Protection against Hypoxia-Reoxygenation Injury of Hippocampal Neurons by H2S via Promoting Phosphorylation of ROCK2 at Tyr722 in Rat Model
Abstract
:1. Introduction
2. Results
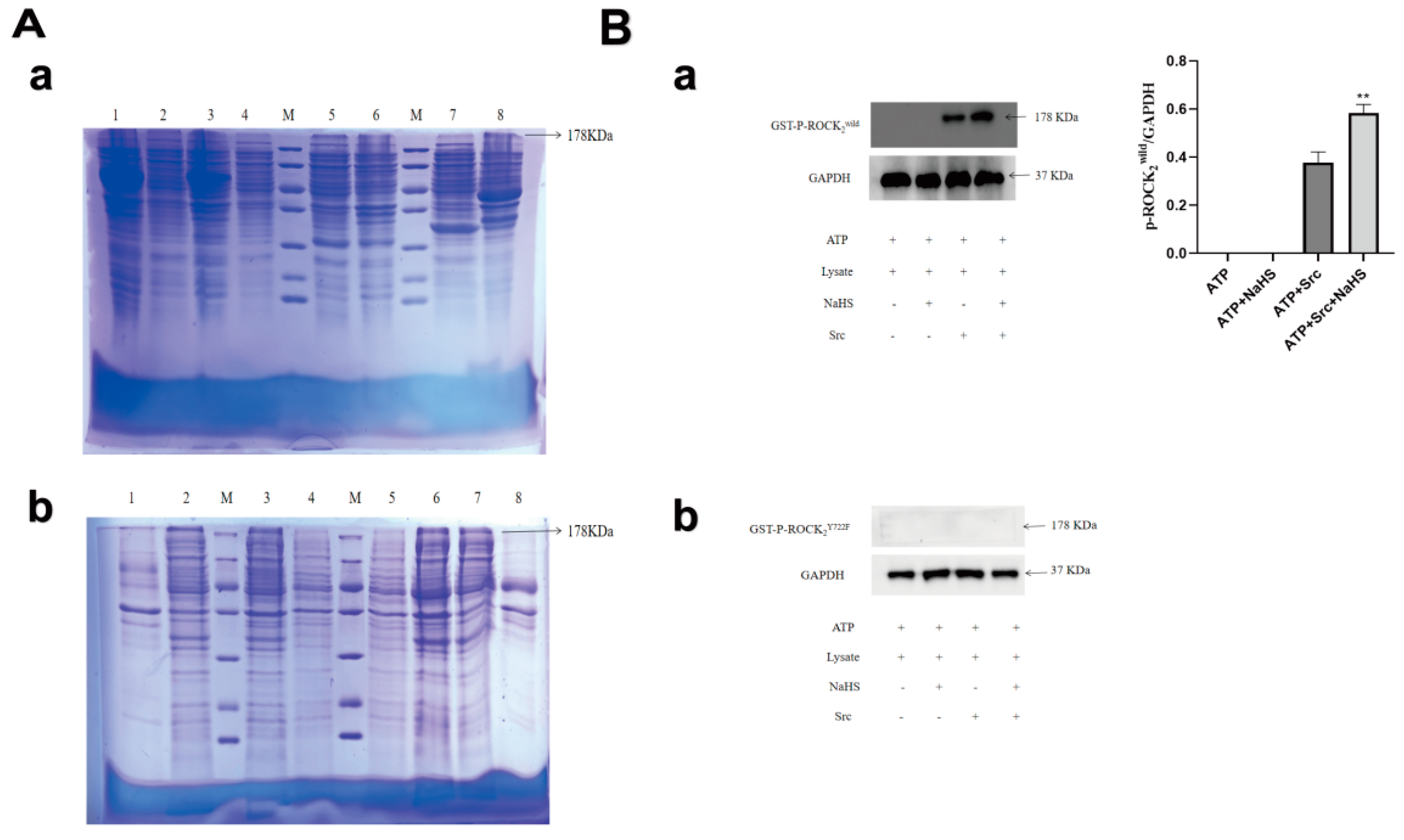
2.1. Expressions of GST-ROCK2wild and GST-ROCK2Y722F in E. coli and Effects of NaHS on Their Phosphorylation In Vitro
2.2. Effects of NaHS on Protein Expression and Activity of ROCK2 and Phosphorylation of ROCK2 at Tyr722 in RHNs
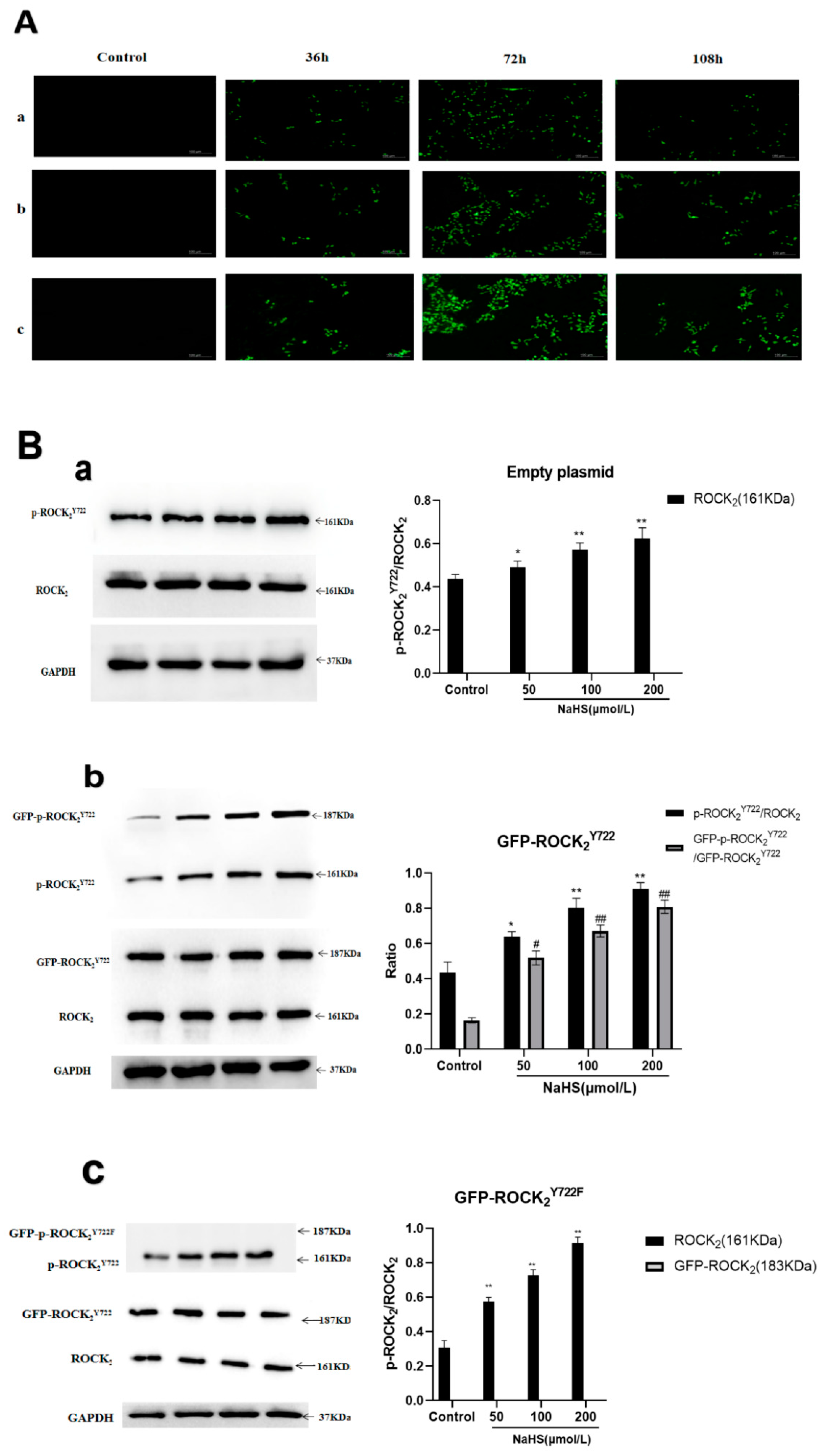
2.3. NaHS-Induced Phosphorylation of ROCK2 or GFP-ROCK2 at Tyr722 in the Transfected RHNs
2.4. Effects of NaHS on ROCK2 Activity in Eukaryotic Plasmid-Transfected RHNs
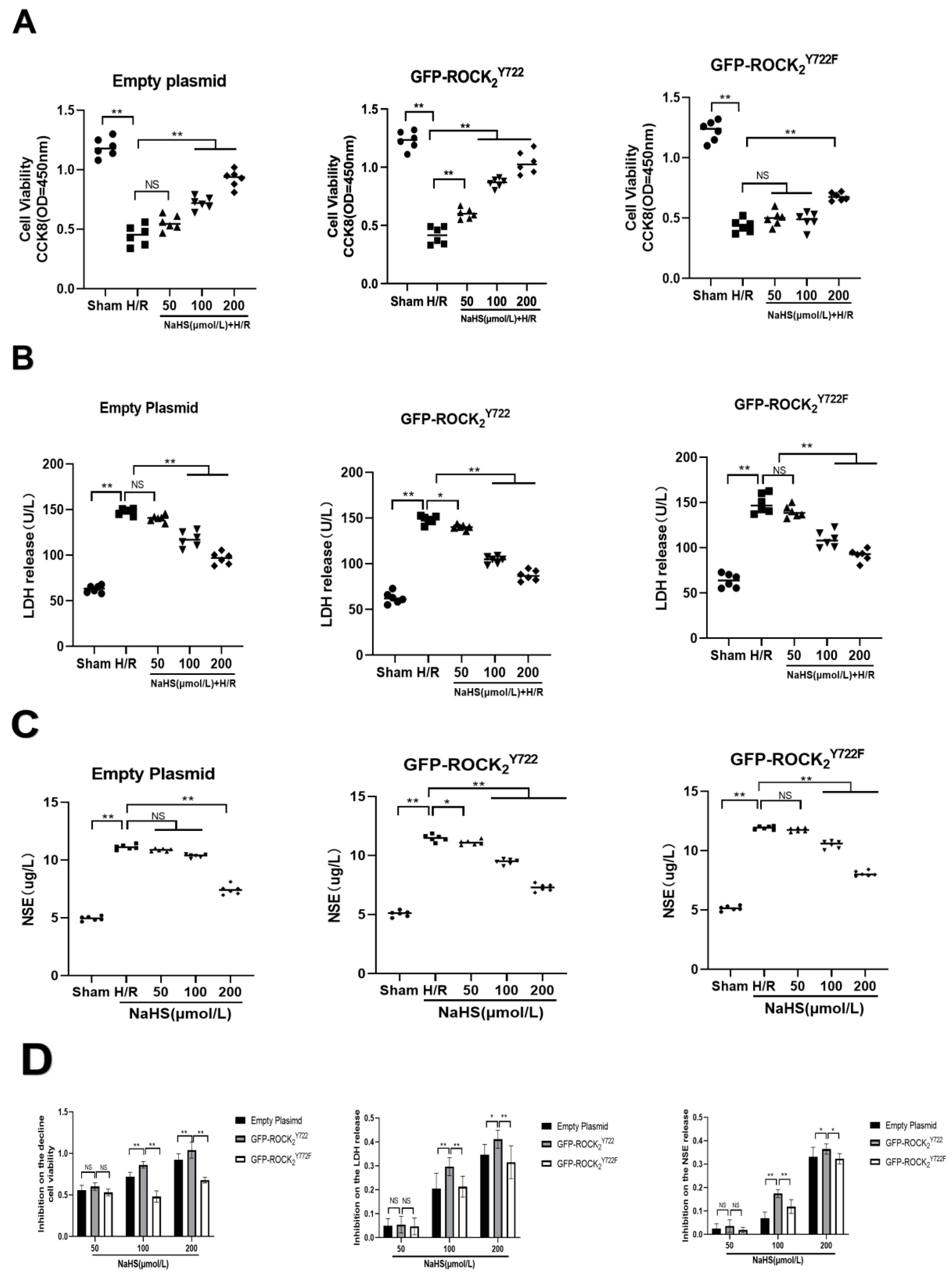
2.5. ROCK2 Tyr722 Mediated Protective Effect of NaHS on H/R Injury in Transfected RHNs
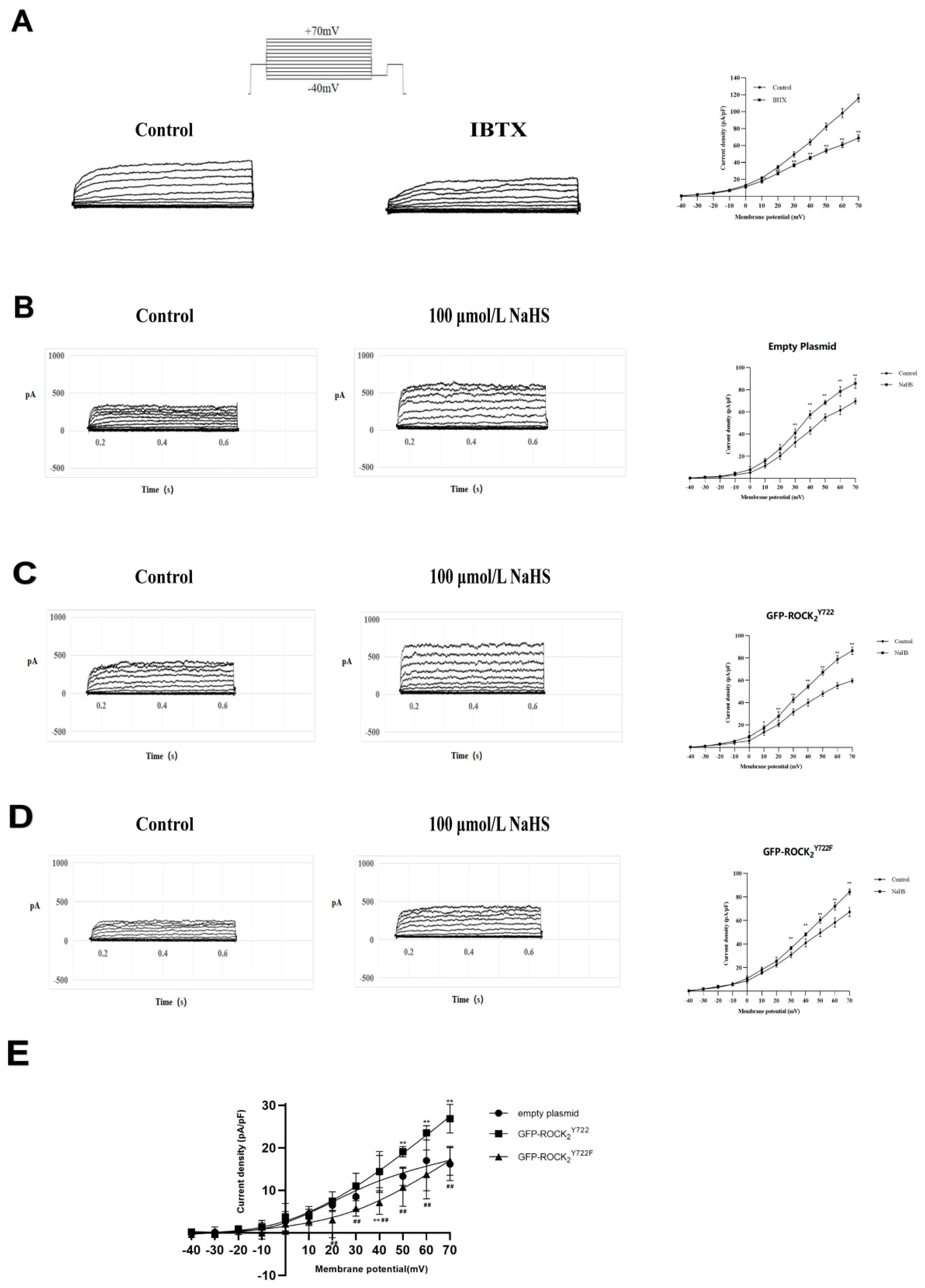
2.6. ROCK2 Tyr722 Mediated the NaHS-Increased Current of Big-Conductance Ca2+-Activated K+ Channel (BKCa) in Transfected RHNs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plasmids and Bacterium
4.3. Expression of Prokaryotic Recombinant Protein GST-ROCK2wild and GST-ROCK2Y722F
4.4. In Vitro Phosphorylation Assay
4.5. Western Blotting
4.6. Determination of ROCK2 Activity
4.7. Lentivirus Transfection
4.8. Determination of Cell Viability
4.9. Determination of LDH
4.10. Determination of NSE
4.11. Whole-Cell Patch-Clamp Technique
4.12. H/R Injury
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandalaneni, K.; Rayi, A.; Jillella, D.V. Stroke Reperfusion Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hussein, A.M.; Khaled, H.K.; Seisa, M.O.; Baiomy, A.; Mohamed, M.A.; Eltantawy, D.; Mahmoud, A.A.; Sheashaa, H.A.; Sobh, M.A. Possible role of nitric oxide in hepatic injury secondary to renal ischemia-reperfusion (I/R) injury. Gen. Physiol. Biophys. 