Andrographolide Inhibits Epstein–Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Andrographolide
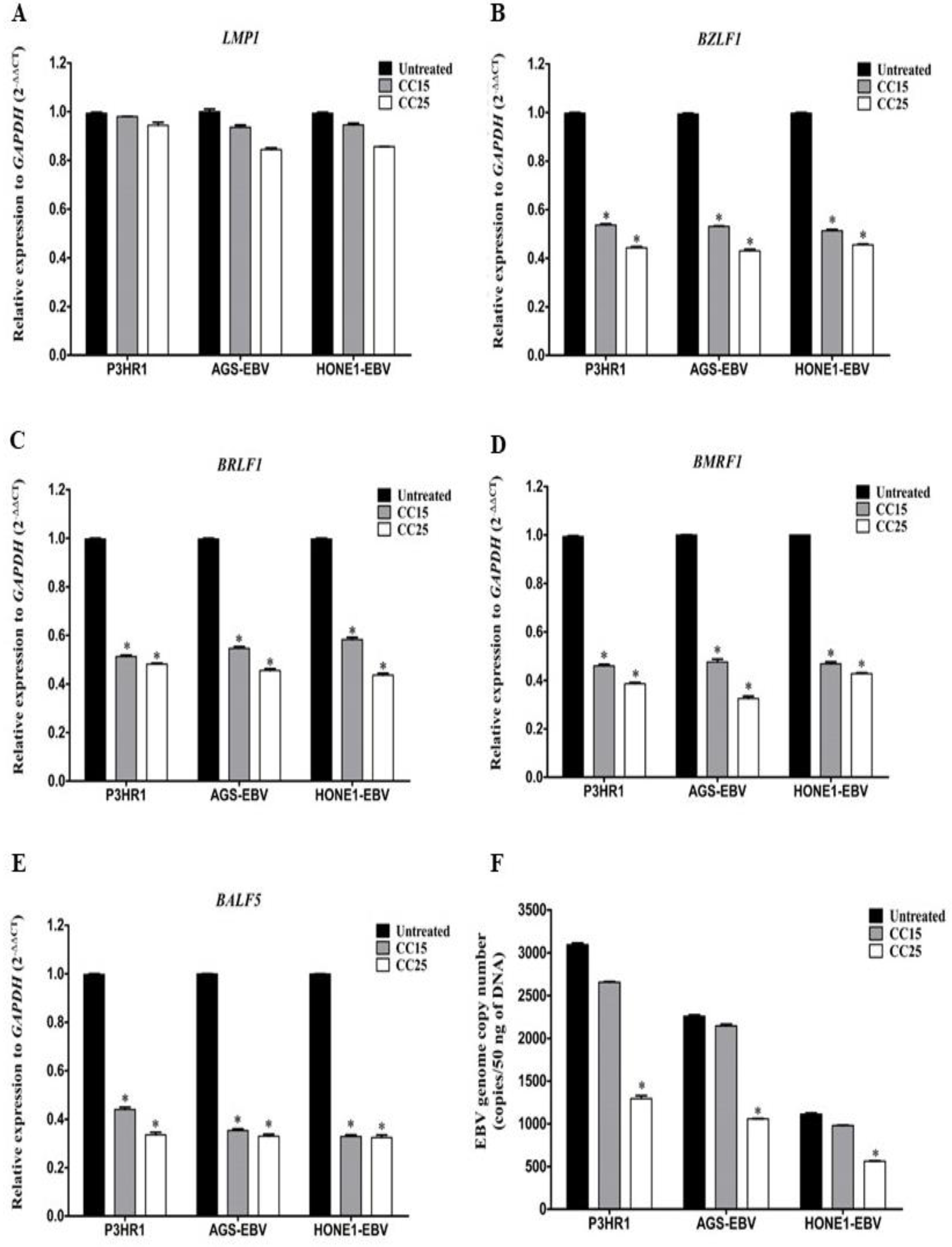
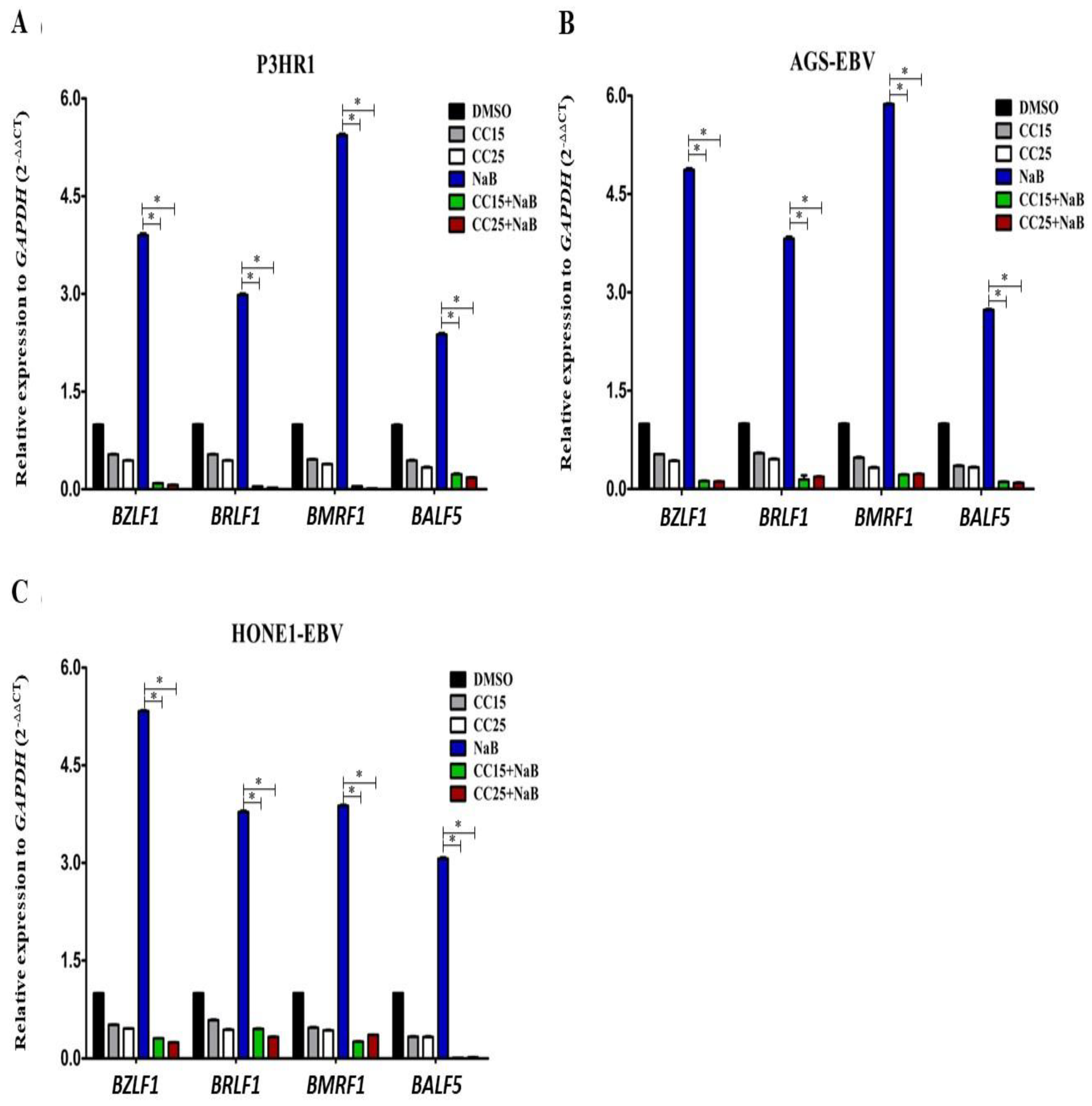
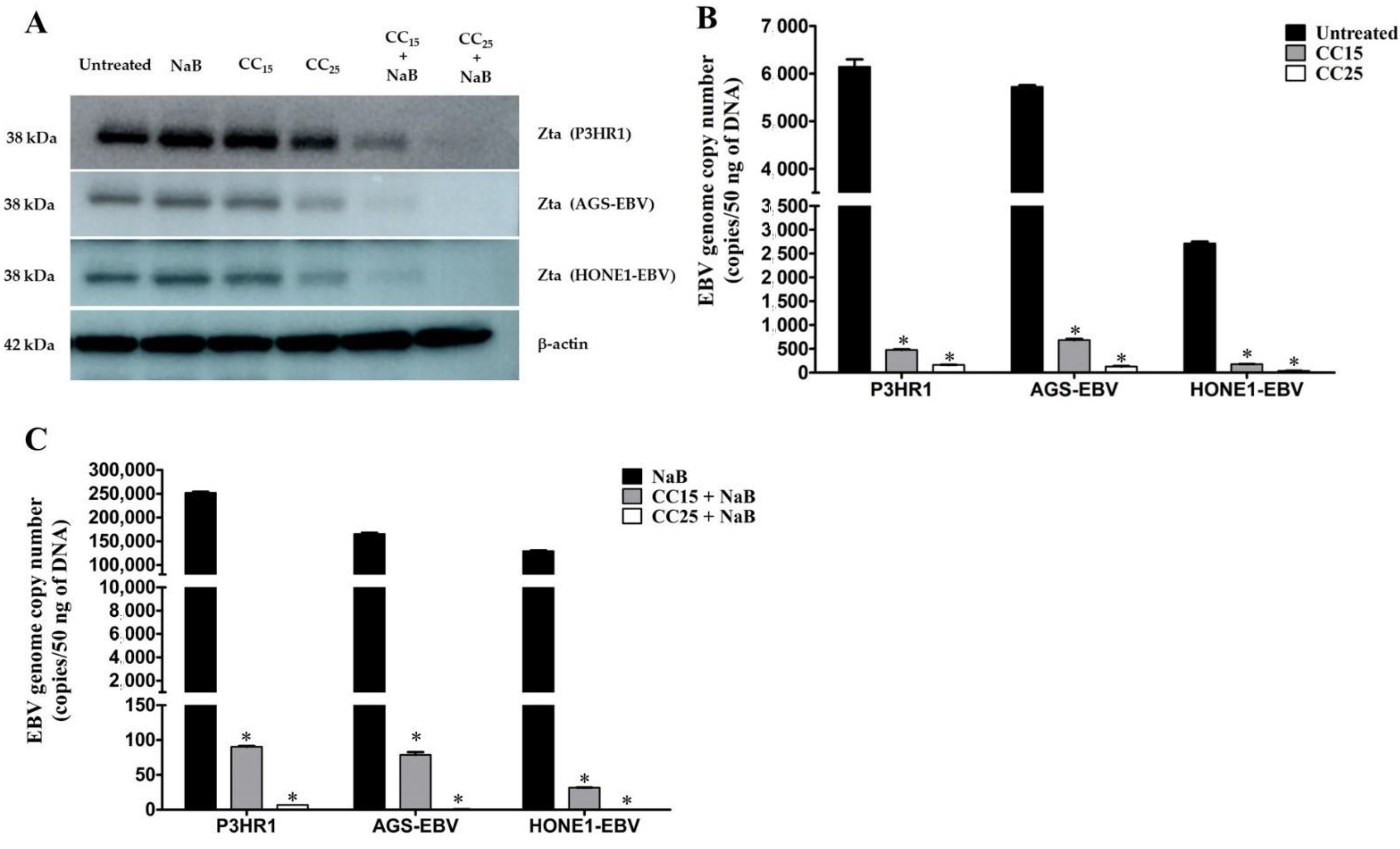
2.2. Andrographolide Inhibit the Spontaneous Lytic Reactivation of EBV
2.3. Andrographolide Treatment and EBV Lytic Gene Induction
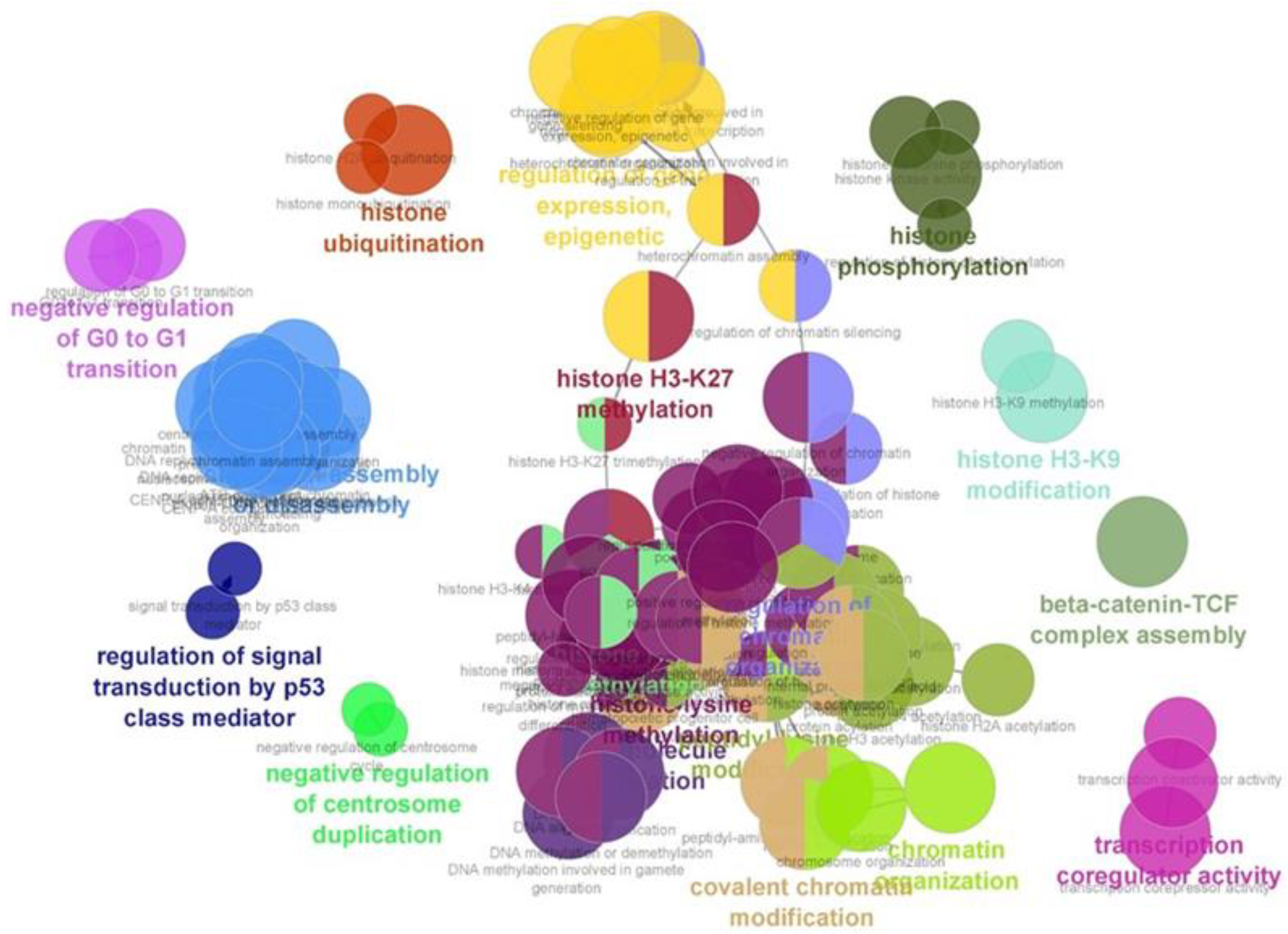
2.4. Andrographolide Induces Histone Modification-Related Proteins in EBV-Positive Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Andrographolide
4.2. Cytotoxicity Assay
4.3. Andrographolide Treatment and EBV Lytic Gene Induction
4.4. Determination of EBV Lytic Protein
4.5. Quantification of EBV Genome Copy Number
4.6. Proteomics and Bioinformatic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Masucci, M.G.; Ernberg, I. Epstein-Barr virus: Adaptation to a life within the immune system. Trends Microbiol. 1994, 24, 125–130. [Google Scholar] [CrossRef]
- Schuster, V.; Kreth, H.W. Epstein-Barr virus infection and associated diseases in children. Eur. J. Pediatr. 1992, 151, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Kieff, E.; Rickinson, A.B. Epstein-Barr virus and its replication. N. Engl. J. Med. 2001, 2, 2603–2654. [Google Scholar]
- Lai, P.K.; Mackay-Scollay, E.M.; Alpers, M.P. Epidemiological studies of Epstein-Barr herpesvirus infection in Western Australia. J. Hyg. 1975, 74, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir. Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Wu, C.C.; Fang, C.-Y.; Hsu, H.-Y.; Chen, Y.-J.; Chou, S.P.; Huang, S.-Y.; Chen, J.Y. Luteolin inhibits Epstein-Barr virus lytic reactivation by repressing the promoter activities of immediate-early genes. Antivir. Res. 2016, 132, 99–110. [Google Scholar] [CrossRef]
