10H20O2 | 172.3 | 1256.1 | 1.49 | 0.45% | 0.16–0.61% | |
| methanol | 67-56-1 | CH4O | 32 | 393.2 | 0.99 | 0.44% | 0.05–0.86% | |
| furfural | 98-01-1 | C5H4O2 | 96.1 | 812.1 | 1.08 | 0.43% | 0.32–0.63% | monomer |
| furfural | 98-01-1 | C5H4O2 | 96.1 | 814.2 | 1.33 | dimer | ||
| methyl acetate | 79-20-9 | C3H6O2 | 74.1 | 537.0 | 1.19 | 0.42% | 0.23–0.88% | |
| 2-methylbutanal | 96-17-3 | C5H10O | 86.1 | 657.6 | 1.40 | 0.31% | 0.17–0.60% | |
| butanoic acid | 107-92-6 | C4H8O2 | 88.1 | 788.9 | 1.17 | 0.30% | 0.13–0.57% | |
| propanal | 123-38-6 | C3H6O | 58.1 | 526.6 | 1.15 | 0.28% | 0.16–0.42% | |
| ethanol | 64-17-5 | C2H6O | 46.1 | 441.8 | 1.05 | 0.27% | 0.11–0.70% | monomer |
| ethanol | 64-17-5 | C2H6O | 46.1 | 442.1 | 1.14 | dimer | ||
| vanillin | 121-33-5 | C8H8O3 | 152.1 | 1408.6 | 1.27 | 0.27% | 0.20–0.43% | |
| butan-2-one | 78-93-3 | C4H8O | 72.1 | 582.7 | 1.25 | 0.27% | 0.11–0.46% | |
| octanal | 124-13-0 | C8H16O | 128.2 | 998.2 | 1.82 | 0.24% | 0.04–0.93% | |
| isopentanol | 123-51-3 | C5H12O | 88.1 | 715.4 | 1.25 | 0.24% | 0.02–0.41% | monomer |
| isopentanol | 123-51-3 | C5H12O | 88.1 | 716.7 | 1.50 | dimer | ||
| benzaldehyde | 100-52-7 | C7H6O | 106.1 | 945.4 | 1.15 | 0.24% | 0.21–0.29% | monomer |
| benzaldehyde | 100-52-7 | C7H6O | 106.1 | 946.7 | 1.47 | dimer | ||
| acetophenone | 98-86-2 | C8H8O | 120.2 | 1073.1 | 1.19 | 0.23% | 0.15–0.31% | |
| butane-2,3-dione | 431-03-8 | C4H6O2 | 86.1 | 572.1 | 1.18 | 0.22% | 0.17–0.26% | |
| propan-1-ol | 67-63-0 | C3H8O | 60.1 | 493.4 | 1.18 | 0.21% | 0.08–0.47% | |
| ethyl decanoate | 110-38-3 | C12H24O2 | 200.3 | 1411.3 | 1.61 | 0.21% | 0.19–0.23% | |
| (Z)-dec-4-enal | 21662-09-9 | C10H18O | 154.3 | 1191.1 | 1.34 | 0.20% | 0.17–0.25% | |
| (methyldisulfanyl)methane | 624-92-0 | C2H6S2 | 94.2 | 726.4 | 0.99 | 0.18% | 0.09–0.45% | |
| (E)-hex-2-en-1-ol | 928-95-0 | C6H12O | 100.2 | 833.9 | 1.18 | 0.17% | 0.02–0.57% | monomer |
| (E)-hex-2-en-1-ol | 928-95-0 | C6H12O | 100.2 | 832.0 | 1.52 | dimer | ||
| pentanoic acid | 109-52-4 | C5H10O2 | 102.1 | 892.7 | 1.22 | 0.14% | 0.05–0.23% | |
| ethyl acetate | 141-78-6 | C4H8O2 | 88.1 | 609.7 | 1.34 | 0.14% | 0.11–0.18% | |
| heptan-2-one | 110-43-0 | C7H14O | 114.2 | 870.2 | 1.26 | 0.14% | 0.05–0.26% | monomer |
| heptan-2-one | 110-43-0 | C7H14O | 114.2 | 870.2 | 1.63 | dimer | ||
| benzyl propionate | 122-63-4 | C10H12O2 | 164.2 | 1347.4 | 1.36 | 0.14% | 0.08–0.29% | |
| methylpropanal | 78-84-2 | C4H8O | 72.1 | 554.7 | 1.28 | 0.14% | 0.06–0.20% | |
| 4-methyl-3-penten-2-one | 141-79-7 | C6H10O | 98.1 | 778.5 | 1.44 | 0.13% | 0.03–0.19% | |
| hexanal | 66-25-1 | C6H12O | 100.2 | 779.6 | 1.56 | 0.13% | 0.03–0.41% | dimer |
| 2-methylbutanoic acid | 116-53-0 | C5H10O2 | 102.1 | 879.9 | 1.20 | 0.12% | 0.07–0.16% | |
| citronellol | 106-22-9 | C10H20O | 156.3 | 1266.1 | 1.85 | 0.11% | 0.09–0.14% | |