2014, 33, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Dandu, C.; Geng, X. Clinical application of nitric oxide in ischemia and reperfusion injury: A literature review. Brain Circ. 2020, 6, 248–253. [Google Scholar] [PubMed]
- Guan, X.; Li, X.; Yang, X.; Yan, J.; Shi, P.; Ba, L.; Cao, Y.; Wang, P. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 2019, 235, 116795. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Wang, H.; Zhu, Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell. Longev. 2018, 2018, 4010395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Cao, L.; Ding, L.; Bian, J.S. A New Hope for a Devastating Disease: Hydrogen Sulfide in Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Wang, X.; Ding, Y.; Ren, Z.; Fang, J.; Sun, T.; Guo, Y.; Chen, Z.; Wen, J. CSE-Derived H2S Inhibits Reactive Astrocytes Proliferation and Promotes Neural Functional Recovery after Cerebral Ischemia/Reperfusion Injury in Mice Via Inhibition of RhoA/ROCK2 Pathway. ACS Chem. Neurosci. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, Z.W.; He, G.W. Acetylcholine- and sodium hydrosulfide-induced endothelium-dependent relaxation and hyperpolarization in cerebral vessels of global cerebral ischemia-reperfusion rat. J. Pharmacol. Sci. 2013, 121, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, Y.; Fan, Y.; Guo, Y.; Chen, F.; Chen, S.; Li, Q.; Chen, Z. Involvement of Hydrogen Sulfide in Endothelium-Derived Relaxing Factor-Mediated Responses in Rat Cerebral Arteries. J. Vasc. Res. 2016, 53, 172–185. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Li, H.; Xue, M.; Ji, A.; Li, Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 186908. [Google Scholar] [CrossRef] [Green Version]
