Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis
Abstract
:1. Introduction
2. SOCS Proteins Expression and JAK/STAT Signaling in AD
2.1. SOCS1
2.2. SOCS3
2.3. SOCS5
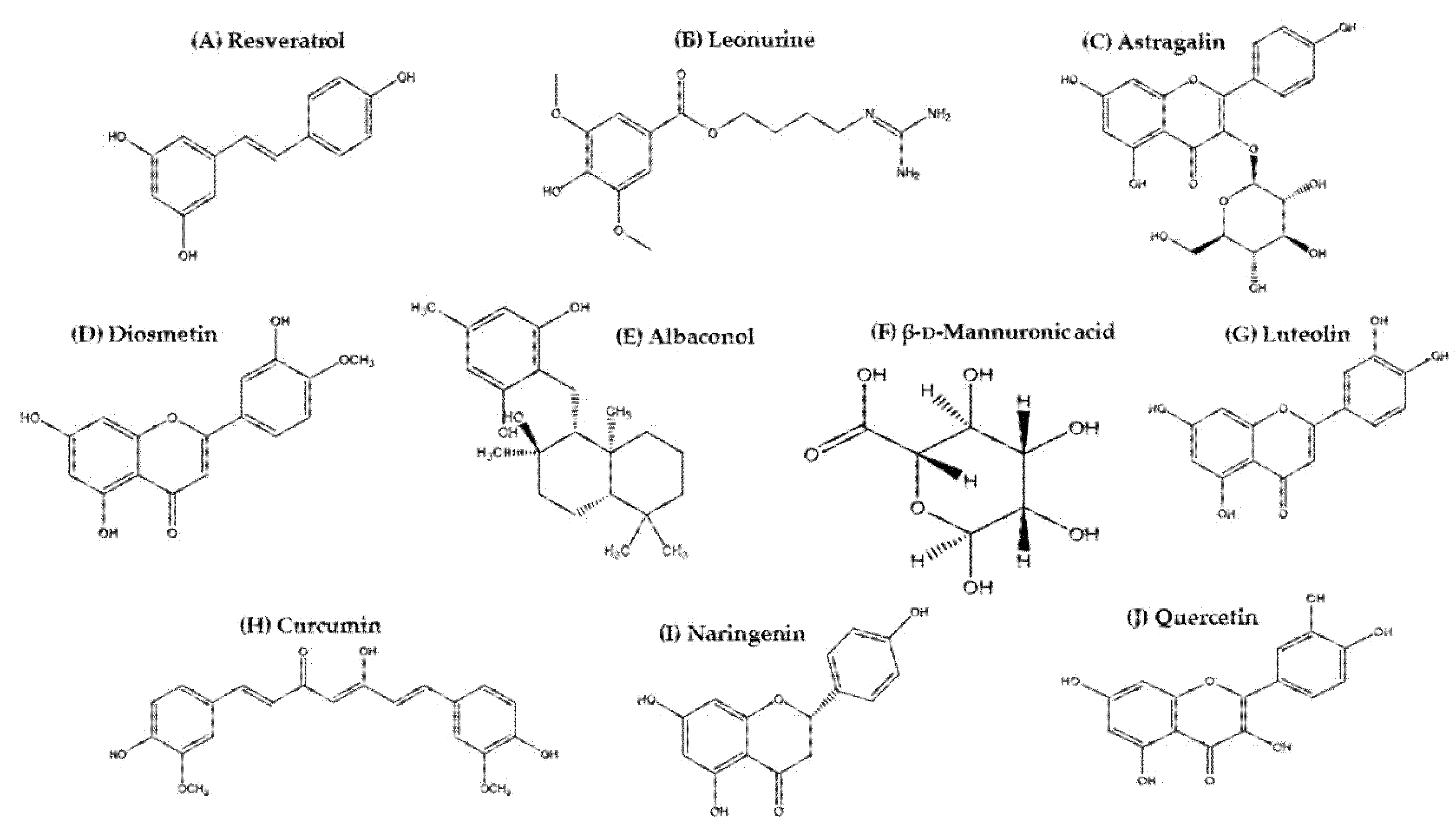
3. Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in AD Management
3.1. Resveratrol
3.2. Leonurine
3.3. Astragalin
3.4. Diosmetin
3.5. Albaconol
3.6. β-d-Mannuronic Acid
3.7. Luteolin
3.8. Curcumin
3.9. Naringenin
3.10. Quercetin
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT 2013, 2, e24137. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Diluvio, L.; Cannizzaro, M.V.; Grelli, S.; Galluzzo, M.; Talamonti, M.; Annicchiarico-Petruzzelli, M.; Mancini, M.; Melino, G.; et al. Skin immunity and its dysregulation in atopic dermatitis, hidradenitis suppurativa and vitiligo. Cell Cycle 2020, 19, 257–267. [Google Scholar] [CrossRef]
- Gavrilova, T. Immune Dysregulation in the Pathogenesis of Atopic Dermatitis. Dermatitis 2018, 29, 57–62. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran. J. Immunol. IJI 2019, 16, 97–107. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Rana, B.M.J.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R.; et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y.M. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y. Thymus and activation-regulated chemokine as a clinical biomarker in atopic dermatitis. J. Dermatol. 2014, 41, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, M.; Shiraishi, H.; Ohta, S.; Suzuki, S.; Arima, K.; Aoki, S.; Toda, S.; Inagaki, N.; Kurihara, Y.; Hayashida, S.; et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012, 122, 2590–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, R.; Miyagaki, T.; Oka, T.; Nakao, M.; Kawaguchi, M.; Suga, H.; Morimura, S.; Kai, H.; Asano, Y.; Tada, Y.; et al. Elevated serum galectin-9 levels in patients with atopic dermatitis. J. Dermatol. 2015, 42, 723–726. [Google Scholar] [CrossRef]
- Esaki, H.; Ewald, D.A.; Ungar, B.; Rozenblit, M.; Zheng, X.; Xu, H.; Estrada, Y.D.; Peng, X.; Mitsui, H.; Litman, T.; et al. Identification of novel immune and barrier genes in atopic dermatitis by means of laser capture microdissection. J. Allergy Clin. Immunol. 2015, 135, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Takemura, M.; Nakahara, T.; Hashimoto-Hachiya, A.; Furue, M.; Tsuji, G. Glyteer, Soybean Tar, Impairs IL-4/Stat6 Signaling in Murine Bone Marrow-Derived Dendritic Cells: The Basis of Its Therapeutic Effect on Atopic Dermatitis. Int. J. Mol. Sci. 2018, 19, 1169. [Google Scholar] [CrossRef] [Green Version]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.F.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L.; et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)+ TH2/TH1 cell imbalance, whereas adults acquire CLA+ TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941–951.e3. [Google Scholar] [CrossRef] [Green Version]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bogaard, E.H.; Bergboer, J.G.M.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.J.J.; Hato, S.V.; van der Valk, P.G.M.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, G.; Ito, T.; Chiba, T.; Mitoma, C.; Nakahara, T.; Uchi, H.; Furue, M. The role of the OVOL1–OVOL2 axis in normal and diseased human skin. J. Dermatol. Sci. 2018, 90, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Li, Y.; Fischer, M.J.M.; Steinhoff, M.; Chen, W.; Wang, J. Th2 Modulation of Transient Receptor Potential Channels: An Unmet Therapeutic Intervention for Atopic Dermatitis. Front. Immunol. 