| heptanal | 111-71-7 | C7H14O | 114.2 | 879.9 | 1.35 | 0.11% | 0.03–0.37% | monomer |
| heptanal | 111-71-7 | C7H14O | 114.2 | 882.1 | 1.70 | dimer | ||
| ethyl propanoate | 105-37-3 | C5H10O2 | 102.1 | 693.4 | 1.45 | 0.11% | 0.03–0.17% | |
| hexan-2-ol | 626-93-7 | C6H14O | 102.2 | 766.6 | 1.29 | 0.10% | 0.04–0.16% | |
| pent-1-en-3-one | 1629-58-9 | C5H8O | 84.1 | 672.5 | 1.31 | 0.10% | 0.02–0.28% | |
| (E)-hept-2-enal | 18829-55-5 | C7H12O | 112.2 | 929.7 | 1.26 | 0.09% | 0.06–0.13% | monomer |
| (E)-hept-2-enal | 18829-55-5 | C7H12O | 112.2 | 942.3 | 1.67 | dimer | ||
| 3-methylbutanal | 590-86-3 | C5H10O | 86.1 | 643.4 | 1.41 | 0.09% | <0.01–0.17% | |
| pentan-1-ol | 71-41-0 | C5H12O | 88.1 | 748.1 | 1.26 | 0.08% | 0.04–0.13% | |
| 1-hydroxypropan-2-one | 116-09-6 | C3H6O2 | 74.1 | 640.2 | 1.22 | 0.08% | 0.05–0.11% | |
| 6-methyl-5-hepten-2-one | 110-93-0 | C8H14O | 126.2 | 972.7 | 1.17 | 0.08% | 0.02–0.18% | |
| ethyl benzoate | 93-89-0 | C9H10O2 | 150.2 | 1179.4 | 1.27 | 0.08% | 0.05–0.13% | |
| 1-penten-3-ol | 616-25-1 | C5H10O | 86.1 | 678.0 | 1.34 | 0.07% | 0.02–0.16% | |
| hexan-1-ol | 111-27-3 | C6H14O | 102.2 | 855.0 | 1.33 | 0.07% | 0.06–0.09% | monomer |
| hexan-1-ol | 111-27-3 | C6H14O | 102.2 | 855.6 | 1.64 | dimer | ||
| isovaleric acid | 503-74-2 | C5H10O2 | 102.1 | 879.9 | 1.23 | 0.07% | 0.04–0.10% | |
| benzeneacetaldehyde | 122-78-1 | C8H8O | 120.2 | 1028.1 | 1.26 | 0.06% | 0.05–0.07% | |
| 3-hydroxybutan-2-one | 513-86-0 | C4H8O2 | 88.1 | 702.5 | 1.33 | 0.06% | 0.03–0.08% | |
| butanal | 123-72-8 | C4H8O | 72.1 | 597.3 | 1.29 | 0.05% | 0.02–0.06% | |
| pyrrole | 109-97-7 | C4H5N | 67.1 | 743.2 | 0.97 | 0.05% | 0.02–0.08% | |
| 3-methylpentan-1-ol | 589-35-5 | C6H14O | 102.2 | 831.3 | 1.60 | 0.04% | 0.02–0.07% | |
| 3-methylpentan-2-one | 565-61-7 | C6H12O | 100.2 | 753.8 | 1.49 | 0.04% | 0.03–0.05% | |
| hexan-2-one | 591-78-6 | C6H12O | 100.2 | 768.7 | 1.20 | 0.04% | 0.02–0.06% | monomer |
| hexan-2-one | 591-78-6 | C6H12O | 100.2 | 768.2 | 1.51 | dimer | ||
| (E)-pent-2-enal | 1576-87-0 | C5H8O | 84.1 | 739.2 | 1.36 | 0.04% | 0.02–0.10% | |
| 3-methylsulfanylpropanal | 3268-49-3 | C4H8OS | 104.2 | 887.8 | 1.09 | 0.04% | 0.02–0.06% | |
| 2-methylfuran-3-thiol | 28588-74-1 | C5H6OS | 114.2 | 871.4 | 1.14 | 0.04% | 0.01–0.09% | |
| 2-oxopropyl acetate | 592-20-1 | C5H8O3 | 116.1 | 848.3 | 1.04 | 0.03% | <0.01–0.12% | |
| ethyl 2-methylbutanoate | 7452-79-1 | C7H14O2 | 130.2 | 853.2 | 1.23 | 0.03% | 0.02–0.04% | |
| pentanal | 110-62-3 | C5H10O | 86.1 | 688.3 | 1.42 | 0.03% | 0.01–0.07% | |
| ethyl 2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 753.4 | 1.56 | 0.02% | <0.01–0.05% | |
| 2-methylbutan-1-ol | 137-32-6 | C5H12O | 88.1 | 736.7 | 1.48 | 0.02% | 0.01–0.03% | |
| 2-ethyl pyrazine | 13925-00-3 | C6H8N2 | 108.1 | 918.8 | 1.12 | 0.02% | 0.01–0.04% | |
| (E)-2-methylpent-2-enal | 623-36-9 | C6H10O | 98.1 | 818.3 | 1.49 | 0.02% | 0.01–0.03% | |
