Synthesis and Biological Activity Screening of Newly Synthesized Trimethoxyphenyl-Based Analogues as Potential Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. Cytotoxic Activity against Liver HepG2 Cell Line
2.2.2. Tubulin Polymerization Inhibition Activity
2.2.3. Cell Cycle Analysis of Compound 9
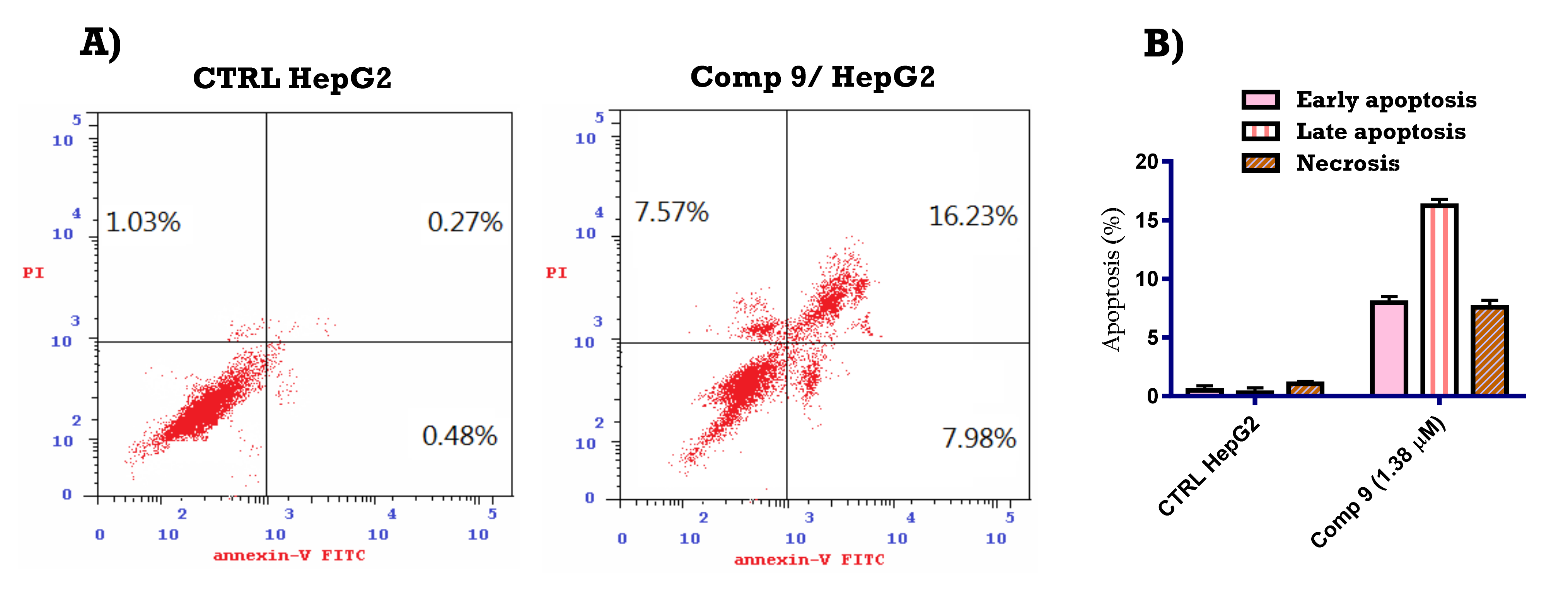
2.2.4. Apoptosis-Inducing Activity of Compound 9
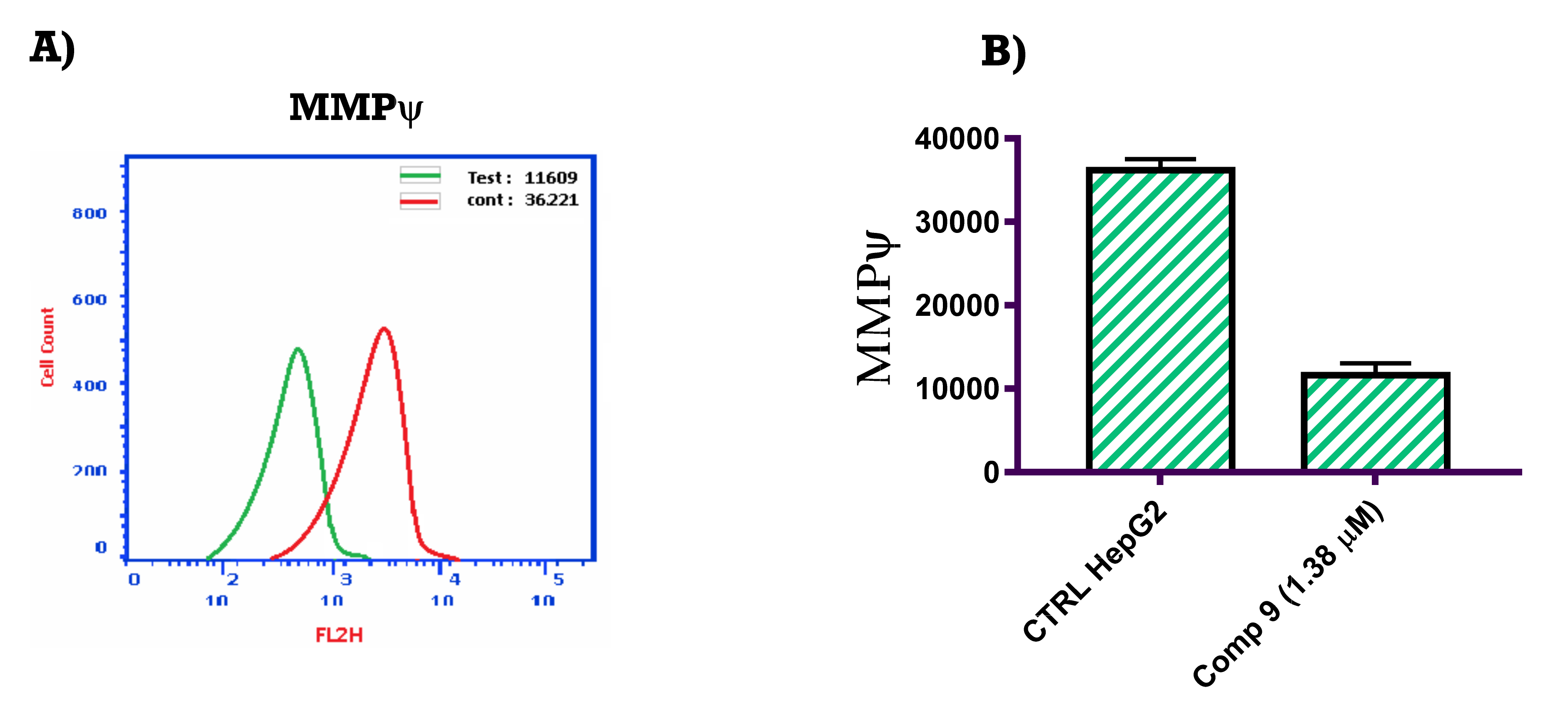
2.2.5. Mitochondrial Membrane Potential
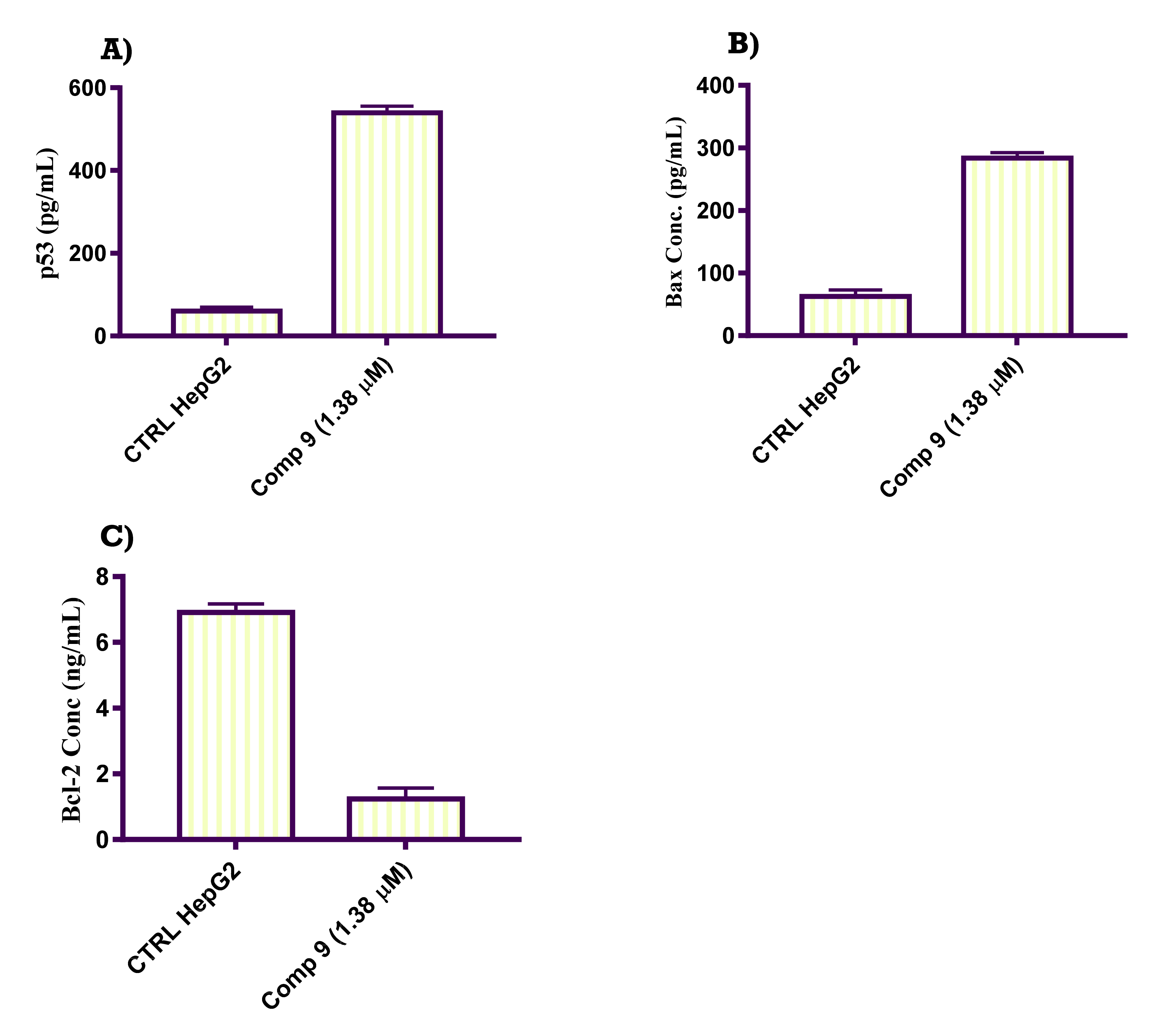
2.2.6. In Vitro ELISA Quantification of the Level of p53, Bax and Bcl-2
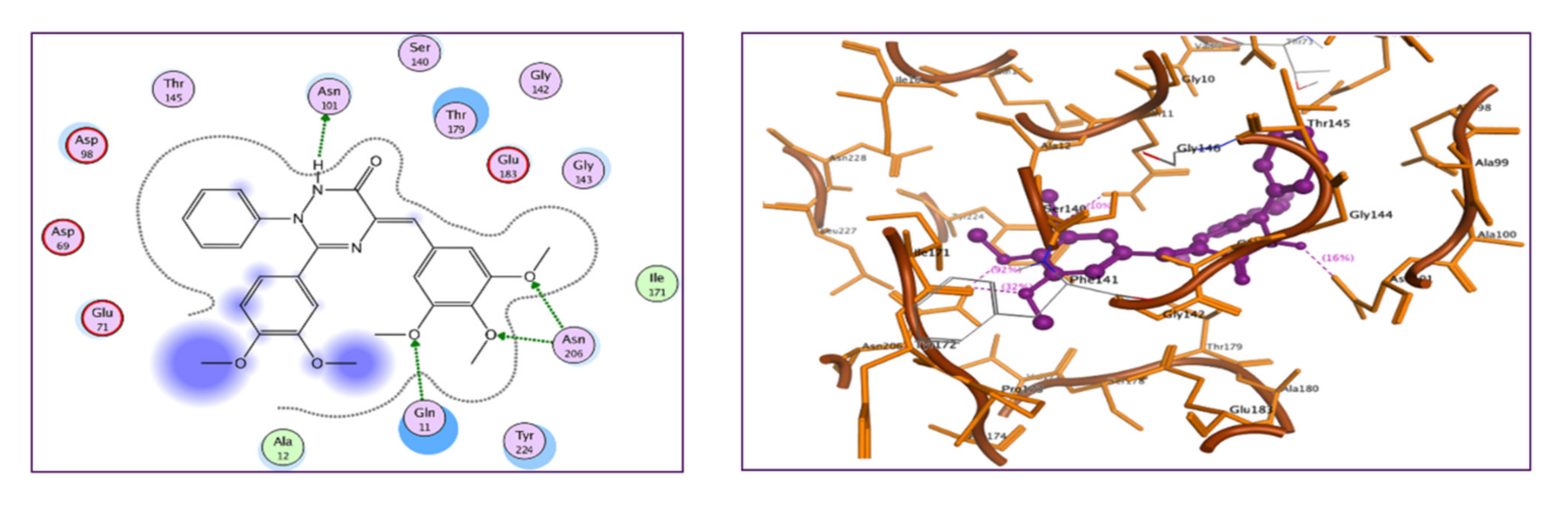
2.2.7. Molecular Docking Study
3. Experimental
3.1. Chemistry: General
3.2. Chemistry
3.2.1. General Procedure for the Preparation of (Z)-2-(3,4-Dimethoxyphenyl)-4-(3,4,5-Trimethoxybenzylidene)oxazol-5(4H)-one (1)
3.2.2. General Procedure for the Preparation of Ester Derivatives 2a,b
(Z)-Methyl 2-(3,4-Dimethoxybenzamido)-3-(3,4,5-Trimethoxyphenyl)acrylate (2a)
(Z)-Ethyl 2-(3,4-Dimethoxybenzamido)-3-(3,4,5-Trimethoxyphenyl)acrylate (2b)
3.2.3. General Procedure for the Preparation of 2-(2-(3,4-Dimethoxybenzamido)-3-(3,4,5-Trimethoxyphenyl)acrylamido)carboxylic Acids 3a,b
(Z)-2-(2-(3,4-Dimethoxybenzamido)-3-(3,4,5-Trimethoxyphenyl)acrylamido)acetic Acid (3a)
(Z)-2-(2-(3,4-Dimethoxybenzamido)-3-(3,4,5-Trimethoxyphenyl)acrylamido)propanoic Acid (3b)
3.2.4. General Procedure for the Preparation of (Z)-N-(3-Aryl-3-oxo-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)-3,4-Dimethoxybenzamides 4a–c
(Z)-3,4-Dimethoxy-N-(3-oxo-3-(M-tolylamino)-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)benzamide (4a)
(Z)-N-(3-(4-Hydroxyphenylamino)-3-oxo-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)-3,4-Dimethoxybenzamide (4b)