2021, 12, 696784. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Phadungsaksawasdi, P.; Ito, T. Atopic dermatitis as Th2 disease revisited. J. Cutan. Immunol. Allergy 2018, 1, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Mitamura, Y.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Yoshihara, T.; Masuoka, M.; Tsuji, G.; Nakahara, T.; Hashimoto-Hachiya, A.; Conway, S.J.; et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy 2018, 73, 1881–1891. [Google Scholar] [CrossRef]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef] [Green Version]
- Aktar, M.K.; Kido-Nakahara, M.; Furue, M.; Nakahara, T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015, 70, 846–854. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Soumelis, V.; Watanabe, N.; Hanabuchi, S.; Antonenko, S.; de Waal-Malefyt, R.; Liu, Y.-J. Human Dendritic Cells Activated by TSLP and CD40L Induce Proallergic Cytotoxic T Cells. J. Exp. Med. 2003, 197, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.-H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.-F.; Yao, Z.; Cao, W.; Liu, Y.-J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Yamamura, K.; Kido-Nakahara, M.; Nakahara, T.; Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2018, 73, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, K.; Uruno, T.; Shiraishi, A.; Tanaka, Y.; Ushijima, M.; Nakahara, T.; Watanabe, M.; Kido-Nakahara, M.; Tsuge, I.; Furue, M.; et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat. Commun. 2017, 8, 13946. [Google Scholar] [CrossRef] [Green Version]
- Szegedi, K.; Kremer, A.E.; Kezic, S.; Teunissen, M.B.M.; Bos, J.D.; Luiten, R.M.; Res, P.C.; Middelkamp-Hup, M.A. Increased frequencies of IL-31-producing T cells are found in chronic atopic dermatitis skin. Exp. Dermatol. 2012, 21, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Dawn, B.; Xuan, Y.-T. Role of the JAK–STAT Pathway in Protection Against Myocardial Ischemia/Reperfusion Injury. Trends Cardiovasc. Med. 2003, 13, 72–79. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Darnell, J.E. STATs and Gene Regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, K.-Y.; Yoon, W.-C.; Park, S.-H.; Park, K.-K.; Yoo, D.-H.; Choe, J.-Y. Melittin enhances apoptosis through suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3 activation and Bcl-2 expression for human fibroblast-like synoviocytes in rheumatoid arthritis. Jt. Bone Spine 2011, 78, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.S. Suppressors of cytokine signalling (SOCS) in the immune system. Nat. Rev. Immunol. 2002, 2, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, C.J.; Miller, M.E.; Hilton, D.J.; Lund, P.K. Suppressors of cytokine signaling: Relevance to gastrointestinal function and disease. Gastroenterology 2002, 123, 2064–2081. [Google Scholar] [CrossRef]
- Sobah, M.L.; Liongue, C.; Ward, A.C. SOCS Proteins in Immunity, Inflammatory Diseases, and Immune-Related Cancer. Front. Med. 2021, 8, 727987. [Google Scholar] [CrossRef]
- Ihle, J.N. Cytokine receptor signalling. Nature 1995, 377, 591–594. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Schreiber, R.D. Cytokine Signaling in 2002. Cell 2002, 109, S121–S131. [Google Scholar] [CrossRef] [Green Version]