| 2,5-dimethylfuran | 625-86-5 | C6H8O | 96.1 | 705.0 | 1.36 | 0.02% | 0.01–0.03% | |
| propanol | 71-23-8 | C3H8O | 60.1 | 540.0 | 1.24 | 0.02% | <0.01–0.04% | |
| furan-2-ylmethanol | 98-00-0 | C5H6O2 | 98.1 | 846.7 | 1.38 | 0.02% | 0.01–0.03% | |
| (Z)-3-hexen-1-ol | 928-96-1 | C6H12O | 100.2 | 844.0 | 1.52 | 0.02% | 0.01–0.05% | |
| isopropyl acetate | 108-21-4 | C5H10O2 | 102.1 | 652.2 | 1.48 | 0.01% | 0.01–0.03% | |
| isoamyl acetate | 123-92-2 | C7H14O2 | 130.2 | 854.9 | 1.75 | 0.01% | 0.01% | |
| ethyl 2-methylbutanoate | 7452-79-1 | C7H14O2 | 130.2 | 826.9 | 1.65 | 0.01% | 0.01% | |
| methyl 3-methylbutanoate | 556-24-1 | C6H12O2 | 116.2 | 756.1 | 1.53 | 0.01% | 0.01 |
| No. | Name | Species | Place of Origin |
|---|---|---|---|
| 1 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 2 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 3 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 4 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 5 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 6 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 7 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 8 | Qu Aurantii Fructus | Citrus changshan-huyou Y.B.Chang | Quzhou City, Zhejiang Province |
| 9 | Aurantii Fructus | Citrus aurantium L. | Qijiang County, Sichuan Province |
| 10 | Aurantii Fructus | Citrus aurantium L. | Chongqing City |
| 11 | Aurantii Fructus | Citrus aurantium ‘Huangpi’ | Yuanjiang City, Hunan Province, |
| 12 | Aurantii Fructus | Citrus aurantium cv. Xiucheng | Jiujiang City, Jiangxi Province |
| 13 | Aurantii Fructus | Citrus aurantium cv. Xiucheng | Zhangshu City, Jiangxi Province |
| 14 | Aurantii Fructus | Citrus aurantium cv. Xiucheng | Sanhu Town, Xingan County, Jiangxi Province |
| 15 | Aurantii Fructus | Citrus aurantium ‘Daidai’ | Quzhou City, Zhejiang Province |
| 16 | Aurantii Fructus | Citrus aurantium ‘Chuluan’ | Dongtou, Wenzhou City, Zhejiang Province |
| 17 | adulterants | Citrus wilsonii Tana-ka, | Hanzhong City, Shanxi Province |
| 18 | adulterants | Citrusreticulata ‘Unshiu’ | Wenzhou City, Zhejiang Province |
| 19 | adulterants | Citrussinensis (Linn.) Osbeck | Quzhou City, Zhejiang Province |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; He, J.; Xiao, Q.; Chen, B.; Zhang, W. Development of the Volatile Fingerprint of Qu Aurantii Fructus by HS-GC-IMS. Molecules 2022, 27, 4537. https://doi.org/10.3390/molecules27144537
Fang C, He J, Xiao Q, Chen B, Zhang W. Development of the Volatile Fingerprint of Qu Aurantii Fructus by HS-GC-IMS. Molecules. 2022; 27(14):4537. https://doi.org/10.3390/molecules27144537
Chicago/Turabian StyleFang, Cuifen, Jia He, Qi Xiao, Bilian Chen, and Wenting Zhang. 2022. "Development of the Volatile Fingerprint of Qu Aurantii Fructus by HS-GC-IMS" Molecules 27, no. 14: 4537. https://doi.org/10.3390/molecules27144537