- Wu, C.C.; Liu, M.T.; Chang, Y.T.; Fang, C.Y.; Chou, S.P.; Liao, H.W.; Chen, J.Y. Epstein-Barr virus DNase (BGLF5) induces genomic instability in human epithelial cells. Nucleic Acids Res. 2010, 38, 1932–1949. [Google Scholar] [CrossRef]
- Lo, D. Quantitative Analysis of Epstein-Barr Virus DNA in Plasma and Serum. Ann. N. Y. Acad. Sci. 2001, 945, 68–72. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chen, J.Y.; Liu, M.Y.; Yang, H.I.; Hsu, M.M.; Chen, C.J.; Yang, C.S. Serologic Markers of Epstein–Barr Virus Infection and Nasopharyngeal Carcinoma in Taiwanese Men. N. Engl. J. Med. 2001, 345, 1877–1882. [Google Scholar] [CrossRef]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Yan, Y.; Fang, L.H.; Du, G.H. Natural Small Molecule Drugs from Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 357–362. [Google Scholar]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.H.; Wu, C.C.; Fang, C.Y.; Yu, S.L.; Hsu, H.Y.; Chow, Y.H.; Chen, J.Y. Epstein-Barr virus BALF3 mediates genomic instability and progressive malignancy in nasopharyngeal carcinoma. Oncotarget 2014, 5, 8583–8601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.Y.; Wu, C.C.; Cheng, Y.J.; Chou, S.P.; Jiang, Y.J.; Chu, K.C.; Chen, J.Y. Epstein-Barr virus BRLF1 induces genomic instability and progressive malignancy in nasopharyngeal carcinoma