(Z)-3,4-Dimethoxy-N-(3-(4-Methoxyphenylamino)-3-oxo-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)benzamide (4c)
3.2.5. General Procedure for the Preparation of (Z)-3,4-Dimethoxy-N-(3-oxo-3-(2-Phenylhydrazinyl)-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)benzamide (5)
3.2.6. General Procedure for the Preparation of (Z)-N-(3-(2-Isonicotinoylhydrazinyl)-3-oxo-1-(3,4,5-Trimethoxyphenyl)prop-1-en-2-yl)-3,4-Dimethoxybenzamide (6)
3.2.7. General Procedure for the Preparation of (Z)-1-Aryl-2-(3,4-Dimethoxyphenyl)-4-(3,4,5-Trimethoxybenzylidene)-1H-Imidazol-5(4H)-ones 7a,b
(Z)-2-(3,4-Dimethoxyphenyl)-1-(4-Hydroxyphenyl)-4-(3,4,5-Trimethoxybenzylidene)-1H-Imidazol-5(4H)-one (7a)
(Z)-2-(3,4-Dimethoxyphenyl)-1-(4-Methoxyphenyl)-4-(3,4,5-Trimethoxybenzylidene)-1H-Imidazol-5(4H)-one (7b)
3.2.8. General Procedure for the Preparation of (Z)-3-(3,4-Dimethoxyphenyl)-6-oxo-5-(3,4,5-Trimethoxybenzylidene)-5,6-Dihydro-1,2,4-Triazine-2(1H)-Carbothioamide (8)
3.2.9. General Procedure for the Preparation of (Z)-3-(3,4-Dimethoxyphenyl)-2-Phenyl-5-(3,4,5-Trimethoxybenzylidene)-1,2-Dihydro-1,2,4-Triazin-6(5H)-one (9)
3.2.10. General Procedure for the Preparation of (Z)-3-(3,4-Dimethoxyphenyl)-2-Isonicotinoyl-5-(3,4,5-Trimethoxybenzylidene)-1,2-Dihydro-1,2,4-Triazin-6(5H)-one (10)
3.2.11. General Procedure for the Preparation of (Z)-3-(3,4-Dimethoxyphenyl)-2-(4-Phenylthiazol-2-yl)-5-(3,4,5-Trimethoxybenzylidene)-1,2-Dihydro-1,2,4-Triazin-6(5H)-one (11)
3.3. Biological Evaluation
3.3.1. MTT Cytotoxicity Assay
3.3.2. Tubulin Polymerization Assay
3.3.3. Cell Cycle Analysis
3.3.4. Annexin V/FITC Staining Assay
3.3.5. Mitochondrial Membrane Potential
3.3.6. Effect on p53, Bax and Bcl-2
3.3.7. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Labrie, M.; Brugge, J.S.; Mills, G.B.; Zervantonakis, I.K. Therapy resistance: Opportunities created by adaptive responses to targeted therapies in cancer. Nat. Rev. Cancer 2022, 22, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.; Zakaria, M.Y.; Munir, M.U.; Ahmad, N.; Elsherif, M.A.; Badr, R.E.; Hassan, A.K.; Almaaty, A.H.A.; Zaki, I. Design, Synthesis, In Vitro Biological Activity Evaluation and Stabilized Nanostructured Lipid Carrier Formulation of Newly Synthesized Schiff Bases-Based TMP Moieties. Pharmaceuticals 2022, 15, 679. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Astore, S.; Ciccarese, C.; Cannella, M.A.; Bove, P.; Iacovelli, V.; Tortora, G. Inside prostate cancer news from the 2021 ASCO Genitourinary Cancers Symposium. Expert Rev. Anticancer Ther. 2021, 21, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Rubio, X.; Martín, M.; Remon, J.; Higuera, O.; Calvo, V.; Jarabo, J.R.; Conde, E.; Luna, J.; Provencio, M.; de Castro, J.; et al. Targeted therapy moves to earlier stages of non-small-cell lung cancer: Emerging evidence, controversies and future challenges. Future Oncol. 2021, 17, 4011–4025. [Google Scholar] [CrossRef]
- Chua, K.C.; El-Haj, N.; Priotti, J.; Kroetz, D.L. Mechanistic insights into the pathogenesis of microtubule-targeting agent-induced peripheral neuropathy from pharmacogenetic and functional studies. Basic Clin. Pharmacol. Toxicol. 2022, 30, 60–74. [Google Scholar] [CrossRef]
- Lin, B.-Y.; Liu, W.-L.; Huang, H.; Hu, Y.-G.; Gong, S.; Meng, Y.-H.; Yan, J.; Lu, Y.-Z.; Chen, H.-L. AQ-4, a deuterium-containing molecule, acts as a microtubule-targeting agent for cancer treatment. Eur. J. Pharmacol. 2020, 877, 173093. [Google Scholar] [CrossRef]