- Tamiya, T.; Kashiwagi, I.; Takahashi, R.; Yasukawa, H.; Yoshimura, A. Suppressors of Cytokine Signaling (SOCS) Proteins and JAK/STAT Pathways. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, H.; Sasaki, A.; Yoshimura, A. Negative Regulation of Cytokine Signaling Pathways. Annu. Rev. Immunol. 2000, 18, 143–164. [Google Scholar] [CrossRef]
- Fujimoto, M.; Naka, T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003, 24, 659–666. [Google Scholar] [CrossRef]
- Cohney, S.J.; Sanden, D.; Cacalano, N.A.; Yoshimura, A.; Mui, A.; Migone, T.S.; Johnston, J.A. SOCS-3 Is Tyrosine Phosphorylated in Response to Interleukin-2 and Suppresses STAT5 Phosphorylation and Lymphocyte Proliferation. Mol. Cell. Biol. 1999, 19, 4980–4988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auernhammer, C.J.; Melmed, S. Interleukin-11 Stimulates Proopiomelanocortin Gene Expression and Adrenocorticotropin Secretion in Corticotroph Cells: Evidence for a Redundant Cytokine Network in the Hypothalamo-Pituitary-Adrenal Axis. Endocrinology 1999, 140, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kubo, M. SOCS proteins in T helper cell differentiation: Implications for allergic disorders? Expert Rev. Mol. Med. 2004, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Larkin, J. Therapeutic Implication of SOCS1 Modulation in the Treatment of Autoimmunity and Cancer. Front. Pharmacol. 2019, 10, 324. [Google Scholar] [CrossRef]
- Marine, J.-C.; Topham, D.J.; McKay, C.; Wang, D.; Parganas, E.; Stravopodis, D.; Yoshimura, A.; Ihle, J.N. SOCS1 Deficiency Causes a Lymphocyte-Dependent Perinatal Lethality. Cell 1999, 98, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Starr, R.; Metcalf, D.; Elefanty, A.G.; Brysha, M.; Willson, T.A.; Nicola, N.A.; Hilton, D.J.; Alexander, W.S. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 1998, 95, 14395–14399. [Google Scholar] [CrossRef] [Green Version]
- Alexander, W.S.; Starr, R.; Fenner, J.E.; Scott, C.L.; Handman, E.; Sprigg, N.S.; Corbin, J.E.; Cornish, A.L.; Darwiche, R.; Owczarek, C.M.; et al. SOCS1 Is a Critical Inhibitor of Interferon γ Signaling and Prevents the Potentially Fatal Neonatal Actions of this Cytokine. Cell 1999, 98, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, A.; Suzuki, M.; Sakaguchi, R.; Hanada, T.; Yasukawa, H. SOCS, Inflammation, and Autoimmunity. Front. Immunol. 2012, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Ryo, A.; Suizu, F.; Yoshida, Y.; Perrem, K.; Liou, Y.-C.; Wulf, G.; Rottapel, R.; Yamaoka, S.; Lu, K.P. Regulation of NF-κB Signaling by Pin1-Dependent Prolyl Isomerization and Ubiquitin-Mediated Proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [Google Scholar] [CrossRef]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef]
- Stoiber, D.; Kovarik, P.; Cohney, S.; Johnston, J.A.; Steinlein, P.; Decker, T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-gamma. J. Immunol. 1999, 163, 2640–2647. [Google Scholar] [PubMed]