- Lai, A.Y.; McLaurin, J. Rho-associated protein kinases as therapeutic targets for both vascular and parenchymal pathologies in Alzheimer’s disease. J. Neurochem. 2018, 144, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Roloff, F.; Scheiblich, H.; Dewitz, C.; Dempewolf, S.; Stern, M.; Bicker, G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS ONE 2015, 10, e0118536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiblich, H.; Bicker, G. Regulation of Microglial Phagocytosis by RhoA/ROCK-Inhibiting Drugs. Cell. Mol. Neurobiol. 2017, 37, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.Y.; Gao, S.S.; Chen, F.L.; Chen, S.; Wang, M.; Chen, Z.W. Role of CSE-Produced H2S on Cerebrovascular Relaxation via RhoA-ROCK Inhibition and Cerebral Ischemia-Reperfusion Injury in Mice. ACS Chem. Neurosci. 2019, 10, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Itoh, H.; Yamashita, J.; Doi, K.; Inoue, M.; Masatsugu, K.; Fukunaga, Y.; Sakaguchi, S.; Sone, M.; Yamahara, K.; et al. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem. Biophys. Res. Commun. 2001, 280, 798–805. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, J.; Chen, Z. H2S protects hippocampal neurons against hypoxia-reoxygenation injury by promoting RhoA phosphorylation at Ser188. Cell Death Discov. 2021, 7, 132. [Google Scholar] [CrossRef]
- Lee, H.H.; Tien, S.C.; Jou, T.S.; Chang, Y.C.; Jhong, J.G.; Chang, Z.F. Src-dependent phosphorylation of ROCK participates in regulation of focal adhesion dynamics. J. Cell Sci. 2010, 123 Pt 19, 3368–3377. [Google Scholar] [CrossRef] [Green Version]