- Whitaker, R.H.; Placzek, W.J. Regulating the BCL2 Family to Improve Sensitivity to Microtubule Targeting Agents. Cells 2019, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat. Rev. Mol. Cell Biol. 2022, 7, 1–18. [Google Scholar] [CrossRef]
- Hirst, W.G.; Biswas, A.; Mahalingan, K.K.; Reber, S. Differences in Intrinsic Tubulin Dynamic Properties Contribute to Spindle Length Control in Xenopus Species. Curr. Biol. 2020, 30, 2184–2190.e5. [Google Scholar] [CrossRef]
- Sallee, M.D.; Feldman, J.L. Microtubule organization across cell types and states. Curr. Biol. 2021, 31, R506–R511. [Google Scholar] [CrossRef]
- Hoffmann, I. Centrosomes in mitotic spindle assembly and orientation. Curr. Opin. Struct. Biol. 2021, 66, 193–198. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Santana-Santos, L.; Shuda, M.; Sobol, R.W.; van Houten, B.; Qian, W. A novel strategy for targeted killing of tumor cells: Induction of multipolar acentrosomal mitotic spindles with a quinazolinone derivative mdivi-1. Mol. Oncol. 2015, 9, 488–502. [Google Scholar] [CrossRef]
- Oskuei, S.R.; Mirzaei, S.; Jafari-Nik, M.R.; Hadizadeh, F.; Eisvand, F.; Mosaffa, F.; Ghodsi, R. Design, synthesis and biological evaluation of novel imidazole-chalcone derivatives as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 2021, 112, 104904. [Google Scholar] [CrossRef]
- Mühlethaler, T.; Gioia, D.; Prota, A.E.; Sharpe, M.E.; Cavalli, A.; Steinmetz, M.O. Comprehensive analysis of binding sites in tubulin. Angew. Chem. 2021, 133, 13443–13454. [Google Scholar] [CrossRef]
- Sun, K.; Sun, Z.; Zhao, F.; Shan, G.; Meng, Q. Recent advances in research of colchicine binding site inhibitors and their interaction modes with tubulin. Future Med. Chem. 2021, 13, 839–858. [Google Scholar] [CrossRef]