cells. Oncotarget 2017, 8, 78948–78964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarti, R.N.; Chakravarti, D. Andrographolide, the active constituent of Andrographis paniculata Nees: A preliminary communication. Ind. Med. Gaz. 1951, 86, 96–97. [Google Scholar]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Islam, M.A.; Shaw, S.; Khan, I.N.; Kamal, M.A. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018, 420, 129–145. [Google Scholar] [CrossRef]
- Malat, P.; Ekalaksananan, T.; Heawchaiyaphum, C.; Suebsasana, S.; Roytrakul, S.; Yingchutrakul, Y.; Pientong, C. Andrographolide Inhibits Lytic Reactivation of Epstein-Barr Virus by Modulating Transcription Factors in Gastric Cancer. Microorganisms 2021, 9, 2561. [Google Scholar] [CrossRef]
- Murata, T.; Kondo, Y.; Sugimoto, A.; Kawashima, D.; Saito, S.; Isomura, H.; Tsurumi, T. Epigenetic Histone Modification of Epstein-Barr Virus BZLF1 Promoter during Latency and Reactivation in Raji Cells. Virol. J. 2012, 86, 4752–4761. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Tsurumi, T. Epigenetic modification of the Epstein-Barr virus BZLF1 promoter regulates viral reactivation from latency. Front. Genet. 2013, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Murata, T. Regulation of Epstein-Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef]
- Murata, T.; Tsurumi, T. Switching of EBV cycles between latent and lytic states. Rev. Med. Virol. 2014, 24, 142–153. [Google Scholar] [CrossRef]
- Wu, C.C.; Fang, C.Y.; Huang, S.Y.; Chiu, S.H.; Lee, C.H.; Chen, J.Y. Perspective: Contribution of Epstein-Barr virus (EBV) Reactivation to the Carcinogenicity of Nasopharyngeal Cancer Cells. Cancers 2018, 10, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.Y.; Lee, C.H.; Wu, C.C.; Chang, Y.T.; Yu, S.L.; Chou, S.P.; Chen, J.Y. Recurrent chemical reactivations of EBV promotes genome instability and enhances tumor progression of nasopharyngeal carcinoma cells. Int. J. Cancer 2009, 124, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.R.; Hsieh, Y.C.; Chang, Y.F.; Lee, K.H.; Wu, Y.C.; Chang, L.K. Inhibition of the Epstein-Barr virus lytic cycle by moronic acid. Antivir. Res. 2010, 85, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.K.; Wei, T.T.; Chiu, Y.F.; Tung, C.P.; Chuang, J.Y.; Hung, S.K.; Liu, S.T. Inhibition of Epstein-Barr virus lytic cycle by (-)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 2003, 301, 1062–1068. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Chang, L.K.; Chiu, Y.F.; Lin, T.P. Inhibitory effects of resveratrol on the Epstein-Barr virus lytic cycle. Molecules 2010, 15, 7115–7124. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.P.; Chen, S.Y.; Duh, P.D.; Chang, L.K.; Liu, Y.N. Inhibition of the Epstein–Barr Virus Lytic Cycle by Andrographolide. Biol. Pharm. Bull. 2008, 31, 2018–2023. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [Green Version]
- Uttekar, M.M.; Das, T.; Pawar, R.S.; Bhandari, B.; Menon, V.; Nutan Bhat, S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012, 56, 368–374. [Google Scholar] [CrossRef]
- Banerjee, M.; Chattopadhyay, S.; Choudhuri, T.; Bera, R.; Kumar, S.; Chakraborty, B.; Mukherjee, S.K. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J. Biomed. Sci. 2016, 23, 40. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Das, B.; Sen, R.; Kundu, P.; Manna, A.; Sarkar, A.; Das, P. Andrographolide Analogue Induces Apoptosis and Autophagy Mediated Cell Death in U937 Cells by Inhibition of PI3K/Akt/mTOR Pathway. PLoS ONE 2015, 10, e0139657. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.C.; Jeon, H.J.; Kee, K.H.; Lee, M.J.; Hong, R.; Han, S.I. Andrographolide induces apoptotic and non-apoptotic death and enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in gastric cancer cells. Oncol. Lett. 2017, 13, 3837–3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Hu, M.; Wu, T.T.; Zhang, C.; Chen, Z.; Huang, S.; Zhou, X.H. Andrographolide Suppresses Proliferation of Nasopharyngeal Carcinoma Cells via Attenuating NF-kB Pathway. Biomed Res. Int. 2015, 2015, 735056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, T.O.; Tang, C.M.; Yu, J. Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: Disease and treatments. World J. Gastroenterol. 2014, 20, 6448–6456. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Okuno, Y.; Sato, Y.; Goshima, F.; Yoshiyama, H.; Kanda, T.; Murata, T. Regulation of Epstein-Barr Virus Life Cycle and Cell Proliferation by Histone H3K27 Methyltransferase EZH2 in Akata Cells. mSphere 2018, 3, e00478-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aromdee, C.; Suebsasana, S.; Ekalaksananan, T.; Pientong, C.; Thongchai, S. Stage of Action of Naturally Occurring Andrographolides and Their Semisynthetic Analogues against Herpes Simplex Virus Type 1 In Vitro. Planta Med. 2011, 77, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Burassakarn, A.; Pientong, C.; Sunthamala, N.; Chuerduangphui, J.; Vatanasapt, P.; Patarapadungkit, N.; Ekalaksananan, T. Aberrant gene promoter methylation of E-cadherin, p16 (INK4a), p14 (ARF), and MGMT in Epstein-Barr virus-associated oral squamous cell carcinomas. Med. Oncol. 2017, 34, 128. [Google Scholar] [CrossRef]
- Chuerduangphui, J.; Proyrungroj, K.; Pientong, C.; Hinkan, S.; Budkaew, J.; Pimson, C.; Ekalaksananan, T. Prevalence and anatomical sites of human papillomavirus, Epstein-Barr virus and herpes simplex virus infections in men who have sex with men, Khon Kaen, Thailand. BMC Infect. Dis. 2018, 18, 509. [Google Scholar] [CrossRef]
- Tenorio, A.; Echevarría, J.E.; Casas, I.; Echevarría, J.M.; Tabarés, E. Detection and typing of human herpesviruses by multiplex polymerase chain reaction. J. Virol Methods. 1993, 44, 261–269. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]

| EBV-Positive Cells | Andrographolide Concentration (μM) | |||
|---|---|---|---|---|
| CC90 | CC50 | CC25 | CC15 | |
| P3HR1 | 116.51 | 69.80 | 38.70 | 26.25 |
| AGS-EBV | 108.20 | 67.72 | 34.40 | 21.07 |
| HONE1-EBV | 115.64 | 69.41 | 36.33 | 23.10 |
| EBV Genes | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| GAPDH | F: TCATCAGCAATGCCTCCTGCA R: TGGGTGGCAGTGATGGCA | 117 |
| BZLF1 | F: TGTTTCAACCGCTCCGACTG R: GGGTTATGTCGGAGACTGGG | 110 |
| BRLF1 | F: TGTTTCAACCGCTCCGACTG R: GGGTTATGTCGGAGACTGGG | 94 |
| BMRF1 | F: ACCTGCCGTTGGATCTTAGTG R: GGCGTTGTTGGAGTCCTGTG | 129 |
| BALF5 | F: GGAGAAGGTCTTCTCGGCCTC R: TTCAGAGAGCGAGACCCTGC | 100 |
| LMP1 | F: TCTCCTTTGGCTCCTCCTGT R: TCGGTAGCTTGTTGAGGGTG | 117 |
| EBNA1 | F: CCACAATGTCGTCTTACACC R: ATAACAGACAATGGACTCCCT | 213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malat, P.; Ekalaksananan, T.; Heawchaiyaphum, C.; Suebsasana, S.; Roytrakul, S.; Yingchutrakul, Y.; Pientong, C. Andrographolide Inhibits Epstein–Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins. Molecules 2022, 27, 4666. https://doi.org/10.3390/molecules27144666
Malat P, Ekalaksananan T, Heawchaiyaphum C, Suebsasana S, Roytrakul S, Yingchutrakul Y, Pientong C. Andrographolide Inhibits Epstein–Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins. Molecules. 2022; 27(14):4666. https://doi.org/10.3390/molecules27144666
Chicago/Turabian StyleMalat, Praphatson, Tipaya Ekalaksananan, Chukkris Heawchaiyaphum, Supawadee Suebsasana, Sittiruk Roytrakul, Yodying Yingchutrakul, and Chamsai Pientong. 2022. "Andrographolide Inhibits Epstein–Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins" Molecules 27, no. 14: 4666. https://doi.org/10.3390/molecules27144666
APA StyleMalat, P., Ekalaksananan, T., Heawchaiyaphum, C., Suebsasana, S., Roytrakul, S., Yingchutrakul, Y., & Pientong, C. (2022). Andrographolide Inhibits Epstein–Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins. Molecules, 27(14), 4666. https://doi.org/10.3390/molecules27144666