- Nakagawa, R.; Naka, T.; Tsutsui, H.; Fujimoto, M.; Kimura, A.; Abe, T.; Seki, E.; Sato, S.; Takeuchi, O.; Takeda, K.; et al. SOCS-1 Participates in Negative Regulation of LPS Responses. Immunity 2002, 17, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Naka, T.; Kawazoe, Y.; Fujimoto, M.; Narazaki, M.; Nakagawa, R.; Fukuyama, H.; Nagata, S.; Kishimoto, T. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor α-induced cell death in fibroblasts. Proc. Natl. Acad. Sci. USA 2000, 97, 5405–5410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, M. A regulatory role for suppressor of cytokine signaling-1 in Th polarization in vivo. Int. Immunol. 2002, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.M.; Cornish, A.L.; Darwiche, R.; Stanley, E.G.; Purton, J.F.; Godfrey, D.I.; Hilton, D.J.; Starr, R.; Alexander, W.S.; Kay, T.W. Suppressor of Cytokine Signaling-1 Is a Critical Regulator of Interleukin-7-Dependent CD8+ T Cell Differentiation. Immunity 2003, 18, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Federici, M.; Giustizieri, M.L.; Scarponi, C.; Girolomoni, G.; Albanesi, C. Impaired IFN-γ-Dependent Inflammatory Responses in Human Keratinocytes Overexpressing the Suppressor of Cytokine Signaling 1. J. Immunol. 2002, 169, 434–442. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, W.; Dai, Y. SOCS3 and its role in associated diseases. Hum. Immunol. 2015, 76, 775–780. [Google Scholar] [CrossRef]
- Carow, B.; Rottenberg, M.E. SOCS3, a Major Regulator of Infection and Inflammation. Front. Immunol. 2014, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Dong, N.; Liu, J.; Wang, Y.; Shi, J.; Wei, Z.; Hu, X.; Gong, L. Inhibitory effects of suppressor of cytokine signaling 3 on inflammatory cytokine expression and migration and proliferation of IL-6/IFN-γ-induced vascular smooth muscle cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 615–622. [Google Scholar] [CrossRef]
- Baetz, A.; Frey, M.; Heeg, K.; Dalpke, A.H. Suppressor of Cytokine Signaling (SOCS) Proteins Indirectly Regulate Toll-like Receptor Signaling in Innate Immune Cells. J. Biol. Chem. 2004, 279, 54708–54715. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Cao, J.; Wu, M.; Zhang, W.; Jiang, T.; Yoshimura, A.; Gao, H. Suppressor of Cytokine Signaling 3 Inhibits LPS-induced IL-6 Expression in Osteoblasts by Suppressing CCAAT/Enhancer-binding Protein β Activity. J. Biol. Chem. 2010, 285, 37227–37239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekelund, E.; Sääf, A.; Tengvall-Linder, M.; Melen, E.; Link, J.; Barker, J.; Reynolds, N.J.; Meggitt, S.J.; Kere, J.; Wahlgren, C.-F.; et al. Elevated Expression and Genetic Association Links the SOCS3 Gene to Atopic Dermatitis. Am. J. Hum. Genet. 2006, 78, 1060–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seki, Y.; Inoue, H.; Nagata, N.; Hayashi, K.; Fukuyama, S.; Matsumoto, K.; Komine, O.; Hamano, S.; Himeno, K.; Inagaki-Ohara, K.; et al. SOCS-3 regulates onset and maintenance of TH2-mediated allergic responses. Nat. Med. 2003, 9, 1047–1054. [Google Scholar] [CrossRef]
- Kinjyo, I.; Inoue, H.; Hamano, S.; Fukuyama, S.; Yoshimura, T.; Koga, K.; Takaki, H.; Himeno, K.; Takaesu, G.; Kobayashi, T.; et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor–β1. J. Exp. Med. 2006, 203, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Trengove, M.C.; Ward, A.C. SOCS proteins in development and disease. Am. J. Clin. Exp. Immunol. 2013, 2, 1–29. [Google Scholar] [PubMed]
- Brender, C.; Columbus, R.; Metcalf, D.; Handman, E.; Starr, R.; Huntington, N.; Tarlinton, D.; Ødum, N.; Nicholson, S.E.; Nicola, N.A.; et al. SOCS5 Is Expressed in Primary B and T Lymphoid Cells but Is Dispensable for Lymphocyte Production and Function. Mol. Cell. Biol. 2004, 24, 6094–6103. [Google Scholar] [CrossRef] [Green Version]
- Kario, E.; Marmor, M.D.; Adamsky, K.; Citri, A.; Amit, I.; Amariglio, N.; Rechavi, G.; Yarden, Y. Suppressors of Cytokine Signaling 4 and 5 Regulate Epidermal Growth Factor Receptor Signaling. J. Biol. Chem. 2005, 280, 7038–7048. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Paul, W.E. Impaired Interleukin 4 Signaling in T Helper Type 1 Cells. J. Exp. Med. 1998, 187, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Kubo, M.; Ransom, J.; Webb, D.; Hashimoto, Y.; Tada, T.; Nakayama, T. T-cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO J. 1997, 16, 4007–4020. [Google Scholar] [CrossRef] [Green Version]
- Seki, Y.; Hayashi, K.; Matsumoto, A.; Seki, N.; Tsukada, J.; Ransom, J.; Naka, T.; Kishimoto, T.; Yoshimura, A.; Kubo, M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc. Natl. Acad. Sci. USA 2002, 99, 13003–13008. [Google Scholar] [CrossRef] [Green Version]
- Masuhara, M.; Sakamoto, H.; Matsumoto, A.; Suzuki, R.; Yasukawa, H.; Mitsui, K.; Wakioka, T.; Tanimura, S.; Sasaki, A.; Misawa, H.; et al. Cloning and Characterization of Novel CIS Family Genes. Biochem. Biophys. Res. Commun. 1997, 239, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linossi, E.M.; Chandrashekaran, I.R.; Kolesnik, T.B.; Murphy, J.M.; Webb, A.I.; Willson, T.A.; Kedzierski, L.; Bullock, A.N.; Babon, J.J.; Norton, R.S.; et al. Suppressor of Cytokine Signaling (SOCS) 5 Utilises Distinct Domains for Regulation of JAK1 and Interaction with the Adaptor Protein Shc-1. PLoS ONE 2013, 8, e70536. [Google Scholar] [CrossRef] [PubMed]
- Keewan, E.; Matlawska-Wasowska, K. The Emerging Role of Suppressors of Cytokine Signaling (SOCS) in the Development and Progression of Leukemia. Cancers 2021, 13, 4000. [Google Scholar] [CrossRef]
- Zhuang, G.; Wu, X.; Jiang, Z.; Kasman, I.; Yao, J.; Guan, Y.; Oeh, J.; Modrusan, Z.; Bais, C.; Sampath, D.; et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012, 31, 3513–3523. [Google Scholar] [CrossRef]
- Toniolo, P.A.; Liu, S.; Yeh, J.E.; Ye, D.Q.; Barbuto, J.A.M.; Frank, D.A. Deregulation of SOCS5 suppresses dendritic cell function in chronic lymphocytic leukemia. Oncotarget 2016, 7, 46301–46314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, A.; Seki, Y.; Fukushima, A.; Kubo, M. The Control of Allergic Conjunctivitis by Suppressor of Cytokine Signaling (SOCS)3 and SOCS5 in a Murine Model. J. Immunol. 2005, 175, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Hatano, Y.; Katagiri, K. Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis. Clin. Exp. Immunol. 2004, 135, 505–510. [Google Scholar] [CrossRef]
- Ohshima, M.; Yokoyama, A.; Ohnishi, H.; Hamada, H.; Kohno, N.; Higaki, J.; Naka, T. Overexpression of suppressor of cytokine signalling-5 augments eosinophilic airway inflammation in mice. Clin. Exp. Allergy 2007, 37, 735–742. [Google Scholar] [CrossRef]
- Knosp, C.A.; Carroll, H.P.; Elliott, J.; Saunders, S.P.; Nel, H.J.; Amu, S.; Pratt, J.C.; Spence, S.; Doran, E.; Cooke, N.; et al. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J. Exp. Med. 2011, 208, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Moreno, A.S.; McPhee, R.; Arruda, L.K.; Howell, M.D. Targeting the T Helper 2 Inflammatory Axis in Atopic Dermatitis. Int. Arch. Allergy Immunol. 2016, 171, 71–80. [Google Scholar] [CrossRef]
- Hilton, D.J. Negative regulators of cytokine signal transduction. Cell. Mol. Life Sci. CMLS 1999, 55, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Xu, J. Resveratrol Exerts Therapeutic Effects on Mice with Atopic Dermatitis. Wounds Compend. Clin. Res. Pract. 2019, 31, 279–284. [Google Scholar]
