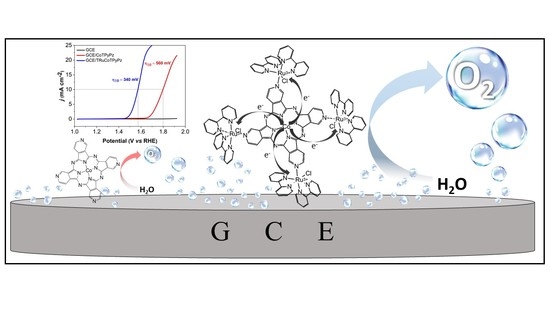
A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results
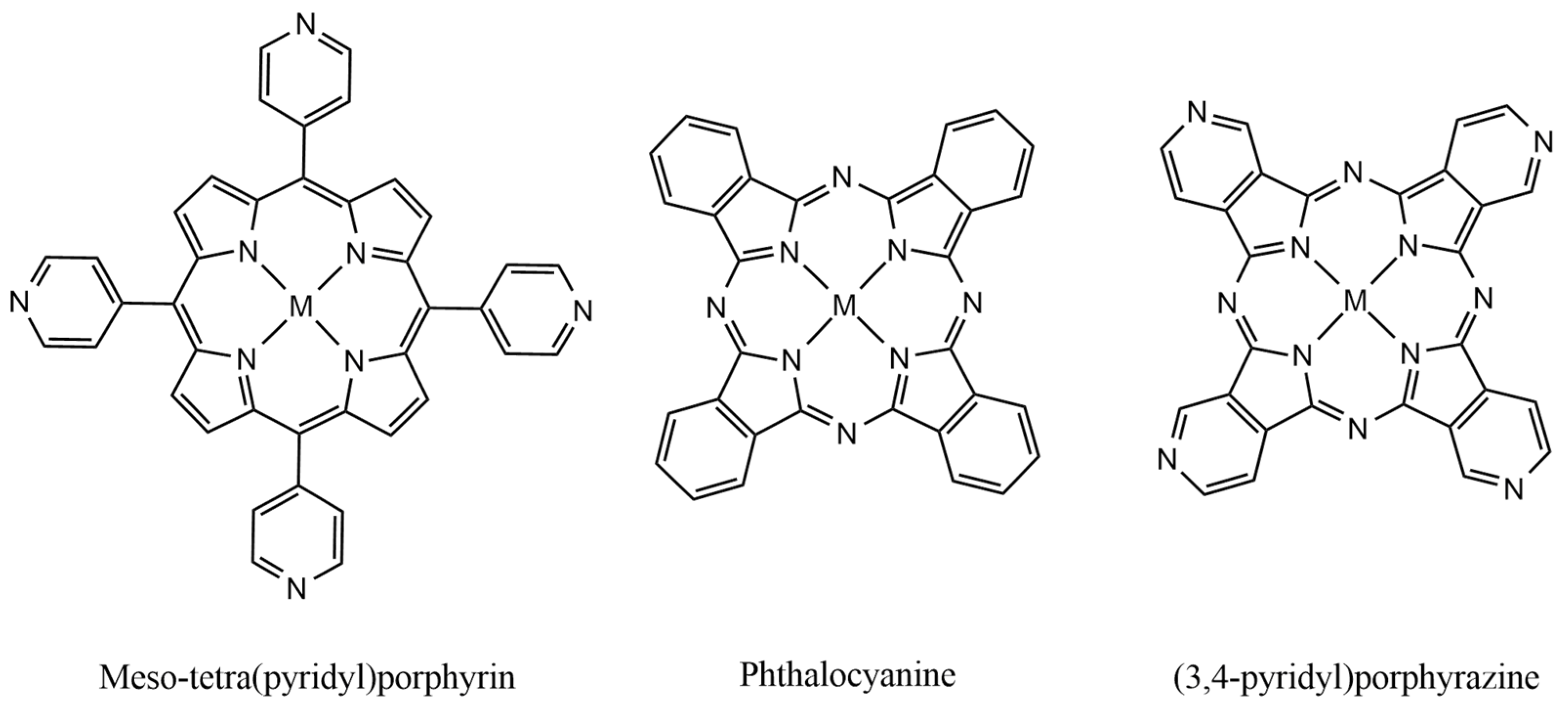
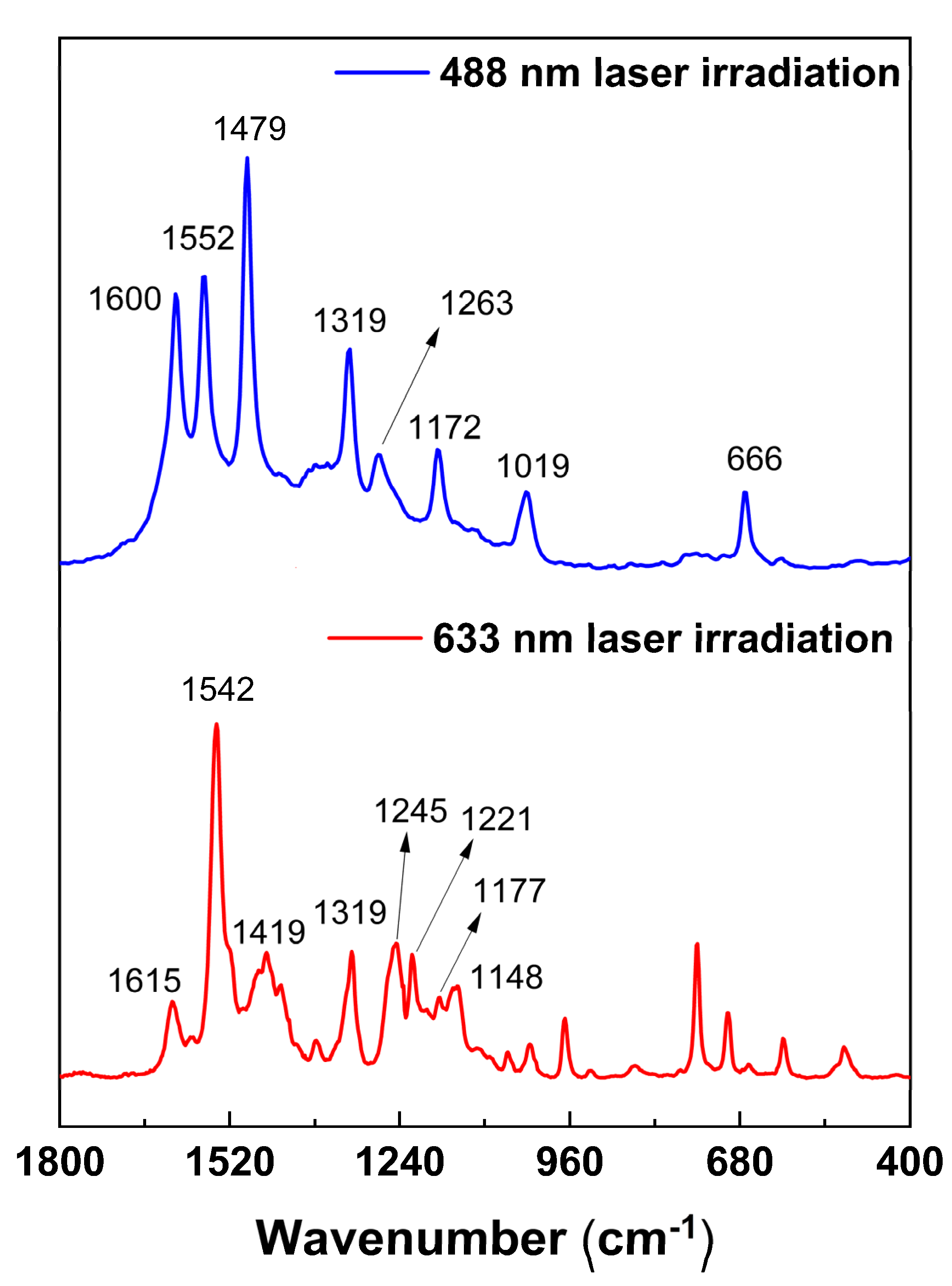
2.1. CoTPyPz Characterization
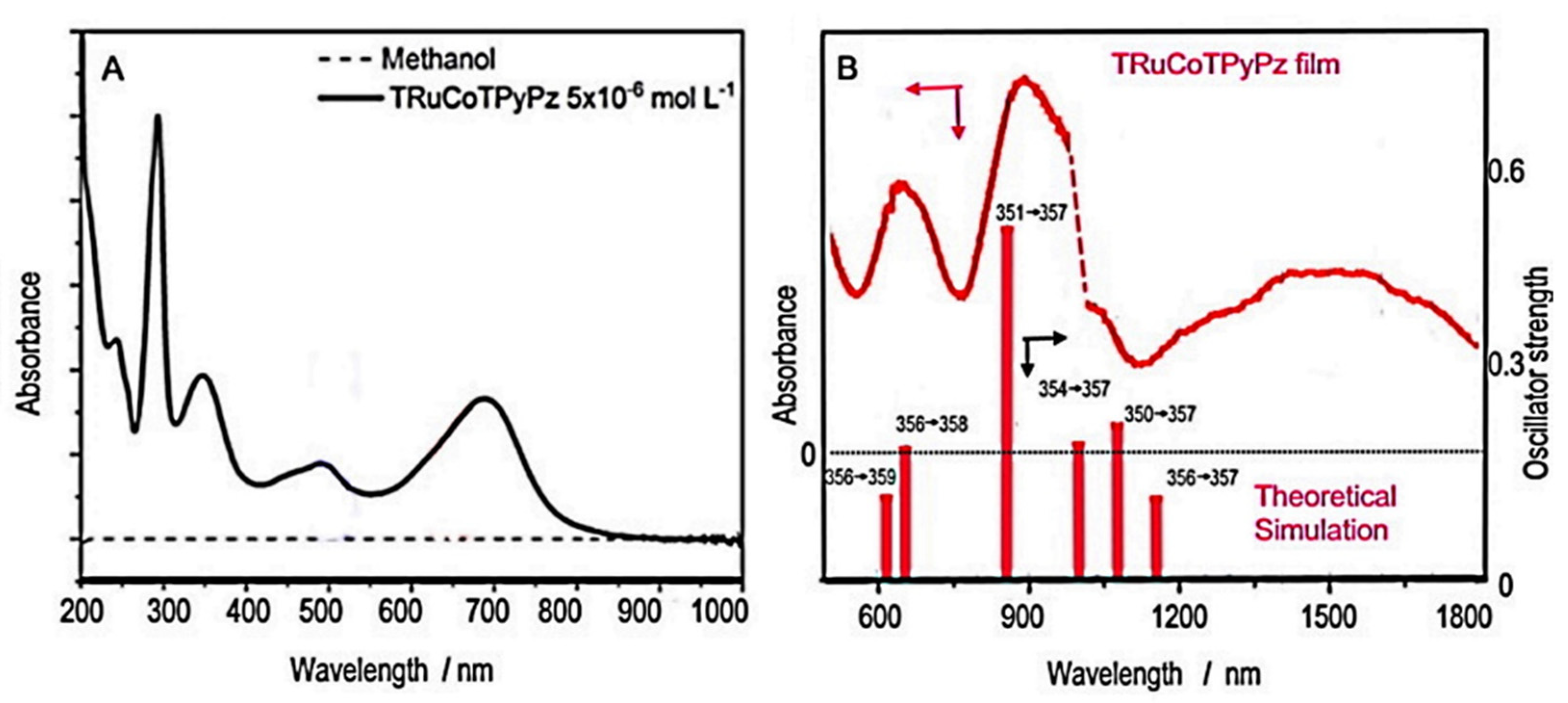
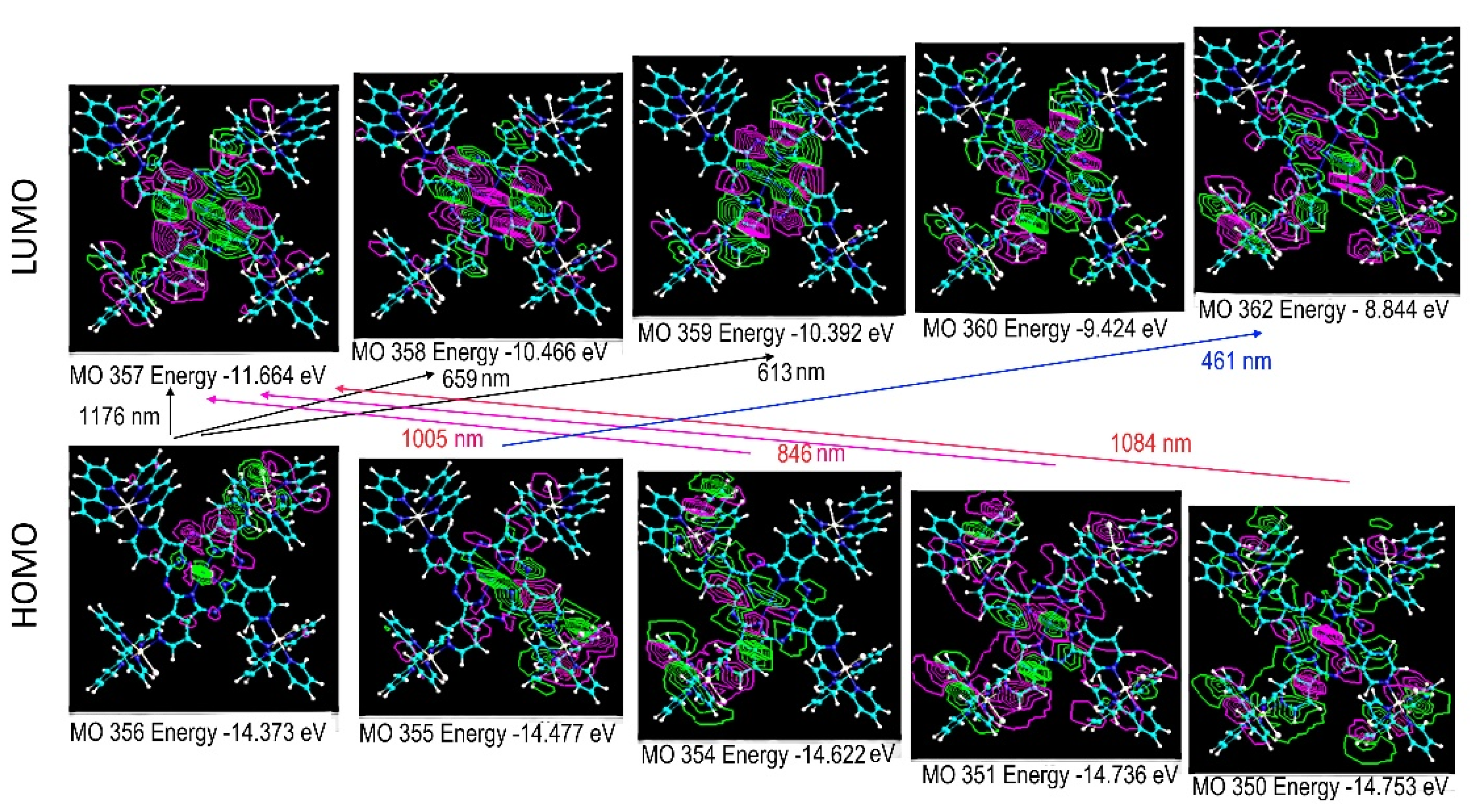
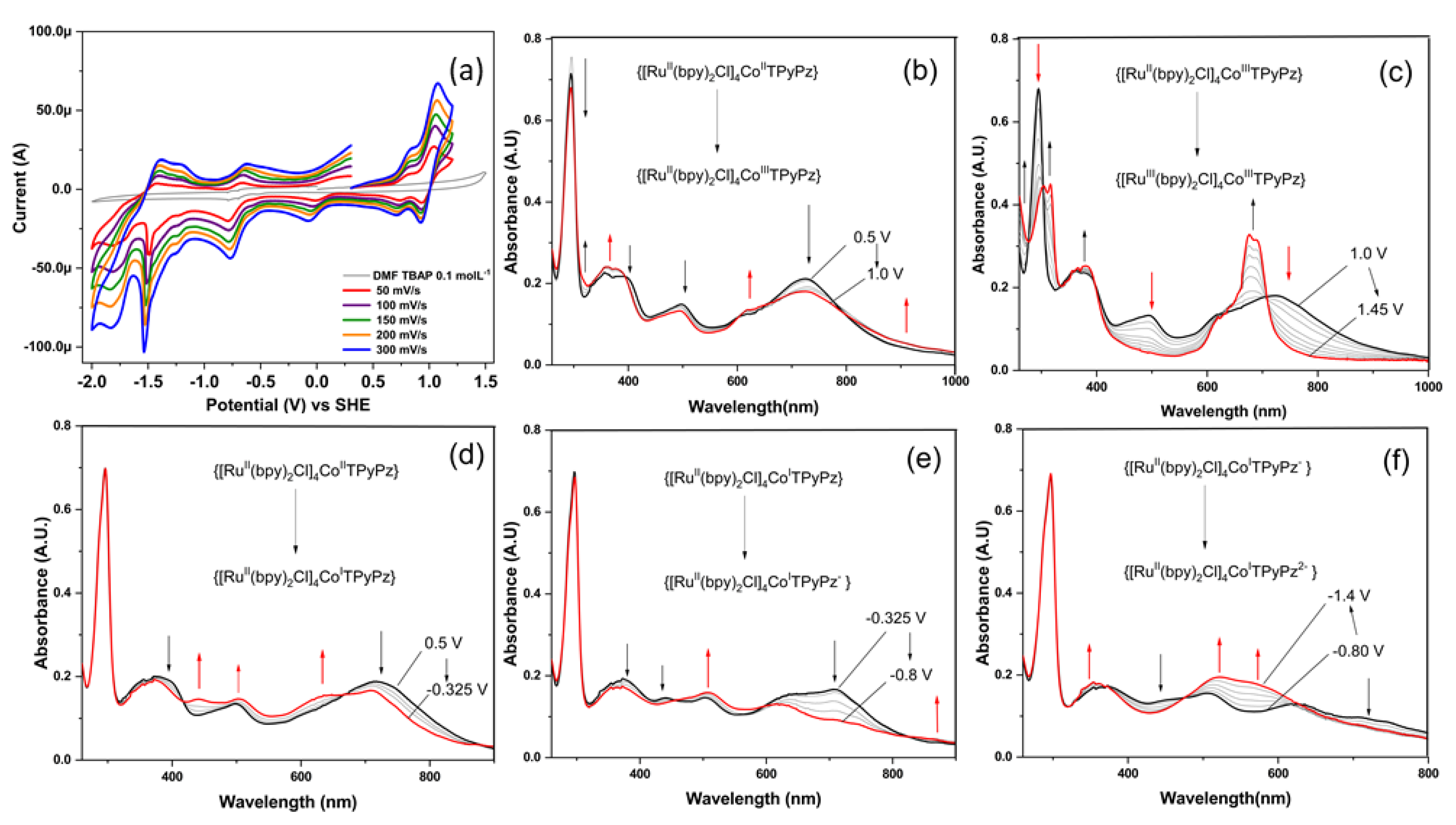
2.2. [TRuCoTPyPz](TFMS)4 Spectroscopy
2.3. Cyclic Voltammetry Behavior
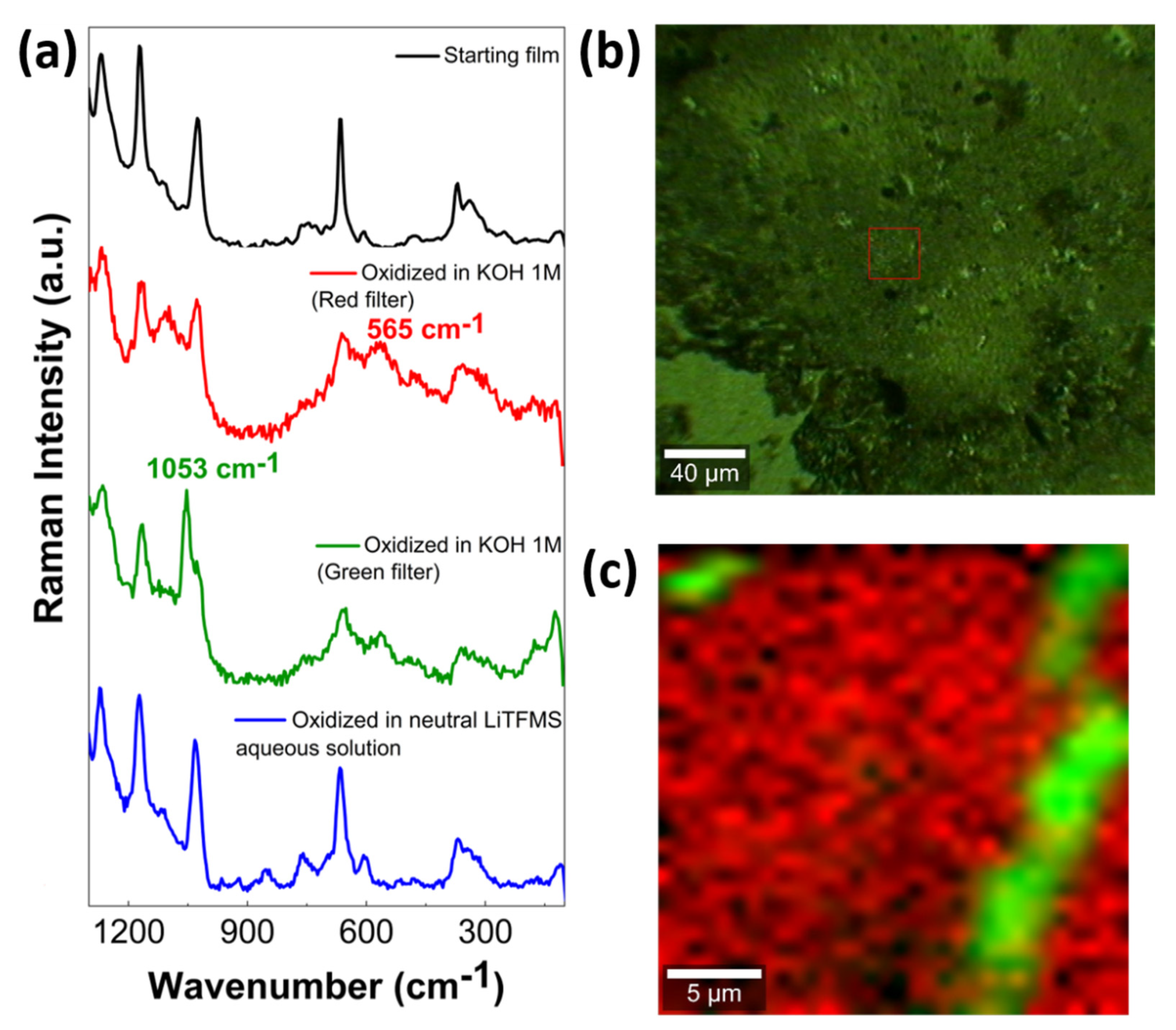
2.4. Preparation and Characterization of TRuCoTPyPz Thin Films
2.5. Electrocatalytic OER
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Precursors Ru(bpy)2Cl2 and CoTPyPz
3.3. Synthesis of TRuCoTPyPz
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and Interface effects in water-splitting electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of water electrolysis and recent progress in cobalt-, nickel-, and iron-based oxides for the oxygen evolution reaction. Angew. Chemie Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.-C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.-T.; Myers, D.; et al. Activity-stability trends for the oxygen evolution reaction on monometallic oxides in acidic environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar Vashistha, V.; Kumar Das, D. Recent development on metal phthalocyanines based materials for energy conversion and storage applications. Coord. Chem. Rev. 2021, 431, 213678. [Google Scholar] [CrossRef]
- Miletin, M.; Zimcik, P.; Novakova, V. Photodynamic properties of aza-analogues of phthalocyanines. Photochem. Photobiol. Sci. 2018, 17, 1749–1766. [Google Scholar] [CrossRef]
- Sun, B.; Ou, Z.; Meng, D.; Fang, Y.; Song, Y.; Zhu, W.; Solntsev, P.V.; Nemykin, V.N.; Kadish, K.M. Electrochemistry and catalytic properties for dioxygen reduction using ferrocene-substituted cobalt porphyrins. Inorg. Chem. 2014, 53, 8600–8609. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Lee, J.; Lee, L.Y.S.; Ng, D.K.P. Tuning the electrochemical properties of polymeric cobalt phthalocyanines for efficient water splitting. Adv. Funct. Mater. 2021, 31, 2103290. [Google Scholar] [CrossRef]
- Toyama, M.M.; Demets, G.J.F.; Araki, K.; Toma, H.E. Highly conductive electrostatically assembled porphyrazine films. Electrochem. Commun. 2000, 2, 749–753. [Google Scholar] [CrossRef]
- Beyene, B.B.; Hung, C.-H. Recent progress on metalloporphyrin-based hydrogen evolution catalysis. Coord. Chem. Rev. 2020, 410, 213234. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Zhao, W.; Shen, X.; Zhu, W. Electrochemical hydrogen and oxygen evolution reactions from a cobalt-porphyrin-based covalent organic polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Toma, H. Molecular materials and devices: Developing new functional systems based on the coordination chemistry approach. J. Braz. Chem. Soc. 2003, 35, 845–869. [Google Scholar] [CrossRef]
- Gonçalves, J.; Matias, T.; Agnes, L.; Martins, P.; Araki, K. Review—Tetraruthenated porphyrins and composites as catalysts and sensor materials: A short review. ECS J. Solid State Sci. Technol. 2020, 9, 061011. [Google Scholar] [CrossRef]
- Mayer, I.; Toma, H.E.; Araki, K. Electrocatalysis on tetraruthenated nickel and cobalt porphyrins electrostatic assembled films. J. Electroanal. Chem. 2006, 590, 111–119. [Google Scholar] [CrossRef]
- Mayer, I.; Nunes, G.S.; Toma, H.E.; Araki, K. Steric and catalytic effects in tetraruthenated manganese porphyrins. Eur. J. Inorg. Chem. 2006, 2006, 850–856. [Google Scholar] [CrossRef]
- Aguirre-Araque, J.S.; Gonçalves, J.M.; Nakamura, M.; Rossini, P.O.; Angnes, L.; Araki, K.; Toma, H.E. GO composite encompassing a tetraruthenated cobalt porphyrin-ni coordination polymer and its behavior as isoniazid BIA sensor. Electrochim. Acta 2019, 2019, 113–122. [Google Scholar] [CrossRef]
- Ferreira, L.M.C.; Martins, P.R.; Araki, K.; Angnes, L. Tuning selectivity and sensitivity of mixed-polymeric tetraruthenated metalloporphyrins modified electrodes as voltammetric sensors of chloramphenicol. Electroanalysis 2019, 31, 688–694. [Google Scholar] [CrossRef]
- Calfumán, K.; Aguirre, M.J.; Cañete-Rosales, P.; Bollo, S.; Llusar, R.; Isaacs, M. Electrocatalytic reduction of nitrite on tetraruthenated metalloporphyrins/nafion glassy carbon modified electrode. Electrochim. Acta 2011, 56, 8484–8491. [Google Scholar] [CrossRef]
- Tomoda, H.; Saito, S.; Ogawa, S.; Shiraishi, S. Synthesis of phthalocyanines from phthalonitrile with organic strong bases. Chem. Lett. 1980, 9, 1277–1280. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lever, A.B.P. Electronic structure and spectra of ruthenium diimine complexes by density functional theory and INDO/S. Comparison of the two methods. J. Organomet. Chem. 2001, 635, 187–196. [Google Scholar] [CrossRef]
- Poizat, O.; Sourisseau, C. Infrared, Raman, and resonance Raman studies of the Ru(2,2′-Bpy)32+ cation in its chloride crystal and as an intercalate in the layered MnPS3 compound. J. Phys. Chem. 1984, 88, 3007–3014. [Google Scholar] [CrossRef]
- Enokida, T.; Hirohashi, R. Cobalt phthalocyanine crystal synthesized at low temperature. Chem. Mater. 1991, 3, 918–921. [Google Scholar] [CrossRef]
- Silverstein, D.W.; Milojevich, C.B.; Camden, J.P.; Jensen, L. Investigation of linear and nonlinear raman scattering for isotopologues of Ru(Bpy)32+. J. Phys. Chem. C 2013, 117, 20855–20866. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Z.; Chen, X.; Nguyen, D.; Mattei, M.; Goubert, G.; Van Duyne, R.P. Investigation of cobalt phthalocyanine at the solid/liquid interface by electrochemical tip-enhanced raman spectroscopy. J. Phys. Chem. C 2019, 123, 9852–9859. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Constantino, V.R.L.; Baldwin, K.J.; Batchelder, D.N.; Pinnavaia, T.J.; Chibwe, M. Raman microspectroscopy of phthalocyanine intercalates: Tetrasulphonated cobalt and nickel phthalocyanines in layered double hydroxide. J. Raman Spectrosc. 1998, 29, 103–108. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Zhang, Y.; Li, R.; Jiang, J. The molecular, electronic structures and vibrational spectra of metal-free, N,N′-dideuterio and magnesium tetra-2,3-pyridino-porphyrazines: Density functional calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.; Luukkanen, S.; Haukka, M.; Ahlgrén, M.; Pakkanen, T.A. Redox and photochemical behaviour of ruthenium(II) complexes with H2dcbpy ligand (H2dcbpy = 2,2′-bipyridine-4,4′-dicarboxylic acid). J. Chem. Soc. Dalt. Trans. 2000, 16, 2745–2752. [Google Scholar] [CrossRef]
- Mayer, I.; Nakamura, M.; Toma, H.E.; Araki, K. Multielectronic redox and electrocatalytic supramolecular films based on a tetraruthenated iron porphyrin. Electrochim. Acta 2006, 52, 263–271. [Google Scholar] [CrossRef]
- Toyama, M.M.; Franco, M.; Catalani, L.H.; Araki, K.; Toma, H.E. Spectroelectrochemical and Photophysical properties of a (3,4-pyridyl) porphyrazine supermolecule containing four [Ru(Bipy)2Cl]+ groups. J. Photochem. Photobiol. A Chem. 1998, 118, 11–17. [Google Scholar] [CrossRef]
- Toyama, M.M.; Araki, K.; Toma, H.E. Absorption and luminescence spectra of tetra (3-Pyridyl)porphyrazine: A convergent spectroscopic method for the elucidation of association reactions in solution. Spectrosc. Lett. 1998, 31, 1065–1074. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, W.; Xiao, Y.; Wang, Y.; Luan, C.; Qin, C.; Dong, Y.; Li, M.; Dai, X.; Zhang, X. One-pot-synthesized cofe-glycerate hollow spheres with rich oxyhydroxides for efficient oxygen evolution reaction. ACS Sustain. Chem. Eng. 2020, 8, 5464–5477. [Google Scholar] [CrossRef]
- Moradi, M.; Hasanvandian, F.; Ghahraman Afshar, M.; Larimi, A.; Khorasheh, F.; Niknam, E.; Rahman Setayesh, S. Incorporation of Fe in mixed CoCu-alkoxide hollow sphere for enhancing the electrochemical water oxidation performance. Mater. Today Chem. 2021, 22, 100586. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Lima, I.S.; Azeredo, N.F.B.; Rocha, D.P.; de Siervo, A.; Angnes, L. NiVCe-Layered double hydroxide as multifunctional nanomaterials for energy and sensor applications. Front. Mater. 2021, 8, 781900. [Google Scholar] [CrossRef]
- Chang, J.; Xiao, Y.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Surface oxidized cobalt-phosphide nanorods as an advanced oxygen evolution catalyst in alkaline solution. ACS Catal. 2015, 5, 6874–6878. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Prabhu, C.P.K.; Hojamberdiev, M.; Sannegowda, L.K. Ni foam-supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2020, 449, 227516. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, Y.; Wang, Q.; Cheng, Y.; Xiang, Z. Bimetal-phthalocyanine based covalent organic polymers for highly efficient oxygen electrode. Appl. Catal. B Environ. 2019, 243, 204–211. [Google Scholar] [CrossRef]
- Qi, D.; Chen, X.; Liu, W.; Liu, C.; Liu, W.; Wang, K.; Jiang, J. A Ni/Fe-based heterometallic phthalocyanine conjugated polymer for the oxygen evolution reaction. Inorg. Chem. Front. 2020, 7, 642–646. [Google Scholar] [CrossRef]
- Barraza Alvarez, I.; Wu, Y.; Sanchez, J.; Ge, Y.; Ramos-Garcés, M.V.; Chu, T.; Jaramillo, T.F.; Colón, J.L.; Villagrán, D. Cobalt porphyrin intercalation into zirconium phosphate layers for electrochemical water oxidation. Sustain. Energy Fuels 2021, 5, 430–437. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Basu, O.; Das, S.K. ZIF-8 MOF encapsulated co-porphyrin, an efficient electrocatalyst for water oxidation in a wide PH range: Works better at neutral PH. ChemCatChem 2020, 12, 5430–5438. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Gao, Y.; Lu, D.; Du, P. Pyrolyzed cobalt porphyrin-based conjugated mesoporous polymers as bifunctional catalysts for hydrogen production and oxygen evolution in water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Bhunia, K.; Patra, B.C.; Das, S.K.; Pradhan, D.; Bhaumik, A.; Pradhan, A.; Bhattacharya, S. Efficacious electrochemical oxygen evolution from a novel Co(II) porphyrin/pyrene-based conjugated microporous polymer. ACS Appl. Mater. Interfaces 2019, 11, 1520–1528. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Li, T.; Zhang, W.-D.; Fu, Q.-T.; Lu, H.-S.; Wang, X.; Gu, Z.-G. Three-dimensional porphyrin-based covalent organic frameworks with tetrahedral building blocks for single-site catalysis. New J. Chem. 2019, 43, 16907–16914. [Google Scholar] [CrossRef]
- Moyer, B.A.; Meyer, T.J. Properties of the oxo/aqua system (bpy)2(py)RuO2+/(bpy)2(py)Ru(OH2)2+. Inorg. Chem. 1981, 20, 436–444. [Google Scholar] [CrossRef]
- Matias, T.A.; Mangoni, A.P.; Toma, S.H.; Rein, F.N.; Rocha, R.C.; Toma, H.E.; Araki, K. Catalytic water-oxidation activity of a weakly coupled binuclear ruthenium polypyridyl complex. Eur. J. Inorg. Chem. 2016, 2016, 5547–5556. [Google Scholar] [CrossRef] [Green Version]
- Alzate-Carvajal, N.; Bolivar-Pineda, L.M.; Meza-Laguna, V.; Basiuk, V.A.; Basiuk, E.V.; Baranova, E.A. Oxygen evolution reaction on single-walled carbon nanotubes noncovalently functionalized with metal phthalocyanines. ChemElectroChem 2020, 7, 428–436. [Google Scholar] [CrossRef]
- Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H.M.; Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: Cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 2020, 142, 11901–11914. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Guzman, K.G.; Dai, X.; Attanayake, N.H.; Strongin, D.R.; Sun, Y. Highly dispersed RuOOH nanoparticles on silica spheres: An efficient photothermal catalyst for selective aerobic oxidation of benzyl alcohol. Nano-Micro Lett. 2020, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, B.P.; Salmon, D.J.; Meyer, T.J. Mixed phosphine 2,2′-bipyridine complexes of ruthenium. Inorg. Chem. 1978, 17, 3334–3341. [Google Scholar] [CrossRef]


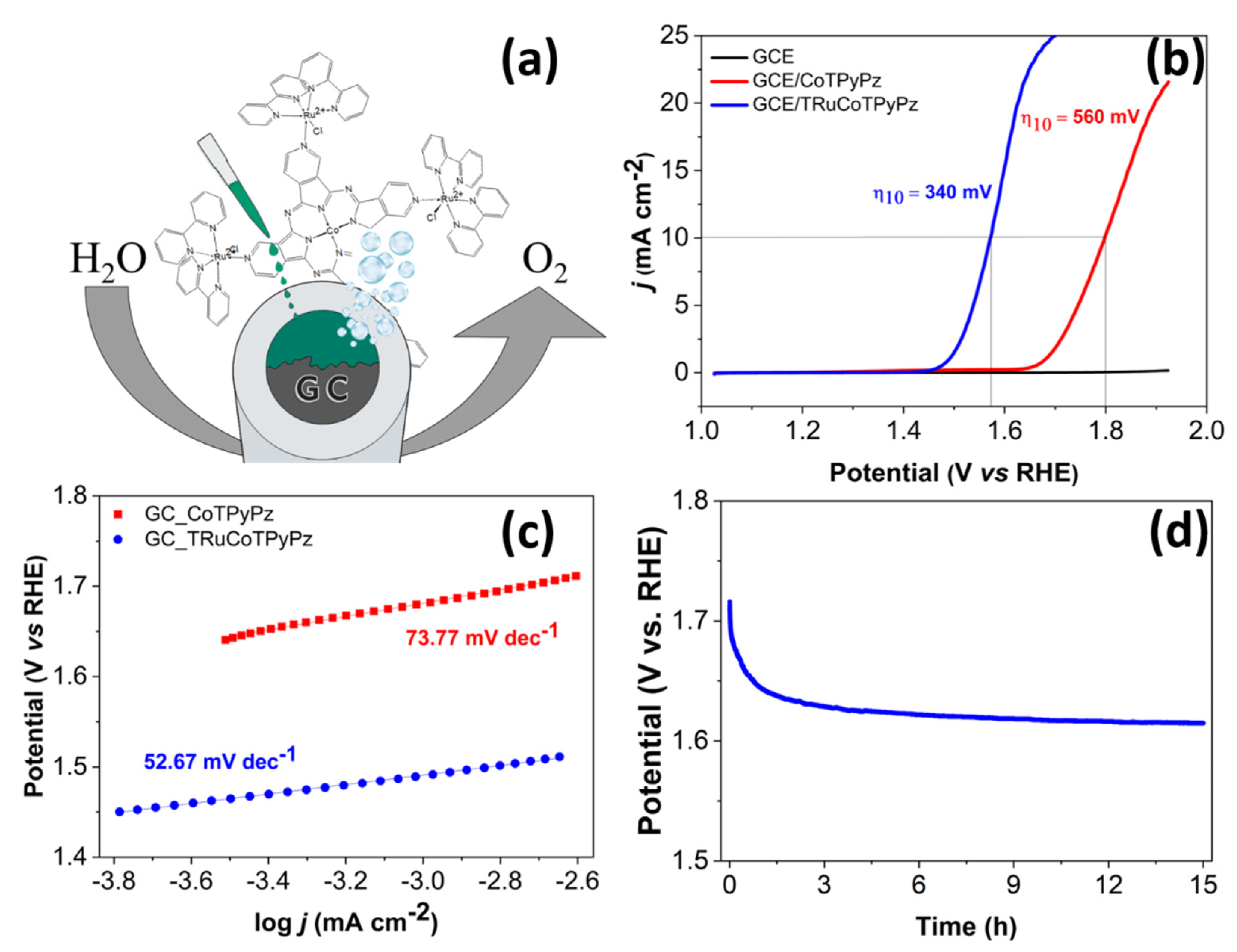
| Catalyst | η10 (mV) | Tafel Slope (mV dec−1) | Electrolyte | Ref. |
|---|---|---|---|---|
| RuO2 | 309 | 62 | 1 M KOH | [34] |
| IrO2 | 320 | 76 | 1 M KOH | [36] |
| 1:1 polyTACoPc + IrO2 | 304 | 39 | 0.1 M KOH | [37] |
| FeNi-COP-800 | 400 | 103 | 0.1 M KOH | [38] |
| Fe0.5Ni0.5Pc-CP | 317 | 116 | 1 M KOH | [39] |
| CoTCPP/ZrP | 476 | 76.4 | 0.1 M KOH | [40] |
| CoTMPP@ZIF-8 | 387 | 210.3 | 0.1 M KCl | [41] |
| CoP-2ph-CMP-800 | 370 | 86 | 1.0 M KOH | [42] |
| Co-MPPy-1 | 420 | 58 | 1.0 M NaOH | [43] |
| PCOF-1-Co | 386 | 89 | 1.0 M KOH | [44] |
| CoTPyPz | 560 | 74 | 1 M KOH | This work |
| TRuCoTPyPz | 340 | 53 | 1 M KOH | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.N.; Toma, S.H.; Hennemann, A.L.; Gonçalves, J.M.; Nakamura, M.; Araki, K.; Toyama, M.M.; Toma, H.E. A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction. Molecules 2022, 27, 4598. https://doi.org/10.3390/molecules27144598
Silva HN, Toma SH, Hennemann AL, Gonçalves JM, Nakamura M, Araki K, Toyama MM, Toma HE. A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction. Molecules. 2022; 27(14):4598. https://doi.org/10.3390/molecules27144598
Chicago/Turabian StyleSilva, Hiago N., Sérgio Hiroshi Toma, Artur Luís Hennemann, Josué M. Gonçalves, Marcelo Nakamura, Koiti Araki, Marcos Makoto Toyama, and Henrique Eisi Toma. 2022. "A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction" Molecules 27, no. 14: 4598. https://doi.org/10.3390/molecules27144598
APA StyleSilva, H. N., Toma, S. H., Hennemann, A. L., Gonçalves, J. M., Nakamura, M., Araki, K., Toyama, M. M., & Toma, H. E. (2022). A New Supramolecular Tetraruthenated Cobalt (II) Porphyrazine Displaying Outstanding Electrocatalytical Performance in Oxygen Evolution Reaction. Molecules, 27(14), 4598. https://doi.org/10.3390/molecules27144598