- Onyike, C.U. Cerebrovascular disease and dementia. Int. Rev. Psychiatry 2006, 18, 423–431. [Google Scholar] [CrossRef]
- Narasimhan, M.; Schwartz, R.; Halliday, G. Parkinsonism and cerebrovascular disease. J. Neurol. Sci. 2022, 433, 120011. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Xu, H.; Zhang, Y.; Sun, H. Electroacupuncture promotes axonal regrowth by attenuating the myelin-associated inhibitors-induced RhoA/ROCK pathway in cerebral ischemia/reperfusion rats. Brain Res. 2020, 1748, 147075. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamashita, T. Axon growth inhibition by RhoA/ROCK in the central nervous system. Front. Neurosci. 2014, 8, 338. [Google Scholar] [CrossRef]
- Iyer, M.; Subramaniam, M.D.; Venkatesan, D.; Cho, S.G.; Ryding, M.; Meyer, M.; Vellingiri, B. Role of RhoA-ROCK signaling in Parkinson’s disease. Eur. J. Pharmacol. 2021, 894, 173815. [Google Scholar] [CrossRef]
- Koch, J.C.; Tonges, L.; Barski, E.; Michel, U.; Bahr, M.; Lingor, P. ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis. 2014, 5, e1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sladojevic, N.; Yu, B.; Liao, J.K. ROCK as a therapeutic target for ischemic stroke. Expert Rev. Neurother. 2017, 17, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, Z.; Wen, J. RhoA/ROCK signaling pathway and astrocytes in ischemic stroke. Metab. Brain Dis. 2021, 36, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.; Wang, Q.; Li, S.; Chen, C.; Wu, W.; Xu, Y.X.Z.; Zhou, P.; Tu, W.; Lou, X.; Rao, G.; et al. Profilin 1 knockdown prevents ischemic brain damage by promoting M2 microglial polarization associated with the RhoA/ROCK pathway. J. Neurosci. Res. 2020, 98, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Sycheva, M.; Sustarich, J.; Zhang, Y.; Selvaraju, V.; Geetha, T.; Gearing, M.; Babu, J.R. Pro-Nerve Growth Factor Induces Activation of RhoA Kinase and Neuronal Cell Death. Brain Sci. 2019, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [Green Version]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Joseph, C.; Buga, A.M.; Vintilescu, R.; Balseanu, A.T.; Moldovan, M.; Junker, H.; Walker, L.; Lotze, M.; Popa-Wagner, A. Prolonged gaseous hypothermia prevents the upregulation of phagocytosis-specific protein annexin 1 and causes low-amplitude EEG activity in the aged rat brain after cerebral ischemia. J. Cereb. Blood Flow Metab. 2012, 32, 1632–1642. [Google Scholar] [CrossRef]
- Lee, H.H.; Chang, Z.F. Regulation of RhoA-dependent ROCKII activation by Shp2. J. Cell Biol. 2008, 181, 999–1012. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, M.J.; Mostafa, M.N.; Park, J.H.; Choi, H.S.; Kim, Y.S.; Choi, E.K. RhoA/ROCK Regulates Prion Pathogenesis by Controlling Connexin 43 Activity. Int. J. Mol. Sci. 2020, 21, 1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loirand, G.; Guilluy, C.; Pacaud, P. Regulation of Rho proteins by phosphorylation in the cardiovascular system. Trends Cardiovasc. Med. 2006, 16, 199–204. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, X.; Kang, B.; Xu, J.; Wu, L.; Hong, J.; Zhang, Y.; Ni, X.; Wang, Z. Hydrogen Sulfide Attenuates Myocardial Hypoxia-Reoxygenation Injury by Inhibiting Autophagy via mTOR Activation. Cell Physiol. Biochem. 