- Hsieh, T.; Wu, S.-T.; Bennett, D.J.; Doonan, B.B.; Wu, E.; Wu, J.M. Functional/activity network (FAN) analysis of gene-phenotype connectivity liaised by grape polyphenol resveratrol. Oncotarget 2016, 7, 38670–38680. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Maepa, M.; Razwinani, M.; Motaung, S. Effects of resveratrol on collagen type II protein in the superficial and middle zone chondrocytes of porcine articular cartilage. J. Ethnopharmacol. 2016, 178, 25–33. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a biological fluid: History, production, and role in disease prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Chen, R.-S.; Wu, P.-L.; Chiou, R.Y.-Y. Peanut Roots as a Source of Resveratrol. J. Agric. Food Chem. 2002, 50, 1665–1667. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, Q.; Wang, X.; Kang, L.; Yang, Y.; Liu, Y.; Yang, L.; Li, J.; Yang, L.; Liu, J.; et al. Metformin potentiates anti-tumor effect of resveratrol on pancreatic cancer by down-regulation of VEGF-B signaling pathway. Oncotarget 2016, 7, 84190–84200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, Y.; Luo, Y.; Niu, W.; Dong, M.; Liu, M.; Dong, H.; et al. Resveratrol alleviate hypoxic pulmonary hypertension via anti-inflammation and anti-oxidant pathways in rats. Int. J. Med. Sci. 2016, 13, 942–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Pu, R.; Xue, X. The effects of resveratrol intervention on risk markers of cardiovascular health in overweight and obese subjects: A pooled analysis of randomized controlled trials. Obes. Rev. 2016, 17, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Meng, Z.; Li, J.; Zhao, H.; Liu, H.; Zhang, G.; Wang, L.; Hu, H.; Li, D.; Liu, M.; Bi, F.; et al. Resveratrol relieves ischemia-induced oxidative stress in the hippocampus by activating SIRT1. Exp. Ther. Med. 2015, 10, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Liang, N.; Zhu, D.; Gao, Q.; Peng, L.; Dong, H.; Yue, Q.; Liu, H.; Bao, L.; Zhang, J.; et al. Resveratrol Inhibits β-Amyloid-Induced Neuronal Apoptosis through Regulation of SIRT1-ROCK1 Signaling Pathway. PLoS ONE 2013, 8, e59888. [Google Scholar] [CrossRef] [Green Version]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 89–94. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Shen, A.; Cai, W. Resveratrol upregulates SOCS1 production by lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting miR-155. Int. J. Mol. Med. 2017, 39, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Ko, J.-H.; Lee, H.; Jung, J.; Kong, M.; Lee, J.; Lee, J.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef]
- Kang, M.; Cho, K.; Lee, J.; Subedi, L.; Yumnam, S.; Kim, S. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gao, L.; Liu, X.; Lu, T.; Xie, C.; Jia, J. Resveratrol Attenuates Microglial Activation via SIRT1-SOCS1 Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8791832. [Google Scholar] [CrossRef]