- Liu, W.; He, M.; Li, Y.; Peng, Z.; Wang, G. A review on synthetic chalcone derivatives as tubulin polymerisation inhibitors. J. Enzyme Inhib. Med. Chem. 2022, 37, 9–38. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization. Int. J. Mol. Sci. 2022, 23, 4001. [Google Scholar] [CrossRef]
- Jie, L.H.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An Insight on Its Pharmacological Activities, Mechanisms of Action and Toxicity. Front. Pharmacol. 2021, 11, 553404. [Google Scholar]
- Ali, M.A.; Nath, A.; Jannat, M.; Islam, M.M. Direct Synthesis of Diamides from Dicarboxylic Acids with Amines Using Nb2O5 as a Lewis Acid Catalyst and Molecular Docking Studies as Anticancer Agents. ACS Omega 2021, 6, 25002–25009. [Google Scholar] [CrossRef]
- Li, N.; Xu, M.; Zhang, L.; Lei, Z.; Chen, C.; Zhang, T.; Chen, L.; Sun, J. Discovery of Novel Celastrol–Imidazole Derivatives with Anticancer Activity In Vitro and In Vivo. J. Med. Chem. 2022, 65, 4578–4589. [Google Scholar] [CrossRef]
- Mohamed, H.; Al-Ghareeb, M.; Abd-Allah, R. Pharmacological Evaluation of Novel 1,2,4-triazine Derivatives Containing Thiazole Ring against Hepatocellular Carcinoma. Curr. Bioact. Compd. 2022, 18, 12–25. [Google Scholar] [CrossRef]
- Xiao, M.; Ahn, S.; Wang, J.; Chen, J.; Miller, D.D.; Dalton, J.T.; Li, W. Discovery of 4-Aryl-2-benzoyl-imidazoles as Tubulin Polymerization Inhibitor with Potent Antiproliferative Properties. J. Med. Chem. 2013, 56, 3318–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Song, J.; Cui, X.-X.; Liu, W.-B.; Li, Y.-R.; Yu, G.-X.; Tian, X.-Y.; Wang, Y.-F.; Liu, Y.; Zhang, S.-Y. Discovery of novel 1,2,4-triazine-chalcone hybrids as anti-gastric cancer agents via an axis of ROS-ERK-DR5 in vitro and in vivo. Arab. J. Chem. 2022, 15, 103644–103660. [Google Scholar] [CrossRef]
- Jung, E.-K.; Leung, E.; Barker, D. Synthesis and biological activity of pyrrole analogues of combretastatin A-4. Bioorg. Med. Chem. Lett. 2016, 26, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Aly, O.M.; Beshr, E.A.; Maklad, R.M.; Mustafa, M.; Gamal-Eldeen, A.M. Synthesis, Cytotoxicity, Docking Study, and Tubulin Polymerization Inhibitory Activity of Novel 1-(3,4-Dimethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole-3-carboxanilides. Arch. Pharm. 2014, 347, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Khan, S.A.; Chawla, G. Design & synthesis of 2-(substituted aryloxy)-5-(substituted benzylidene)-3-phenyl-2,5-dihydro-1H-[1,2,4] triazin-6-one as potential anticonvulsant agents. Eur. J. Med. Chem. 2010, 45, 3960–3969. [Google Scholar]
- Xue, J.; Wu, G.; Ejaz, U.; Akhtar, F.; Wan, X.; Zhu, Y.; Geng, A.; Chen, Y.; He, S. A novel histone deacetylase inhibitor LT-548-133-1 induces apoptosis by inhibiting HDAC and interfering with microtubule assembly in MCF-7 cells. Investig. New Drugs 2021, 39, 1222–1231. [Google Scholar] [CrossRef]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anti-Cancer Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Prakashkumar, N.; Sivamaruthi, B.S.; Chaiyasut, C.; Suganthy, N. Decoding the Neuroprotective Potential of Methyl Gallate-Loaded Starch Nanoparticles against Beta Amyloid-Induced Oxidative Stress-Mediated Apoptosis: An In Vitro Study. Pharmaceutics 2021, 13, 299. [Google Scholar] [CrossRef]
- Ebadollahi, S.H.; Pouramir, M.; Zabihi, E.; Golpour, M.; Aghajanpour-Mir, M. The Effect of Arbutin on The Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J. 2021, 22, 532–541. [Google Scholar]



| Comp No | IC50 Value (μM) | |
|---|---|---|
| HepG2 | HL-7702 | |
| 2a | 14.39 ± 0.82 | NT |
| 2b | 11.09 ± 0.77 | NT |
| 3a | 8.13 ± 0.54 | NT |
| 3b | 19.41 ± 1.43 | NT |
| 4a | 45.53 ± 2.89 | NT |
| 4b | 17.69 ± 1.10 | NT |
| 4c | 23.72 ± 1.37 | NT |
| 5 | 9.24 ± 0.52 | NT |
| 6 | 8.74 ± 0.57 | NT |
| 7a | 4.53 ± 0.29 | NT |
| 7b | 6.45 ± 0.24 | NT |
| 8 | 4.02 ± 0.09 | NT |
| 9 | 1.38 ± 0.15 | 29.07 ± 2.03 |
| 10 | 2.52 ± 0.24 | NT |
| 11 | 3.21 ± 0.22 | NT |
| podo | 2.08 ± 0.04 | 18.59 ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Warhi, T.; Abualnaja, M.; Abu Ali, O.A.; Althobaiti, F.; Alharthi, F.; Elsaid, F.G.; Shati, A.A.; Fayad, E.; Elghareeb, D.; Abu Almaaty, A.H.; et al. Synthesis and Biological Activity Screening of Newly Synthesized Trimethoxyphenyl-Based Analogues as Potential Anticancer Agents. Molecules 2022, 27, 4621. https://doi.org/10.3390/molecules27144621
Al-Warhi T, Abualnaja M, Abu Ali OA, Althobaiti F, Alharthi F, Elsaid FG, Shati AA, Fayad E, Elghareeb D, Abu Almaaty AH, et al. Synthesis and Biological Activity Screening of Newly Synthesized Trimethoxyphenyl-Based Analogues as Potential Anticancer Agents. Molecules. 2022; 27(14):4621. https://doi.org/10.3390/molecules27144621
Chicago/Turabian StyleAl-Warhi, Tarfah, Matokah Abualnaja, Ola A. Abu Ali, Fayez Althobaiti, Fahad Alharthi, Fahmy G. Elsaid, Ali A. Shati, Eman Fayad, Doaa Elghareeb, Ali H. Abu Almaaty, and et al. 2022. "Synthesis and Biological Activity Screening of Newly Synthesized Trimethoxyphenyl-Based Analogues as Potential Anticancer Agents" Molecules 27, no. 14: 4621. https://doi.org/10.3390/molecules27144621
APA StyleAl-Warhi, T., Abualnaja, M., Abu Ali, O. A., Althobaiti, F., Alharthi, F., Elsaid, F. G., Shati, A. A., Fayad, E., Elghareeb, D., Abu Almaaty, A. H., & Zaki, I. (2022). Synthesis and Biological Activity Screening of Newly Synthesized Trimethoxyphenyl-Based Analogues as Potential Anticancer Agents. Molecules, 27(14), 4621. https://doi.org/10.3390/molecules27144621