2015, 37, 2444–2453. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, X.M.; Chen, S.; Wen, J.Y.; Chen, Z.W. Total flavones of Rhododendron simsii Planch flower protect rat hippocampal neuron from hypoxia-reoxygenation injury via activation of BKCa channel. J. Pharm. Pharmacol. 2020, 72, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gueguinou, M.; Chantome, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca(2+) channels: The complex thought. Biochim. Biophys. Acta 2014, 1843, 2322–2333. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.L.; Bai, Y.L.; Cai, B.Z. Calcium-Activated Potassium Channels: Potential Target for Cardiovascular Diseases. Adv. Protein Chem. Struct. Biol. 2016, 104, 233–261. [Google Scholar] [PubMed]
- Zhang, I.; Hu, H. Store-Operated Calcium Channels in Physiological and Pathological States of the Nervous System. Front. Cell Neurosci. 2020, 14, 600758. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.A.; Li, H.Y.; Shin, K.O.; Oh, G.T.; Lee, Y.M.; Oh, S.; Pewzner-Jung, Y.; Futerman, A.H.; Suh, S.H. KCa 3.1 upregulation preserves endothelium-dependent vasorelaxation during aging and oxidative stress. Aging Cell 2016, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sitdikova, G.F.; Weiger, T.M.; Hermann, A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 2010, 459, 389–397. [Google Scholar] [CrossRef]
- Sitdikova, G.F.; Fuchs, R.; Kainz, V.; Weiger, T.M.; Hermann, A. Phosphorylation of BK channels modulates the sensitivity to hydrogen sulfide (H2S). Front. Physiol. 2014, 5, 431. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yue, Z.; Wang, Q.; Lv, Q.; Liu, H.; Bai, Y.; Li, S.; Xie, M.; Bao, J.; Ma, J.; et al. BKCa compensates impaired coronary vasoreactivity through RhoA/ROCK pathway in hind-limb unweighted rats. FASEB J. 2019, 33, 13358–13366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.Y.; Zhang, J.; Chen, S.; Chen, Y.; Zhang, Y.; Ma, Z.Y.; Zhang, F.; Xie, W.M.; Fan, Y.F.; Duan, J.S.; et al. Endothelium-derived hydrogen sulfide acts as a hyperpolarizing factor and exerts neuroprotective effects via activation of large-conductance Ca(2+) -activated K(+) channels. Br. J. Pharmacol. 2021, 178, 4155–4175. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Dumitrascu, D.I.; Capitanescu, B.; Petcu, E.B.; Surugiu, R.; Fang, W.H.; Dumbrava, D.A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural. Regen Res. 2020, 15, 394–400. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Chen, S.; Xi, J.; Guan, Q.; Chen, W.; Guo, Y.; Chen, Z. Protection against Hypoxia-Reoxygenation Injury of Hippocampal Neurons by H2S via Promoting Phosphorylation of ROCK2 at Tyr722 in Rat Model. Molecules 2022, 27, 4567. https://doi.org/10.3390/molecules27144567
Xue M, Chen S, Xi J, Guan Q, Chen W, Guo Y, Chen Z. Protection against Hypoxia-Reoxygenation Injury of Hippocampal Neurons by H2S via Promoting Phosphorylation of ROCK2 at Tyr722 in Rat Model. Molecules. 2022; 27(14):4567. https://doi.org/10.3390/molecules27144567
Chicago/Turabian StyleXue, Meng, Shuo Chen, Jiaojiao Xi, Qianjun Guan, Wei Chen, Yan Guo, and Zhiwu Chen. 2022. "Protection against Hypoxia-Reoxygenation Injury of Hippocampal Neurons by H2S via Promoting Phosphorylation of ROCK2 at Tyr722 in Rat Model" Molecules 27, no. 14: 4567. https://doi.org/10.3390/molecules27144567