- Yang, D.; Jia, W.; Zhu, Y.Z. Leonurine, a Potential Agent of Traditional Chinese Medicine: Recent Updates and Future Perspectives. Nat. Prod. Commun. 2016, 11, 1757–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.-L.; Zhou, Q.-M.; Peng, C.; Liu, Z.-H.; Xiong, L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: A comprehensive overview. Biomed. Pharmacother. 2019, 117, 109060. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Zhang, L.; Cai, M.-H.; Guo, H.; Yuan, H.-H. Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway. Pharm. Biol. 2015, 53, 1684–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S. A Contribution to Our Knowledge of Leonurus L., I-mu-ts’ao, the Chinese Motherwort. Am. J. Chin. Med. 1976, 4, 219–237. [Google Scholar] [CrossRef]
- Xu, R.; Wu, J.D. Study on protecting effect of Leonurus japonicus Houtt (LSH) on rat skin photoaging induced by ultraviolet ray. Liaoning J. Tradit. Chin. Med. 2012, 39, 1421–1422. [Google Scholar]
- Li, F.; Li, X.; Peng, X.; Sun, L.; Jia, S.; Wang, P.; Ma, S.; Zhao, H.; Yu, Q.; Huo, H. Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes. Exp. Ther. Med. 2017, 14, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Gupta, K.; Biswas, S. A mechanistic insight of phytoestrogens used for Rheumatoid arthritis: An evidence-based review. Biomed. Pharmacother. 2021, 133, 111039. [Google Scholar] [CrossRef]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Białas, A.J.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed Root Extract of Leonurus sibiricus Induces Apoptosis through Intrinsic and Extrinsic Pathways in Various Grades of Human Glioma Cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Liu, H.-M.; Guo, C.-L.; Zhang, Y.-F.; Chen, J.-F.; Liang, Z.-P.; Yang, L.-H.; Ma, Y.-P. Leonurine-Repressed miR-18a-5p/SOCS5/JAK2/STAT3 Axis Activity Disrupts CML malignancy. Front. Pharmacol. 2021, 12, 657724. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wu, W.; Zhu, Q.; Liu, X. Discovery of Leonuri and therapeutical applications: From bench to bedside. Pharmacol. Ther. 2018, 188, 26–35. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–322. [Google Scholar]
- Kim, G.-E.; Kang, H.-K.; Seo, E.-S.; Jung, S.-H.; Park, J.-S.; Kim, D.-H.; Kim, D.-W.; Ahn, S.-A.; Sunwoo, C.; Kim, D. Glucosylation of the flavonoid, astragalin by Leuconostoc mesenteroides B-512FMCM dextransucrase acceptor reactions and characterization of the products. Enzym. Microb. Technol. 2012, 50, 50–56. [Google Scholar] [CrossRef]
- Svobodová, A.; Psotová, J.; Walterová, D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed. Pap. 2003, 147, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Kim, S.-H. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. Arch. Pharmacal Res. 2011, 34, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-B.; Kim, E.-K.; Park, S.-J.; Bang, S.; Kim, T.G.; Chung, D. Isolation and anti-inflammatory effect of astragalin synthesized by enzymatic hydrolysis of tea seed extract. J. Sci. Food Agric. 2011, 91, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kotani, M.; Fujita, A.; Higa, S.; Kishimoto, T.; Suemura, M.; Tanaka, T. Oral administration of persimmon leaf extract ameliorates skin symptoms and transepidermal water loss in atopic dermatitis model mice, NC/Nga. Br. J. Dermatol. 2002, 146, 221–227. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Zhang, X.; Zhang, X.; Chen, S.; Hu, Z.; Zhou, C.; Zhang, E.; Ma, S. Astragalin Attenuates Allergic Inflammation in a Murine Asthma Model. Inflammation 2015, 38, 2007–2016. [Google Scholar] [CrossRef]
- Jang, S.-W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [Green Version]
- Androutsopoulos, V.P.; Mahale, S.; Arroo, R.R.; Potter, G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol. