Characteristics of Gaseous/Liquid Hydrocarbon Adsorption Based on Numerical Simulation and Experimental Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental
2.1.1. Materials
2.1.2. Hydrocarbon Vapor Adsorption Methodology
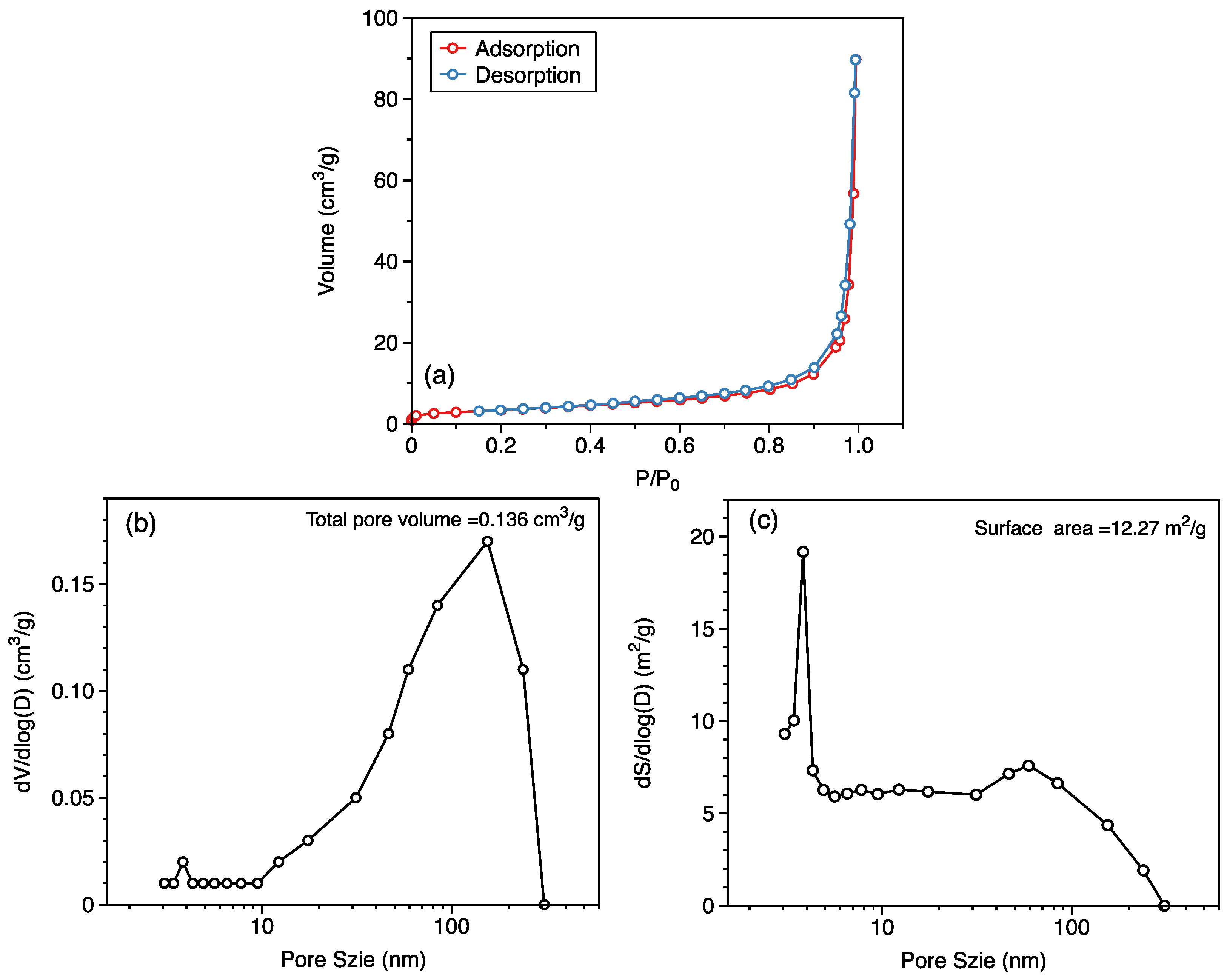
2.1.3. Data Analysis
2.2. Molecular Simulation Details
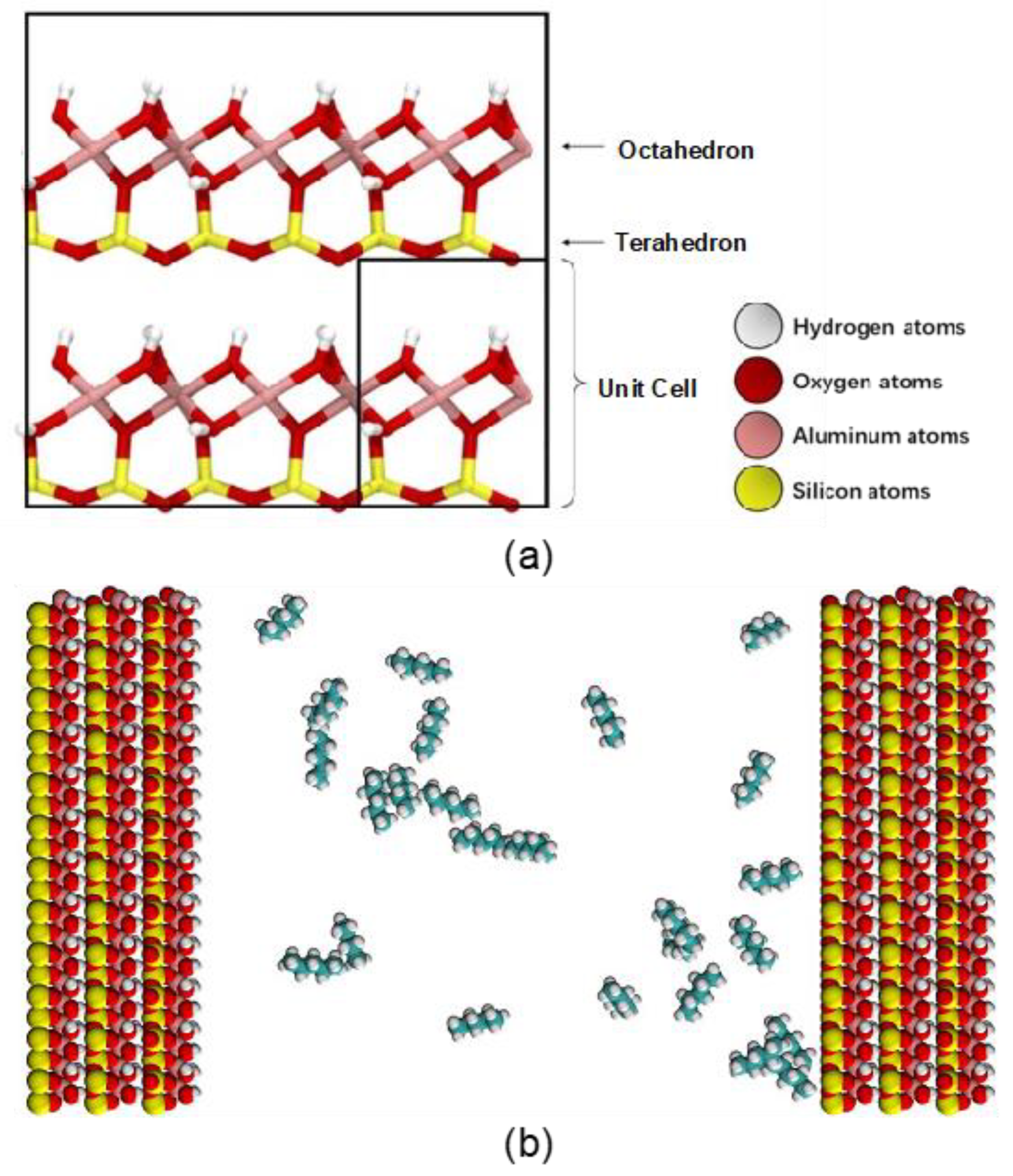
2.2.1. Molecular Structures
2.2.2. Force Field Parameters
2.2.3. Simulation Protocol
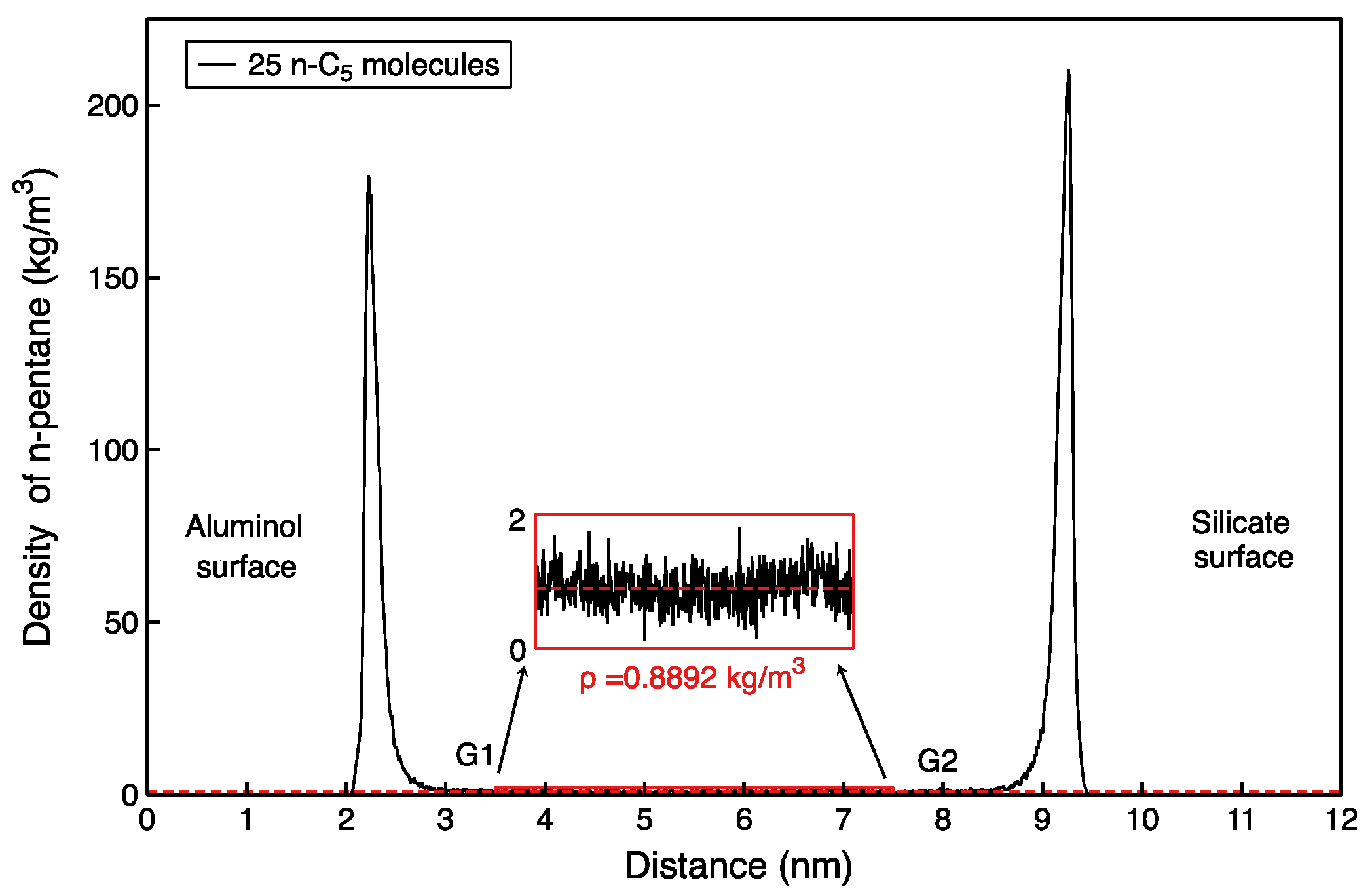
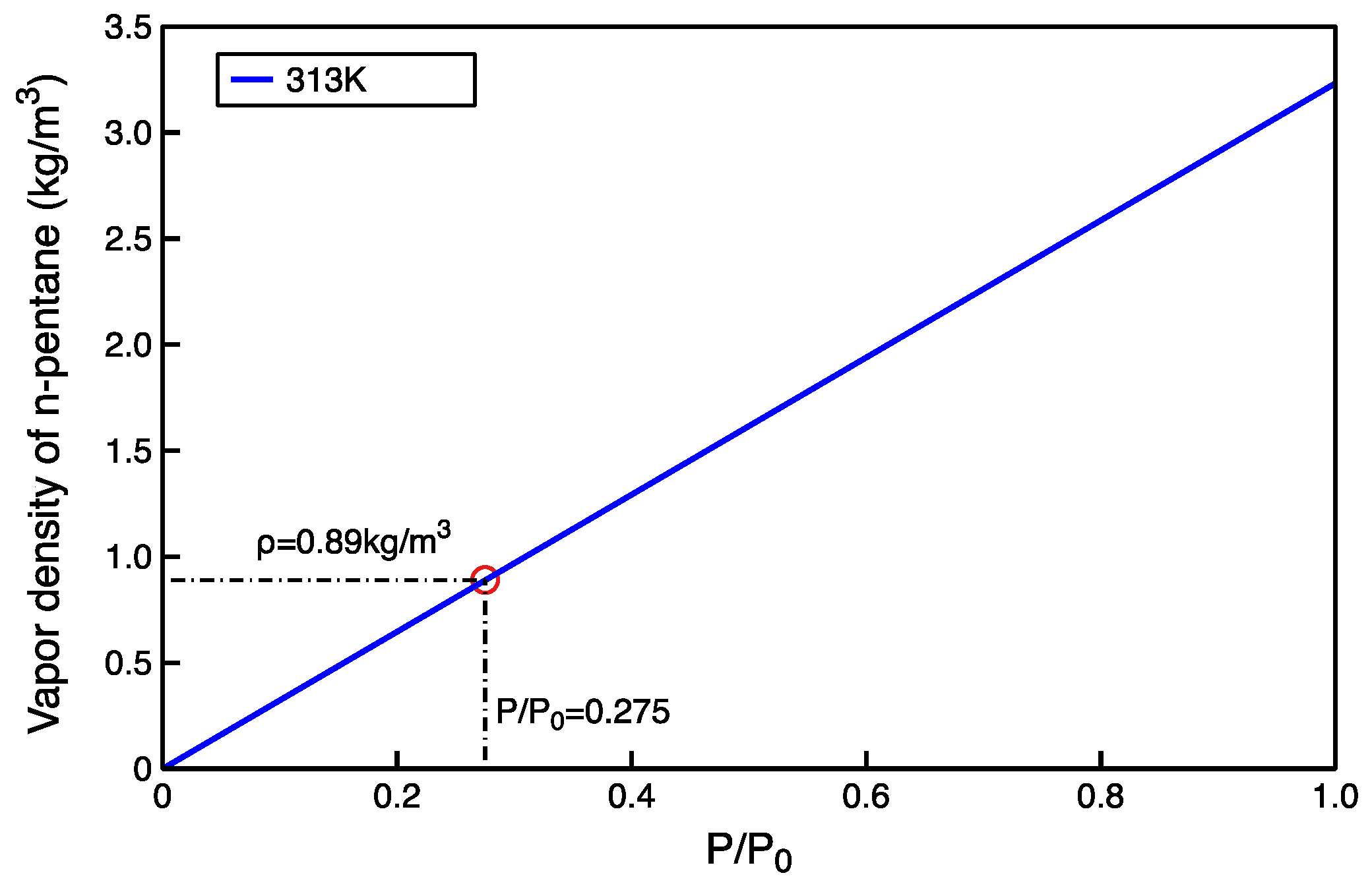
2.2.4. Data Analysis
3. Result and Discussion
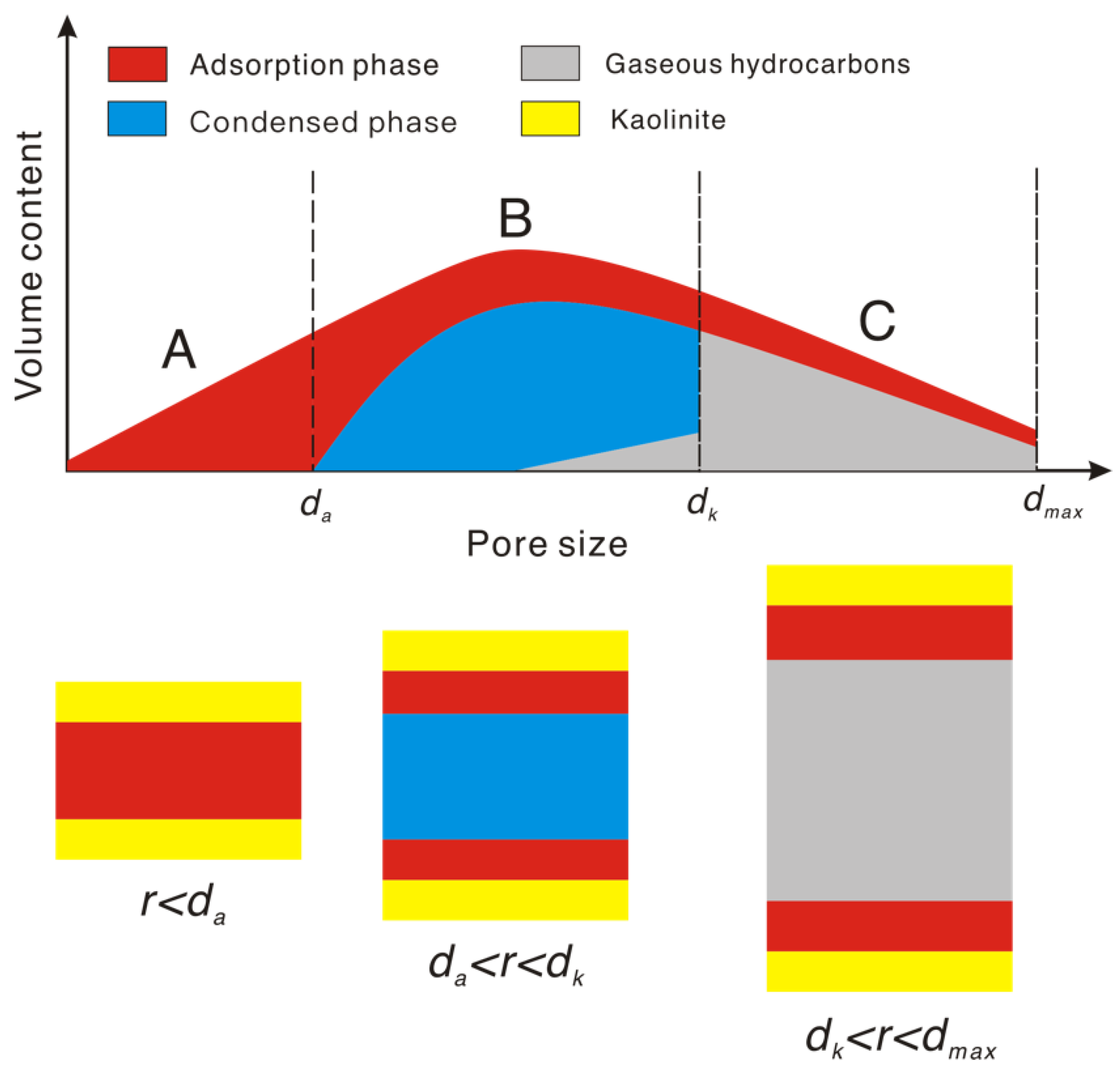
3.1. Comparison of Adsorption Characteristics of Gaseous and Liquid Hydrocarbons
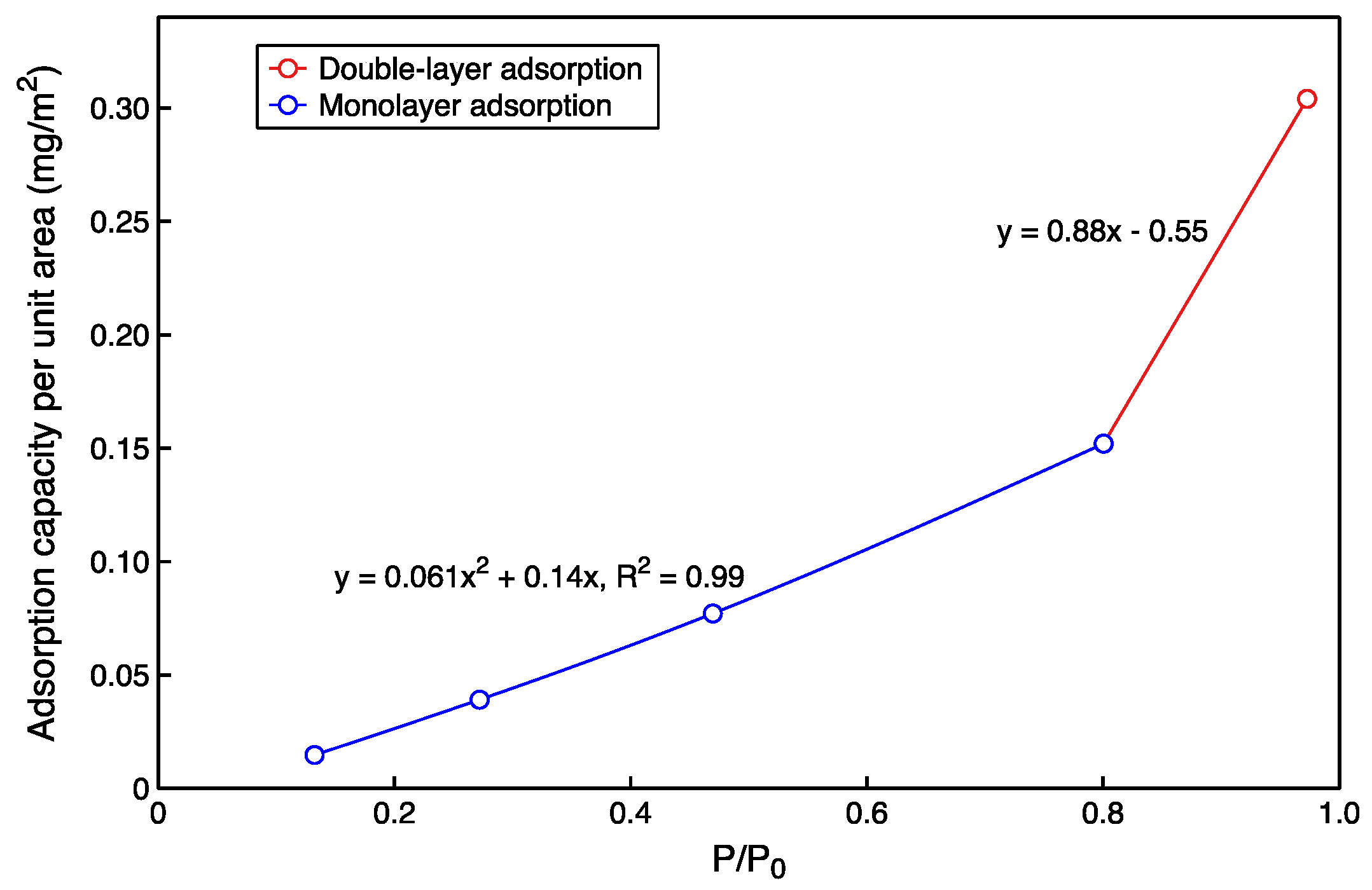
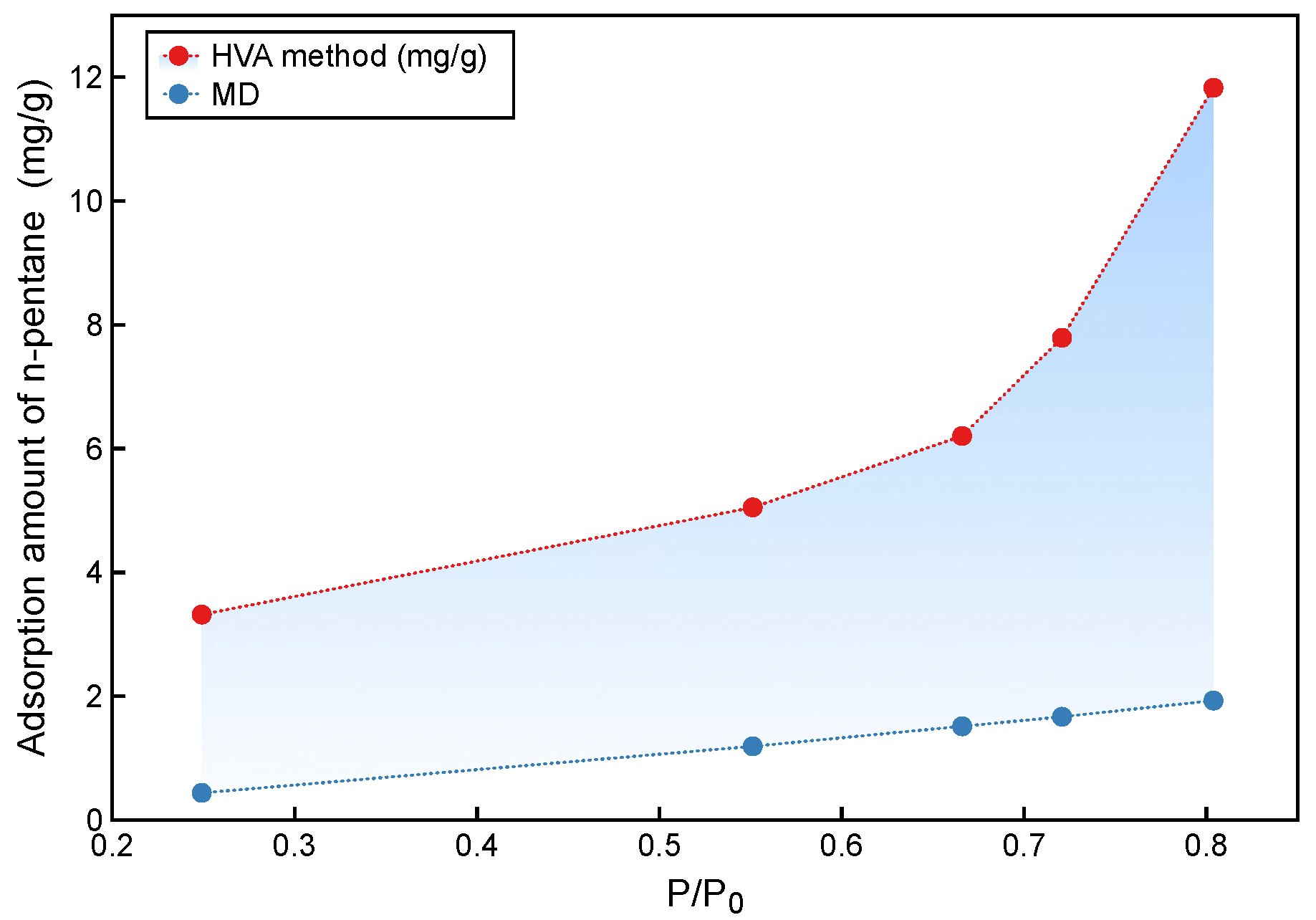
3.2. Comparison of Experimental and Simulated Gaseous Hydrocarbon Adsorption Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Sun, J.; Zhang, Y.; He, J.; Fu, X.; Liang, Y.; Xing, J.; Zhao, X. Reservoir space and enrichment model of shale oil in the first member of Cretaceous Qingshankou Formation in the Changling sag, southern Songliao Basin, NE China. Pet. Explor. Dev. 2021, 48, 608–624. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Xie, L.; Zhang, J.; Xue, H.; Zhang, P.; Tian, S. Modeling of hydrocarbon adsorption on continental oil shale: A case study on n-alkane. Fuel 2017, 206, 603–613. [Google Scholar] [CrossRef]
- Wang, S.; Javadpour, F.; Feng, Q. Molecular dynamics simulations of oil transport through inorganic nanopores in shale. Fuel 2016, 171, 74–86. [Google Scholar] [CrossRef]
- Tian, S.; Xue, H.; Lu, S.; Zeng, F.; Xue, Q.; Chen, G.; Wu, C.; Zhang, S. Molecular simulation of oil mixture adsorption character in shale system. J. Nanosci. Nanotechnol. 2017, 17, 6198–6209. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Zhang, L.; Sun, H.; Zhang, K.; Ma, J. Pore-scale simulation of shale oil flow based on pore network model. Fuel 2019, 251, 683–692. [Google Scholar] [CrossRef]
- Jarvie, D.M. Shale resource systems for oil and gas: Part 2—Shale-oil resource systems. In Shale Reservoirs—Giant Resources for the 21st Century; AAPG, Memoir; Breyer, J.A., Ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2012; Volume 97, pp. 89–119. [Google Scholar]
- Wright, M.C.; Court, R.W.; Kafantaris, F.-C.A.; Spathopoulos, F.; Sephton, M.A. A new rapid method for shale oil and shale gas assessment. Fuel 2015, 153, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Washburn, K.E.; Birdwell, J.E. Updated methodology for nuclear magnetic resonance characterization of shales. J. Magn. Reson. 2013, 233, 17–28. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Jin, X.; Cao, D. Molecular dynamics simulation of diffusion of shale oils in montmorillonite. J. Phys. Chem. C 2016, 120, 8986–8991. [Google Scholar] [CrossRef]
- Pernyeszi, T.; Patzkó, Á.; Berkesi, O.; Dékány, I. Asphaltene adsorption on clays and crude oil reservoir rocks. Colloids Surf. A Physicochem. Eng. Asp. 1998, 137, 373–384. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Huang, W.-L. Selective adsorption of hydrocarbon gases on clays and organic matter. Org. Geochem. 2004, 35, 413–423. [Google Scholar] [CrossRef]
- Luo, X.; Zhao, Z.; Hou, L.; Lin, S.; Sun, F.; Zhang, L.; Zhang, Y. Experimental Methods for the Quantitative Assessment of the Volume Fraction of Movable Shale Oil: A Case Study in the Jimsar Sag, Junggar Basin, China. Front. Earth Sci. 2021, 9, 218. [Google Scholar] [CrossRef]
- Drits, V.A.; Lindgreen, H.; Salyn, A.L. Determination of the content and distribution of fixed ammonium in illite-smectite by X-ray diffraction: Application to North Sea illite-smectite. Am. Mineral. 1997, 82, 79–87. [Google Scholar] [CrossRef]
- Xie, X.; Li, M.; Littke, R.; Huang, Z.; Ma, X.; Jiang, Q.; Snowdon, L.R. Petrographic and geochemical characterization of microfacies in a lacustrine shale oil system in the Dongying Sag, Jiyang Depression, Bohai Bay Basin, eastern China. Int. J. Coal Geol. 2016, 165, 49–63. [Google Scholar] [CrossRef]
- Pang, H.; Pang, X.; Dong, L.; Zhao, X. Factors impacting on oil retention in lacustrine shale: Permian Lucaogou Formation in Jimusaer Depression, Junggar Basin. J. Pet. Sci. Eng. 2018, 163, 79–90. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xie, X.; Lv, Q.; Huang, X.; Ye, J. Assessment of shale oil potential using a new free hydrocarbon index. Int. J. Coal Geol. 2016, 156, 74–85. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, K.; Bastia, R.; Garg, K.; Das, S.; Mohanty, D. Evaluation of Eocene source rock for potential shale oil and gas generation in north Cambay Basin, India. Mar. Pet. Geol. 2017, 88, 141–154. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Ma, X.; Cao, T.; Li, Z.; Jiang, Q. A numerical method for calculating total oil yield using a single routine Rock-Eval program: A case study of the Eocene Shahejie Formation in Dongying Depression, Bohai Bay Basin, China. Int. J. Coal Geol. 2018, 191, 49–65. [Google Scholar] [CrossRef]
- Li, M.; Chen, Z.; Qian, M.; Ma, X.; Jiang, Q.; Li, Z.; Tao, G.; Wu, S. What are in pyrolysis S1 peak and what are missed? Petroleum compositional characteristics revealed from programed pyrolysis and implications for shale oil mobility and resource potential. Int. J. Coal Geol. 2020, 217, 103321. [Google Scholar] [CrossRef]
- Tian, S.; Erastova, V.; Lu, S.; Greenwell, H.C.; Underwood, T.R.; Xue, H.; Zeng, F.; Chen, G.; Wu, C.; Zhao, R. Understanding model crude oil component interactions on kaolinite silicate and aluminol surfaces: Toward improved understanding of shale oil recovery. Energy Fuels 2018, 32, 1155–1165. [Google Scholar] [CrossRef]
- Li, Z.; Zou, Y.-R.; Xu, X.-Y.; Sun, J.-N.; Li, M. Adsorption of mudstone source rock for shale oil–Experiments, model and a case study. Org. Geochem. 2016, 92, 55–62. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Correia, J.C.G.; Seidl, P.R. The influence of different minerals on the mechanical resistance of asphalt mixtures. J. Pet. Sci. Eng. 2009, 65, 171–174. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Li, J.; Zhang, P.; Xue, H.; Zhao, X.; Xie, L. Adsorption properties of hydrocarbons (n-decane, methyl cyclohexane and toluene) on clay minerals: An experimental study. Energies 2017, 10, 1586. [Google Scholar] [CrossRef] [Green Version]
- Schlangen, L.J.; Koopal, L.K.; Stuart, M.A.C.; Lyklema, J.; Robin, M.; Toulhoat, H. Thin hydrocarbon and water films on bare and methylated silica: Vapor adsorption, wettability, adhesion, and surface forces. Langmuir 1995, 11, 1701–1710. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A chemical data resource on the internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Baseline studies of The Clay Minerals Society Source Clays: Powder X-ray diffraction analyses. Clays Clay Miner. 2001, 49, 398–409. [Google Scholar] [CrossRef]
- Borden, D.; Giese, R.F. Baseline studies of The Clay Minerals Society Source Clays: Cation exchange capacity measurements by the ammonia-electrode method. Clays Clay Miner. 2001, 49, 444–445. [Google Scholar] [CrossRef]
- Wu, W.J. Baseline studies of The Clay Minerals Society Source Clays: Colloid and surface phenomena. Clays Clay Miner. 2001, 49, 446–452. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Dogan, A.U.; Dogan, M.; Onal, M.; Sarikaya, Y.; Aburub, A.; Wurster, D.E. Baseline studies of the clay minerals society source clays: Specific surface area by the Brunauer Emmett Teller (BET) method. Clays Clay Miner. 2006, 54, 62–66. [Google Scholar] [CrossRef]
- Qian, X.; Chen, L.; Yin, L.; Liu, Z.; Pei, S.; Li, F.; Hou, G.; Chen, S.; Song, L.; Thebo, K.H. CdPS3 nanosheets-based membrane with high proton conductivity enabled by Cd vacancies. Science 2020, 370, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-W.; Wei, Y.-S.; Dong, X.-Y.; Wu, X.-H.; Du, C.-X.; Zang, S.-Q.; Mak, T.C. Hypersensitive dual-function luminescence switching of a silver-chalcogenolate cluster-based metal–organic framework. Nat. Chem. 2017, 9, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.G.T.; Horn, J.C.; Thommes, M.; Van Zee, R.D.; Espinal, L. Experimental aspects of buoyancy correction in measuring reliable high-pressure excess adsorption isotherms using the gravimetric method. Meas. Sci. Technol. 2017, 28, 125802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Underwood, T.; Erastova, V.; Greenwell, H.C. Wetting effects and molecular adsorption at hydrated kaolinite clay mineral surfaces. J. Phys. Chem. C 2016, 120, 11433–11449. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Shi, J.; Fu, X.; Lyu, Y.; Sun, X.; Lei, G.; Bai, Y. Petrological characteristics and shale oil enrichment of lacustrine fine-grained sedimentary system: A case study of organic-rich shale in first member of Cretaceous Qingshankou Formation in Gulong Sag, Songliao Basin, NE China. Pet. Explor. Dev. 2018, 45, 884–894. [Google Scholar] [CrossRef]
- Dong, Z.; Xue, H.; Li, B.; Tian, S.; Lu, S.; Lu, S. Molecular Dynamics Simulations of Oil-Water Wetting Models of Organic Matter and Minerals in Shale at the Nanometer Scale. J. Nanosci. Nanotechnol. 2021, 21, 85–97. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Neria, E.; Fischer, S.; Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 1996, 105, 1902–1921. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond perception and atom typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Underwood, T.; Erastova, V.; Greenwell, H.C. Ion adsorption at clay-mineral surfaces: The Hofmeister series for hydrated smectite minerals. Clays Clay Miner. 2016, 64, 472–487. [Google Scholar] [CrossRef] [Green Version]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; Van Der Spoel, D. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]




| P0/bar | M/g/mol | VL/10−6 m3/mol | ρ/g/cm3 | |
|---|---|---|---|---|
| n-Pentane | 1.1509 | 72.15 | 115.2 | 0.626 |
| System | Molecular Load | P/P0 | n | h (nm) | ρal (kg/m3) | ρSi (kg/m3) | ρbulk (kg/m3) |
|---|---|---|---|---|---|---|---|
| Gas | 10 | 0.27 | 1 | 0.32 | 100.79 | 73.91 | 0.89 |
| 25 | 0.13 | 1 | 0.33 | 167.98 | 208.30 | 0.42 | |
| 50 | 0.46 | 1 | 0.54 | 470.36 | 295.66 | 1.53 | |
| 100 | 0.79 | 1 | 0.76 | 792.90 | 571.16 | 2.67 | |
| 200 | 0.97 | 2 | 1.05 | 1128.88 | 1122.17 | 3.26 | |
| Liquid | 2000 | -- | 3 | 1.30 | 1471.58 | 1417.83 | 625.71 |
| System | Molecular Load | P/P0 | CAl (mg/m2) | CSi (mg/m2) | Cave (mg/m2) |
|---|---|---|---|---|---|
| gas | 10 | 0.27 | 0.012 | 0.016 | 0.014 |
| 25 | 0.13 | 0.034 | 0.044 | 0.039 | |
| 50 | 0.46 | 0.083 | 0.071 | 0.077 | |
| 100 | 0.79 | 0.18 | 0.12 | 0.15 | |
| 200 | 0.97 | 0.31 | 0.29 | 0.30 | |
| Liquid | 2000 | -- | 0.80 | 0.82 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Dong, Z.; Liu, B.; Xue, H.; Erastova, V.; Wang, M.; Yan, H. Characteristics of Gaseous/Liquid Hydrocarbon Adsorption Based on Numerical Simulation and Experimental Testing. Molecules 2022, 27, 4590. https://doi.org/10.3390/molecules27144590
Tian S, Dong Z, Liu B, Xue H, Erastova V, Wang M, Yan H. Characteristics of Gaseous/Liquid Hydrocarbon Adsorption Based on Numerical Simulation and Experimental Testing. Molecules. 2022; 27(14):4590. https://doi.org/10.3390/molecules27144590
Chicago/Turabian StyleTian, Shansi, Zhentao Dong, Bo Liu, Haitao Xue, Valentina Erastova, Min Wang, and Haiyang Yan. 2022. "Characteristics of Gaseous/Liquid Hydrocarbon Adsorption Based on Numerical Simulation and Experimental Testing" Molecules 27, no. 14: 4590. https://doi.org/10.3390/molecules27144590
APA StyleTian, S., Dong, Z., Liu, B., Xue, H., Erastova, V., Wang, M., & Yan, H. (2022). Characteristics of Gaseous/Liquid Hydrocarbon Adsorption Based on Numerical Simulation and Experimental Testing. Molecules, 27(14), 4590. https://doi.org/10.3390/molecules27144590







