Styrylpyrone Derivative (SPD) Extracted from Goniothalamus umbrosus Binds to Dengue Virus Serotype-2 Envelope Protein and Inhibits Early Stage of Virus Replication
Abstract
:1. Introduction
2. Results
2.1. Isolation of SPD
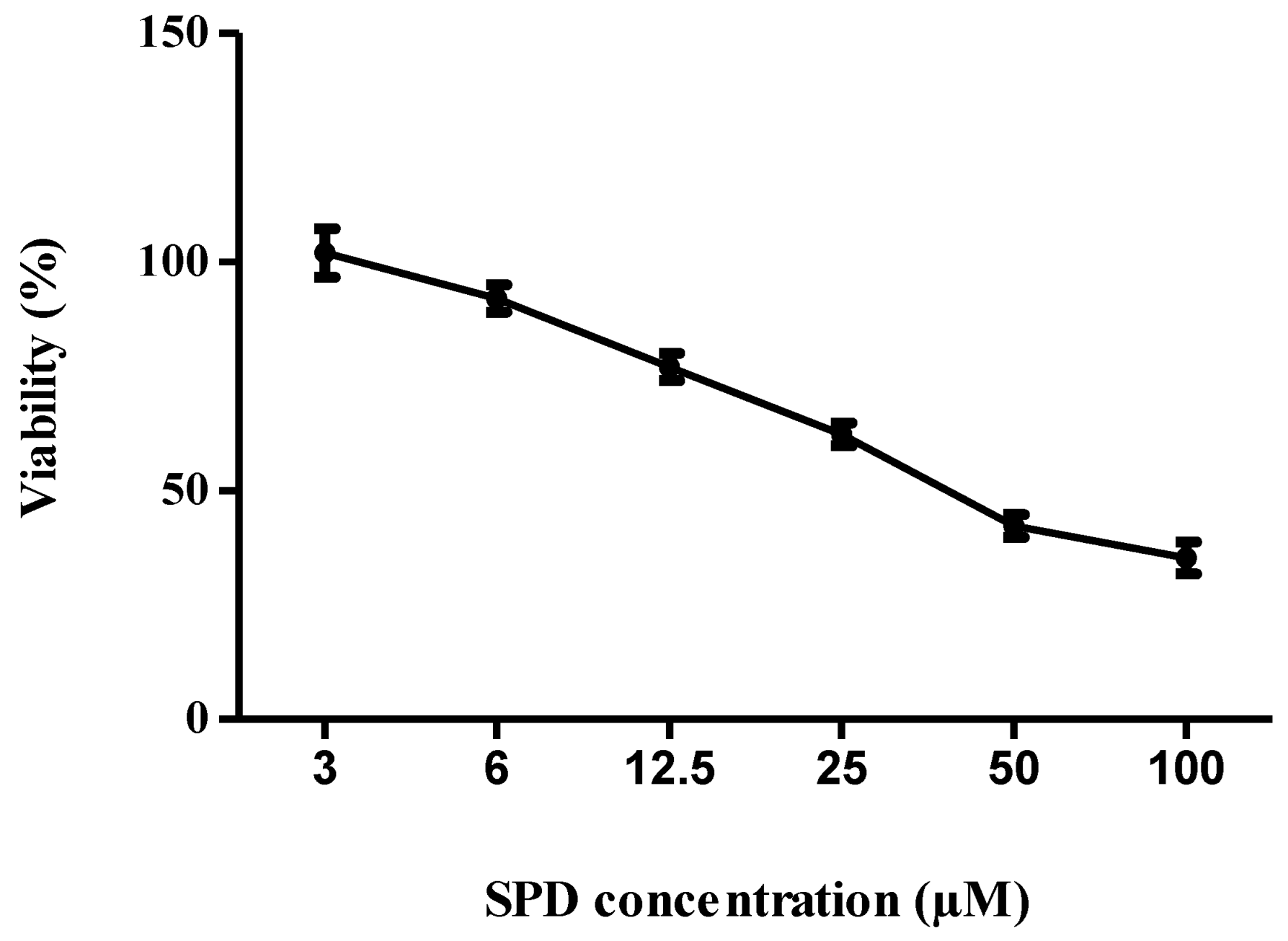
2.2. Cytotoxicity of SPD
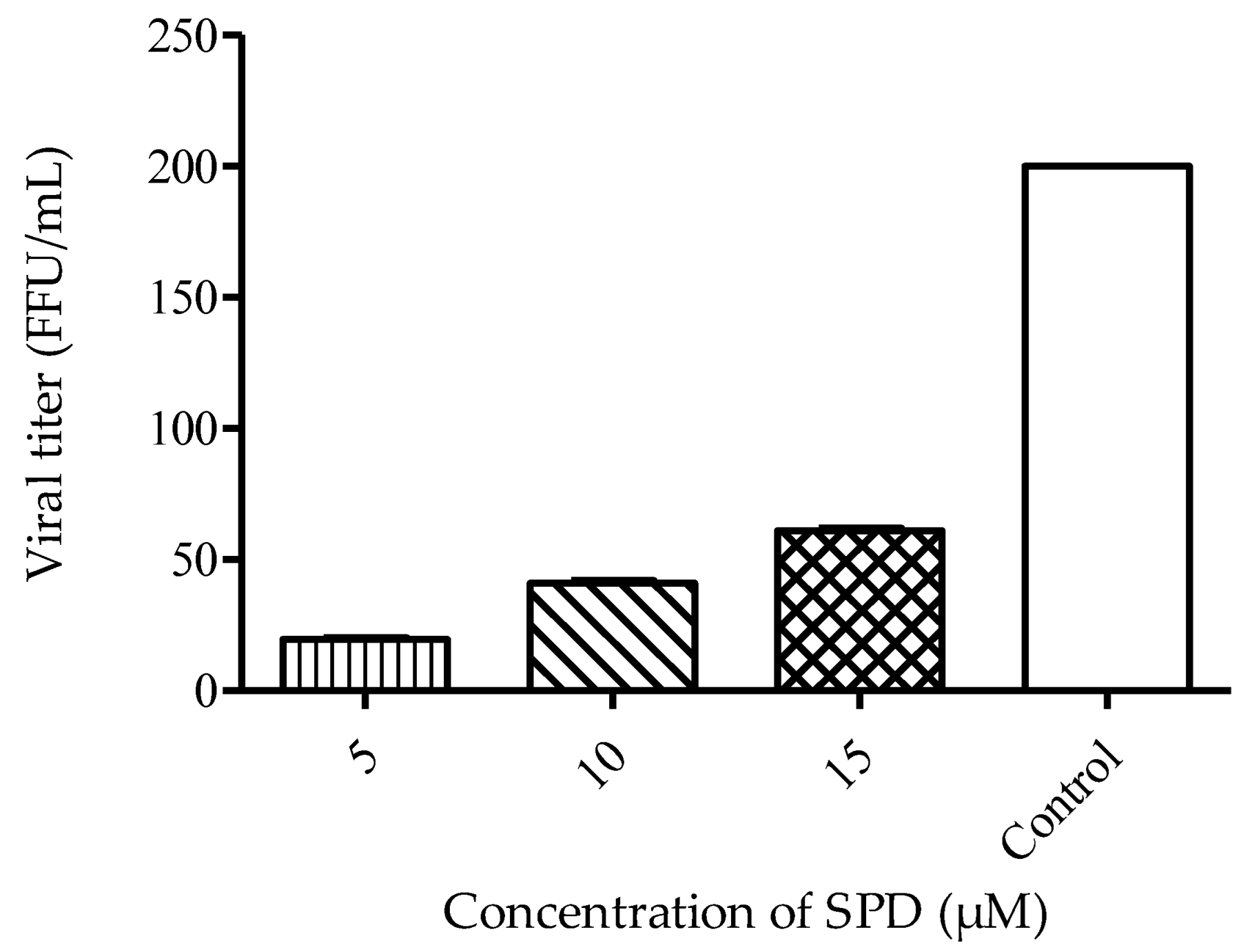
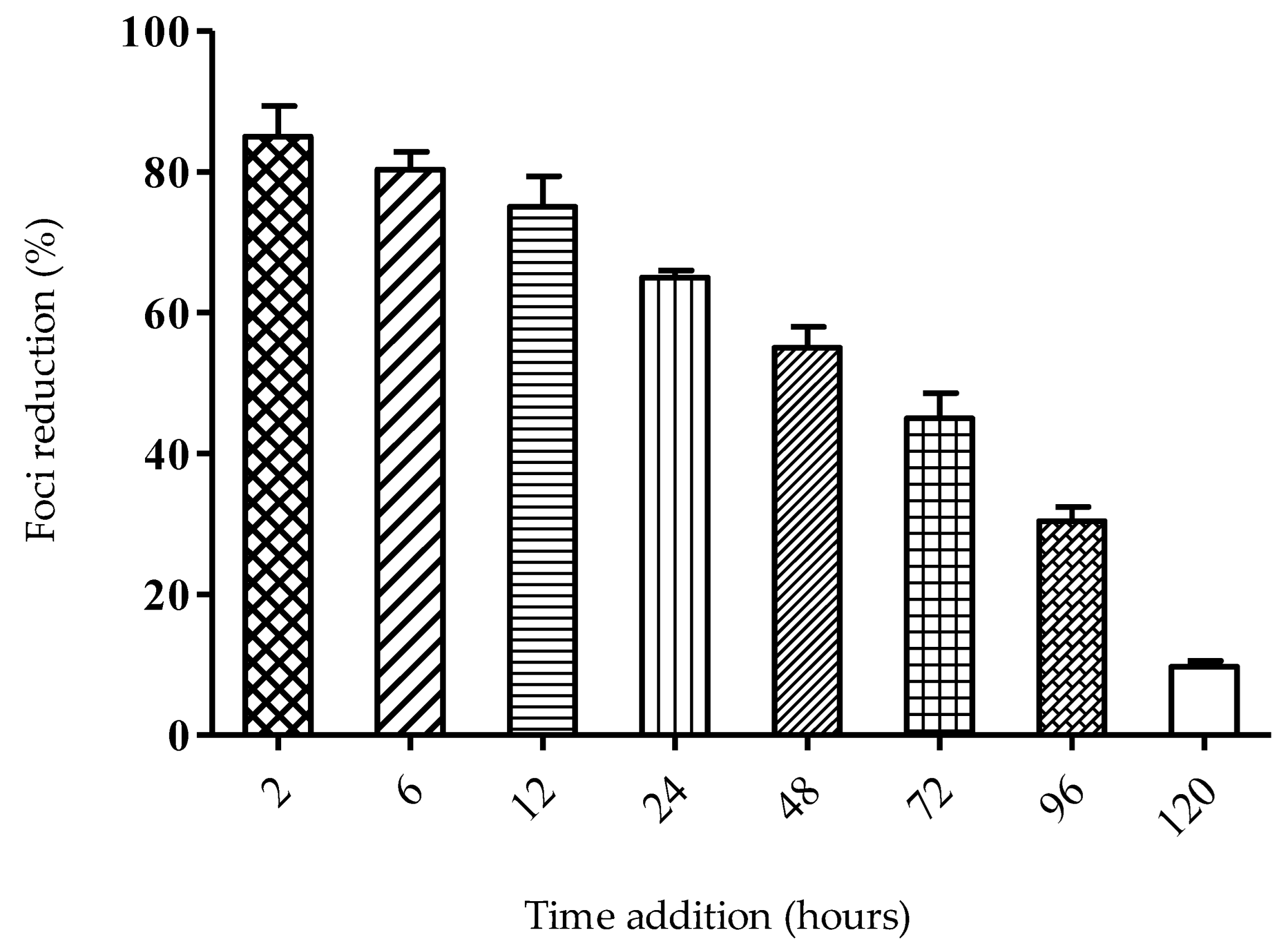
2.3. Mode of Action of SPD
2.4. SPD Alters E Gene during DENV-2 Replication
2.5. SPD Inhibits DENV-2 E Protein
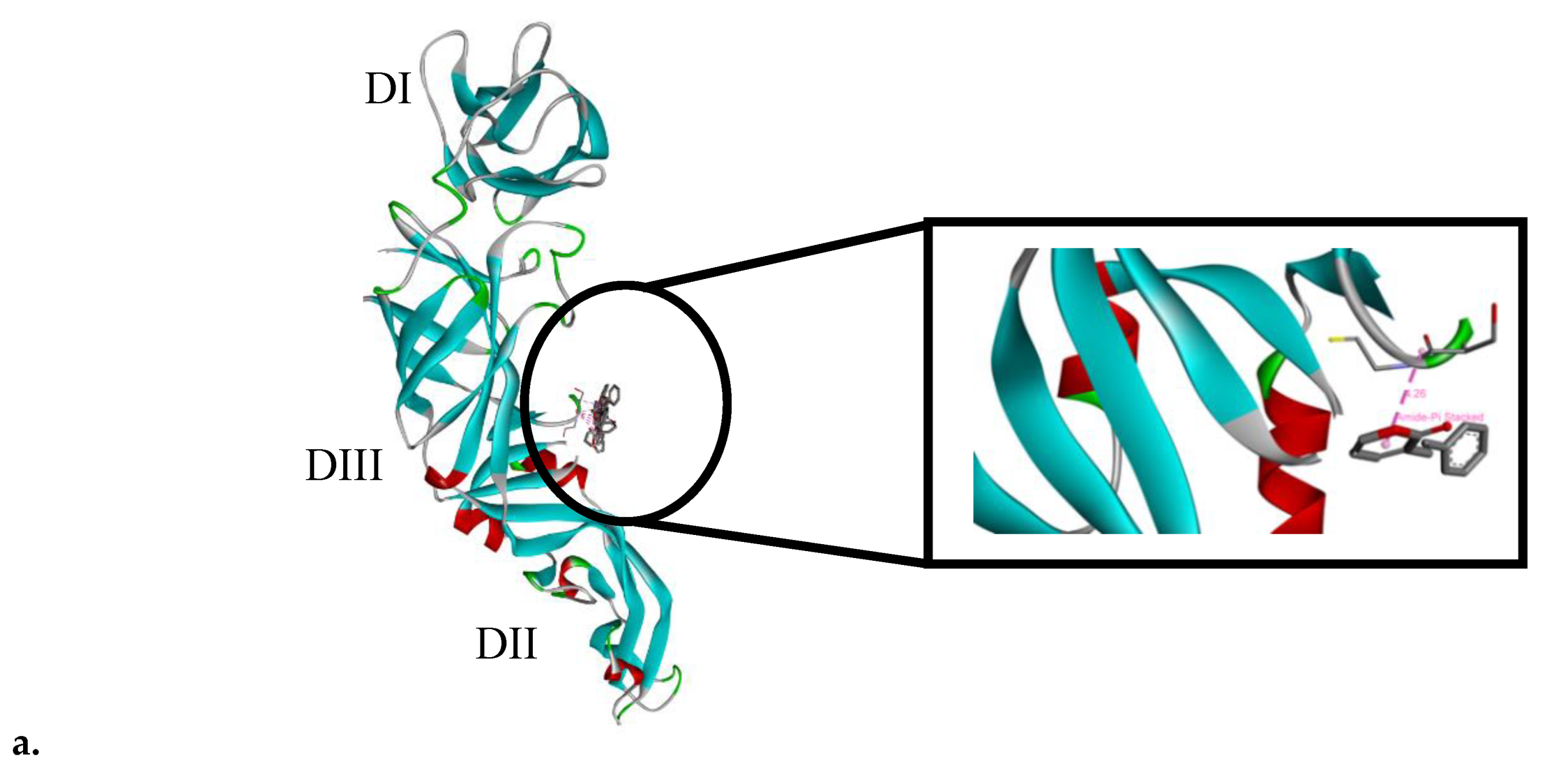
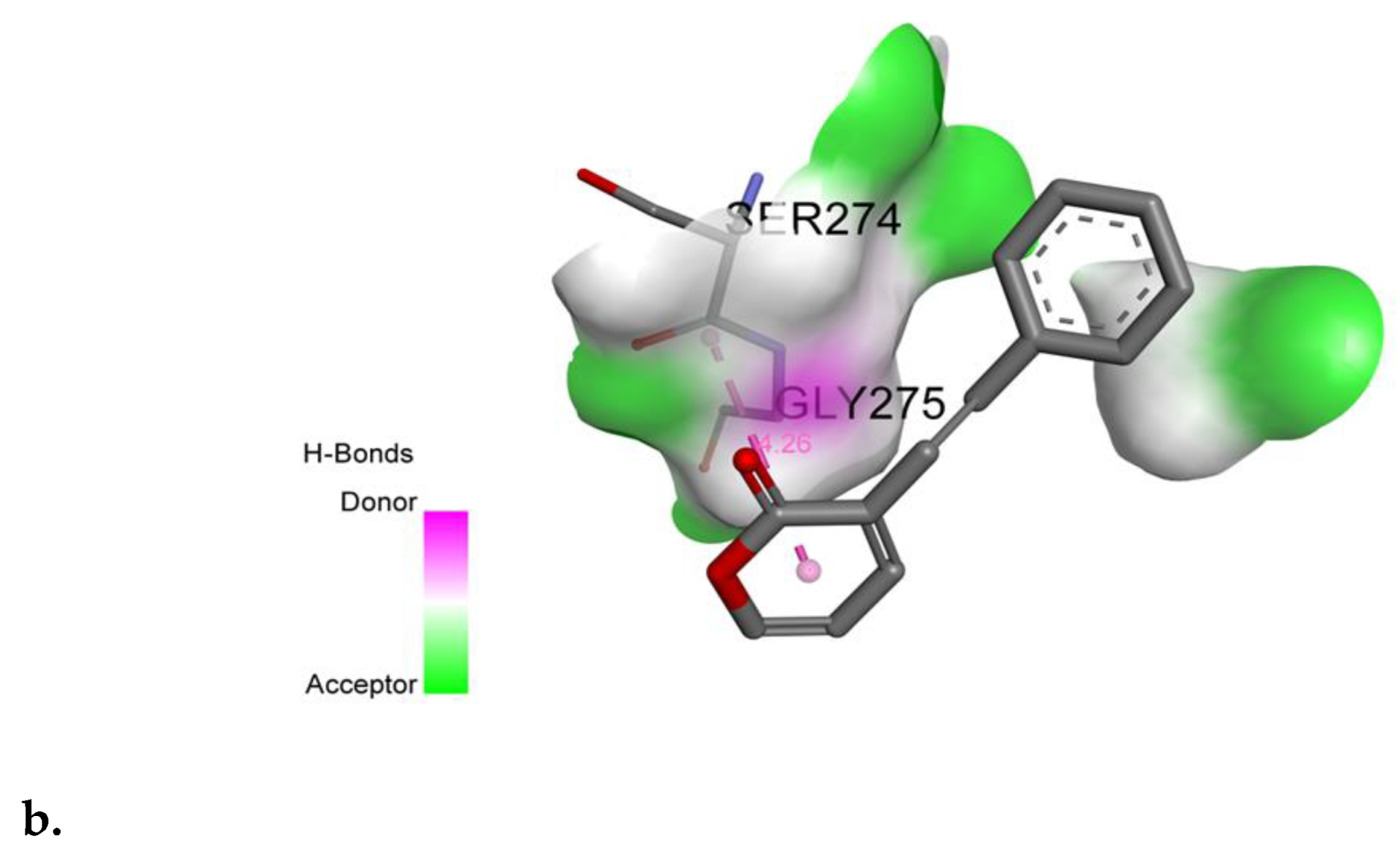
2.6. SPD Binds to Domain III of DENV-2 E Protein
3. Discussion
4. Materials and Methods
4.1. Synthesis of SPD
4.2. Cytotoxicity Assay
4.3. Anti-viral Mechanism Assay
4.3.1. Virus Infectivity Assay
4.3.2. Time-of-Addition Assay
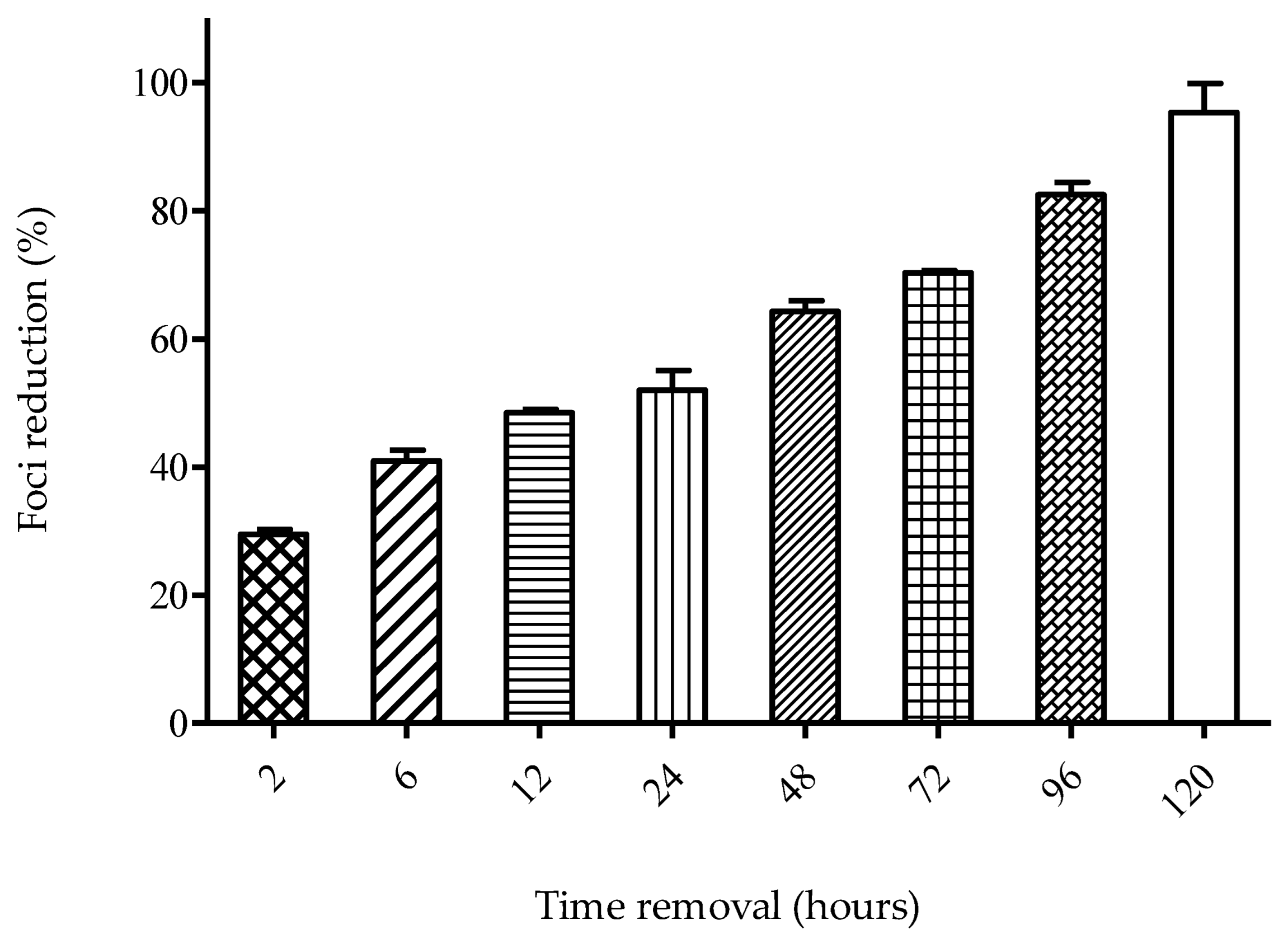
4.3.3. Time-Removal Assay
4.4. Quantitative Real-Time–Polymerase Chain Reaction (qRT-PCR)
4.5. Molecular Docking
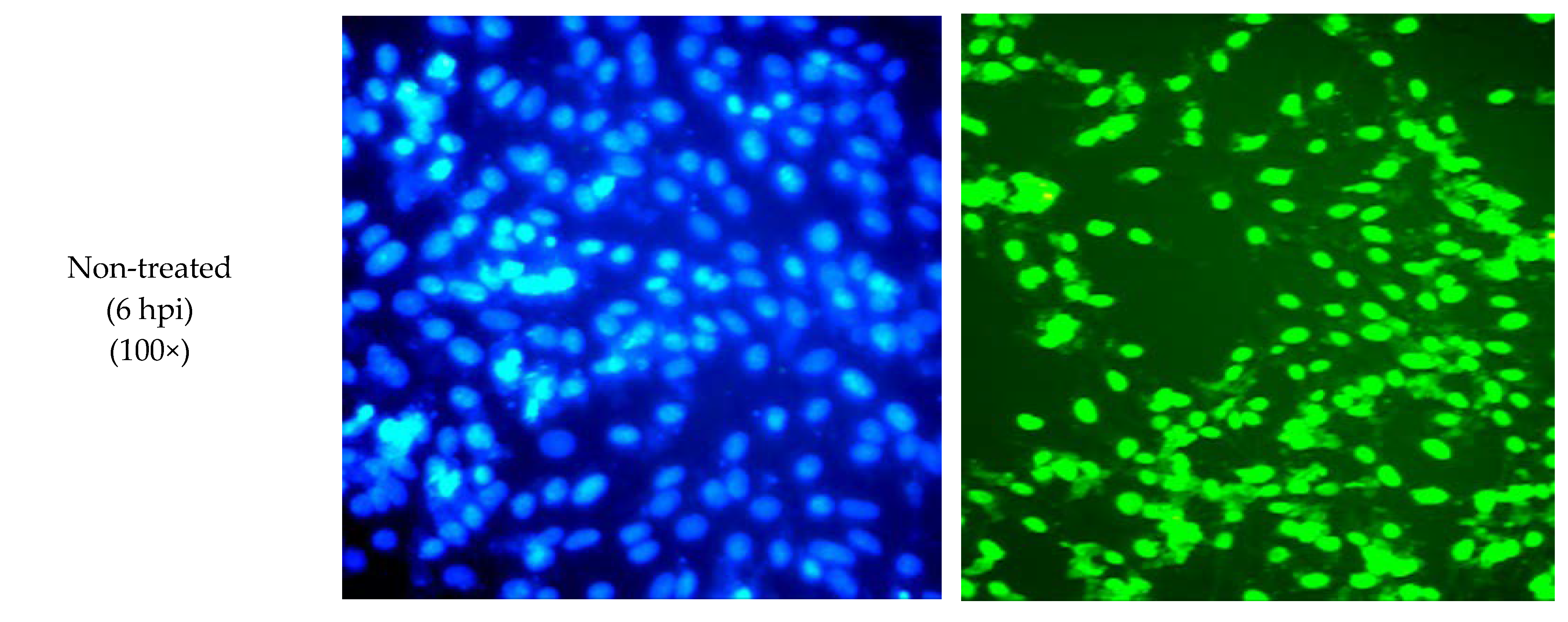
4.6. Immunofluorescent Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, W.; Ping, Z. Recent Advances in The Identification of The Host Factors Involved in Dengue Virus Replication. Virol. Sin. 2017, 32, 23–31. [Google Scholar] [CrossRef] [Green Version]
- King, C.A.; Wegman, A.D.; Endy, T.P. Mobilization and Activation of the Innate Immune Response to Dengue Virus. Front. Cell. Infect. Microbiol. 2020, 10, 574417. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Urbina, A.N.; Chang, M.R.; Assavalapsakul, W.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. Dengue Hemorrhagic Fever—A Systemic Literature Review of Current Perspectives on Pathogenesis, Prevention and Control. J. Microbiol. Immunol. Infect. 2020, 53, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Abd Kadir, S.L.; Yaakob, H.; Zulkifli, R.M. Potential Anti-Dengue Medicinal Plants: A Review. J. Nat. Med. 2013, 67, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, K.P.; Sharma, N.; Diwaker, D.; Ganju, L.; Singh, S.B. Plant Derived Antivirals: A Potential Source of Drug Development. J. Virol. Antivir. Res. 2013, 2, 2. [Google Scholar]
- Sharma, N.; Mishra, K.P.; Ganju, L. Salidroside Exhibits Anti-Dengue Virus Activity by Upregulating Host Innate Immune Factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef]
- Rajapakse, S.; de Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Sigera, C.; Fernando, S.D. Carica papaya Extract in Dengue: A Systematic Review and Meta-Analysis. BMC Complement. Altern. Med. 2019, 19, 265. [Google Scholar] [CrossRef] [Green Version]
- Azeredo de, E.L.; Monteiro, R.Q.; de-Oliveira Pinto, L.M. Thrombocytopenia in Dengue: Interrelationship Between Virus and The Imbalance Between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediat. Inflamm. 2015, 2015, 313842. [Google Scholar] [CrossRef] [Green Version]
- Honda, S.; Saito, M.; Dimaano, E.M.; Morales, P.A.; Alonzo, M.T.; Suarez, L.A.; Koike, N.; Inoue, S.; Kumatori, A.; Matias, R.R.; et al. Increased Phagocytosis of Platelets from Patients with Secondary Dengue Virus Infection by Human Macrophages. Am. J. Trop. Med. Hyg. 2009, 80, 841–845. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of Anti-Dengue Activity of Carica papaya Aqueous Leaf Extract and Its Role in Platelet Augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Sharma, D.K.; Tiwari, B.; Singh, R.K.; Sahu, S.; Mathur, S.C.; Singh, R.M.; Singh, G.N. Estimation of Minerals in Carica papaya L. Leaf Found in Northern India by Using ICP-OES Technique. Int. J. Sci. Eng. Res. 2013, 4, 2012–2019. [Google Scholar]
- Sarala, N.; Paknikar, S.S. Papaya Extract to Treat Dengue: A Novel Therapeutic Option? Ann. Med. Health Sci. 2014, 4, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://malaya.com.ph/business-news/living/tawa-tawa-dengue-cure-capsule (accessed on 30 June 2022).
- Azimahtol, H.L.P.; Johnson, S.; Laily, B.D. Non-Steroid Receptor-Mediated Anti-Proliferative Activity of Styrylpyrone Derivative in Human Breast Cancer Cell Lines. Anticancer Res. 1998, 18, 1739–1744. [Google Scholar]
- Azimahtol, H.L.P.; Munawer, M.; Laily, B.D. Antifertility Effect of SPD: A Styrylpyrone Isolated from Goniothalamus tapis Miqo. Asia Pac. J. Pharmacol. 1994, 9, 273–277. [Google Scholar]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological Activities of Alkaloids: From Toxicology to Pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koduru, S.; Grierson, D.S.; van de Venter, M.; Afolayan, A.J. Anticancer Activity of Steroid Alkaloids Isolated from Solanum aculeastrum. Pharm. Biol. 2007, 45, 613–618. [Google Scholar] [CrossRef]
- Wu, S.-B.; Bao, Q.-Y.; Wang, W.-X.; Zhao, Y.; Xia, G.; Zhao, Z.; Zeng, H.; Hu, J.-F. Cytotoxic Triterpenoids and Steroids from the Bark of Melia azedarach. Planta Med. 2011, 77, 922–928. [Google Scholar] [CrossRef]
- Patel, S.S.; Savjani, J.K. Systematic Review of Plant Steroids as Potential Antiinflammatory Agents: Current Status and Future Perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Taleb-Contini, S.H.; Salvador, M.J.; Watanabe, E.; Ito, I.Y.; Oliveira, D.C.R. Antimicrobial Activity of Flavonoids and Steroids Isolated from Two Chromolaena species. Braz. J. Pharm. Sci. 2003, 39, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H. Antibacterial Action of Several Tannins Against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Juan, A.A.V.; Jose, J.B.F.; Antonio, A.C.; Arely, P.B.; Raul, R.H.; Cristobal, N.A. Ellagitannins: Biosynthesis, Biodegradation and Biological Properties. J. Med. Plants Res. 2011, 5, 4696–4703. [Google Scholar] [CrossRef]
- Yildirim, I.; Kutlu, T. Anticancer Agents: Saponin and Tannin. Int. J. Biol. Chem. 2015, 9, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Oerther, S.E. 2011. Plant Poisonings: Common Plants that Contain Cardiac Glycosides. J. Emerg. Nurs. 2011, 37, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Abdul, A.B.; Elhassan, M.M.; Al-zubairi, A.S.; Alhaj, N.A.; Abdullah, R.; Adam, A. Biological and Phytochemical Investigations of Goniothalamus umbrosus Leaves Hexane Extract. J. Med. Plants Res. 2009, 3, 883–888. [Google Scholar]
- Jewers, K.; Davis, J.B.; Dougan, J.; Manchanda, A.H.; Blunden, G.; Kyi, A.; Wetchapinan, S. Goniothalamin and Its Distribution in Four Goniothalamus Species. Phytochemistry 1972, 11, 2025–2030. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products: Synthesis, Function and Application; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kubitzki, K.; Rohwer, J.G.; Bittrich, V. Flowering Plants· Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Springer: Berlin/Heidelberg/Hamburg, Germany, 1993. [Google Scholar]
- Lee, I.K.; Seo, G.S.; Jeon, N.B.; Kang, H.W.; Yun, B.S. Phellinins A1 and A2, New Styrylpyrones from The Culture Broth of Phellinus sp. KACC93057P: I. Fermentation, Taxonomy, Isolation and Biological Properties. J. Antibiot. 2009, 62, 631–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazquez, M.A.; Bermejo, A.; Zafra-Polo, M.C.; Cortes, D. Styryl-lactones from Goniothalamus Species-A review. Phytochem. Anal. 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Ibrahim, N.; Shahar, S.; Wahab, N.Z.A.; Nor, N.S.M. Effect of Styrylpyrone Derivative (SPD) and SPD/Foscarnet Combination Towards Virus Infected Cell. AIP Conf. Proc. 2019, 2111, 040002. [Google Scholar] [CrossRef]
- Fouzi, S.S.; Abd Wahab, N.Z.; Yan, L.C.; Ibrahim, N. Styrylpyrone Derivative from Goniothalamus sp.: A Powerful Drug for Fighting Against Herpes Simplex Virus Type 1. Pharmacogn. J. 2021, 13, 1598–1606. [Google Scholar] [CrossRef]
- Yip, C.-W.; Nagaoka, Y.M.d.; Nor, N.S.; Ibrahim, N. In vitro Evaluation of Anticancer Effect and Neurotoxicity of Styrylpyrone Derivative (SPD). AIP Conf. Proc. 2016, 1784, 020013. [Google Scholar] [CrossRef]
- Schoenberger, T. Determination of Standard Sample Purity Using the High-Precision 1H-NMR Process. Anal. Bioanal. Chem. 2012, 403, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.A. Gas Chromatography-Mass Spectrometry (GC-MS): Applications in Archaeology. In Encyclopedia of Global Archaeology; Springer: New York, NY, USA, 2018; pp. 2953–2959. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Abd Wahab, N.Z.; Ibrahim, N. In vitro Study, Antiviral Activity of Styrylpyrone Derivative Against Dengue Virus Type 2. Asian J. Plant Sci. 2020, 19, 438–442. [Google Scholar]
- Bajaj, S.; Singla, D.; Sakhuja, N.J. Stability Testing of Pharmaceutical Products. J. Appl. Pharm. Sci. 2012, 02, 129–138. [Google Scholar]
- Fasihuddin, B.A.; Nisa, N.K.; Zaini, A. Chemical Constituents and Antiviral Study of Goniothalamus velutinus. J. Fundam. Sci. 2010, 6, 72–75. [Google Scholar]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of The Dengue Virus Envelope Protein After Membrane Fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef]
- Zheng, A.; Umashankar, M.; Kielian, M. In vitro and in vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions. PLoS Pathog. 2010, 6, e1001157. [Google Scholar] [CrossRef]
- Shukla, D.; Spear, P.G. Herpesviruses and Heparan Sulfate: An Intimate Relationship in Aid of Viral Entry. J. Clin. Investig. 2001, 108, 503–510. [Google Scholar] [CrossRef]
- Hisham, A.; Toubi, M.; Shuaily, W.; Ajitha, M.D.B.; Fujimoto, Y. Cardiobutanolide, A Styryl-Lactone from G. cardiopetalus. Phytochemistry 2003, 62, 597–600. [Google Scholar] [CrossRef]
- Wu, Y.C.; Duh, C.Y.; Chang, F.R.; Chang, G.Y.; Wang, S.K.; Chang, J.J.; McPhail, D.R.; McPhail, A.T.; Lee, K.-H. The Crystal Structure and Cytotoxicity of Goniodiol-7- Monoacetate from G. amuyon. J. Nat. Prod. 1991, 54, 1077–1081. [Google Scholar] [CrossRef]
- Mu, Q.; Tang, W.D.; Liu, R.Y.; Li, C.M.; Lou, L.G.; Sun, H.D.; Hu, C.Q. Constituents from The Stems of G. griffithii. Planta Med. 2003, 69, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Hasan, C.M.; Mia, M.Y.; Rashid, M.A.; Connolly, J.D. 5-Acetoxyisogoniothalamin Oxide, An Epoxystyryl Lactone from G. sesquipedalis. Phytochemistry 1994, 37, 1763–1764. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Mohajeri, S.A.; Seifi, M. Extraction and Purification of Crocin from Saffron Stigmas Employing a Simple and Efficient Crystallization Method. Pak. J. Biol. Sci. 2010, 13, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Nzikou, J.M.; Matouba, E.; Pandzou-Yembe, V.N.; Mapepoulou, T.G.; Linder, M.; Desobry, S. Studies of Irvingia gabonensis Seed Kernels: Oil Technological Applications. Pak. J. Nutr. 2009, 8, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Ahamad Bahtiar, A.; Md. Nor, N.S.; Ibrahim, N. tability Evaluation of Styrylpyrone Derivative (SPD) Incorporated Products. AIP Conf. Proc. 2015, 1678, 030005. [Google Scholar] [CrossRef]
- Lopes, V.R.; Schmidtke, M.; Fernandes, M.H.; Martins, R.; Vasconcelos, V. Cytotoxicity in L929 Fibroblasts and Inhibition of Herpes Simplex Virus Type 1 Kupka by Estuarine Cyanobacteria Extracts. Toxicol. Vitr. 2011, 25, 944–950. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lee, C.C.; Houghton, P. Cytotoxicity of Plants from Malaysia and Thailand Used Traditionally to Treat Cancer. J. Ethnopharmacol. 2005, 100, 237–243. [Google Scholar] [CrossRef]
- Prichard, M.N.; Turk, S.R.; Coleman, L.A.; Engelhardt, S.L.; Shipman, C.; Drach, J.C. A Microtiter Virus Yield Reduction Assay for The Evaluation of Antiviral Compounds Against Human Cytomegalovirus and Herpes Simplex Virus. J. Virol. Methods 1990, 28, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Suga, S.; Yoshikawa, T.; Yazaki, T.; Ozaki, T.; Asano, Y. Dose-Dependent Effects of Oral Acyclovir in The Incubation Period of Varicella. Acta Pædiatr. 1996, 85, 1418–1421. [Google Scholar] [CrossRef]
- Ståhle, E.L.; Schloss, L.; Sundqvist, V.A.; Brytting, M.; Hökeberg, I.; Cox, S.; Wahren, B.; Linde, A. Solid Phase ELISA for Determination of the Virus Dose Dependent Sensitivity of Human Cytomegalovirus to Antiviral Drugs in vitro. Antivir. Res. 1998, 40, 105–112. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Liu, M.C.; Lin, C.C. In vitro Antiviral Activities of Caesalpinia pulcherrima and Its Related Flavonoids. J. Antimicrob. Chemother. 2003, 52, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Md Nor, N.S.; Ibrahim, N. Styrylpyrone Derivative of Goniothalamus umbrosus Inhibit HSV-1 Infection During Viral Early Replication Cycle. Antivir. Res. 2011, 90, A21–A78. [Google Scholar] [CrossRef]
- Daelemans, D.; Pauwels, R.; De Clercq, E.; Pannecouque, C. A time-of-drug Addition Approach to Target Identification of Antiviral Compounds. Nat. Protoc. 2011, 6, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Era, S.; Watanabea, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.P.J.; Suzuki, T. Antiviral Activity of Chondroitin Sulphate E Targeting Dengue Virus Envelope Protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.O.; Costin, J.M.; Rowe, D.K.; Lin, L.; Jenwitheesuk, E.; Samudrala, R.; Isern, S.; Michael, S.F. Viral Entry Inhibitors Block Dengue Antibody-Dependent Enhancement in vitro. Antivir. Res. 2011, 89, 71–74. [Google Scholar] [CrossRef]
- Grinter, S.Z.; Zou, X. Challenges, Applications, and Recent Advances of Protein-Ligand Docking in Structure-Based Drug Design. Molecules 2014, 19, 10150–10176. [Google Scholar] [CrossRef] [Green Version]
- Abd Wahab, N.Z.; Azizul, A.; Ibrahim, N. Phytochemistry, Cytotoxicity and Antiviral Activity of Catharanthus roseus. Iran. J. Microbiol. 2020, 12, 460–465. [Google Scholar] [CrossRef]
- Schmidtke, M.; Schnittler, U.; Jahn, B.; Dahse, H.; Stelzner, A. A Rapid Assay for Evaluation of Antiviral Activity Against Coxsackie Virus B3, Influenza Virus A, and Herpes Simplex Virus Type 1. J. Virol. Methods 2001, 95, 133–143. [Google Scholar] [CrossRef]
- Moghaddam, E.; Teoh, B.T.; Sam, S.S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, A Metabolite of Baicalein with Antiviral Activity Against Dengue Virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [Green Version]
- Saddi, M.; Sanna, A.; Cottiglia, F.; Chisu, L.; Casu, L.; Bonsignore, L.; De Logu, A. Antiherpevirus Activity of Artemisia arborescens Essential Oil and Inhibition of Lateral Diffusion in Vero Cells. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, F.; Ishida, Y.; Oishi, S.; Fujii, N.; Watanabe, S.; Vasudevan, S.G.; Tajima, S.; Takasaki, T.; Suzuki, Y.; Ichiyama, K.; et al. Novel Antiviral Activity of Bromocriptine Against Dengue Virus Replication. Antivir. Res. 2016, 131, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Wahab, N.Z.; Ibrahim, N. Efficacy of Catharanthus Roseus Extract Against Dengue Virus Type 2 Infection in vitro. Indian J. Public Health Res. Dev. 2020, 11, 1320–1325. [Google Scholar] [CrossRef]
- Zhen, H.; Fang, F.; Ye, D.Y.; Shu, S.N.; Zhou, Y.F.; Dong, Y.S.; Nie, X.C.; Li, G. Experimental Study on The Action of Allitridin Against Human Cytomegalovirus in vitro: Inhibitory Effects on Immediate-Early Genes. Antivir. Res. 2006, 72, 68–74. [Google Scholar] [CrossRef]
- Iberahim, R.; Nor, N.S.M.; Yaacob, W.A.; Ibrahim, N. Eleusine indica Inhibits Early and Late Phases of Herpes Simplex Virus Type 1 Replication Cycle and Reduces Progeny Infectivity. Sains Malays. 2018, 47, 1431–1438. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with A New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Zhu, X.; Pitts, J.D.; Wang, J.; Potisopon, S.; Zhang, J.; Wojciechowski, A.; Justin, J.H.C.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef] [Green Version]
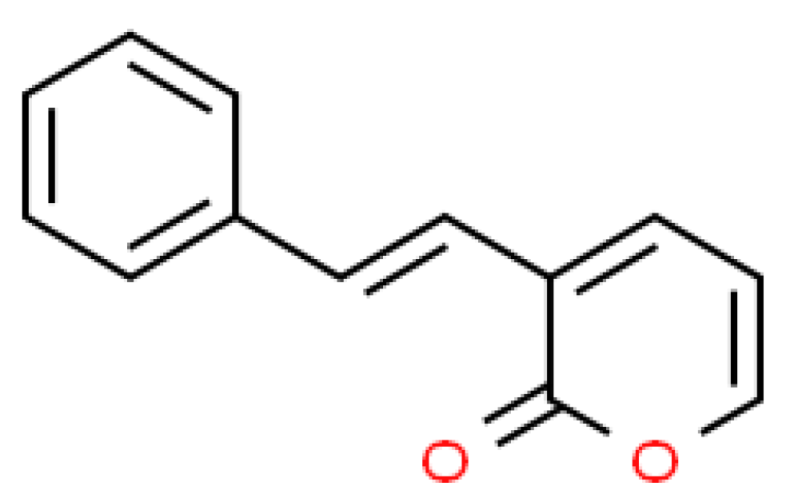



| Isolated SPD | Fasihuddin et al. [10] |
|---|---|
| 200 | 200 |
| 172 | 172 |
| 131 | 131 |
| 115 | 115 |
| 104 | 104 |
| 91 | 91 |
| 63 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Wahab, N.Z.; Ibrahim, N. Styrylpyrone Derivative (SPD) Extracted from Goniothalamus umbrosus Binds to Dengue Virus Serotype-2 Envelope Protein and Inhibits Early Stage of Virus Replication. Molecules 2022, 27, 4566. https://doi.org/10.3390/molecules27144566
Abd Wahab NZ, Ibrahim N. Styrylpyrone Derivative (SPD) Extracted from Goniothalamus umbrosus Binds to Dengue Virus Serotype-2 Envelope Protein and Inhibits Early Stage of Virus Replication. Molecules. 2022; 27(14):4566. https://doi.org/10.3390/molecules27144566
Chicago/Turabian StyleAbd Wahab, Noor Zarina, and Nazlina Ibrahim. 2022. "Styrylpyrone Derivative (SPD) Extracted from Goniothalamus umbrosus Binds to Dengue Virus Serotype-2 Envelope Protein and Inhibits Early Stage of Virus Replication" Molecules 27, no. 14: 4566. https://doi.org/10.3390/molecules27144566
APA StyleAbd Wahab, N. Z., & Ibrahim, N. (2022). Styrylpyrone Derivative (SPD) Extracted from Goniothalamus umbrosus Binds to Dengue Virus Serotype-2 Envelope Protein and Inhibits Early Stage of Virus Replication. Molecules, 27(14), 4566. https://doi.org/10.3390/molecules27144566






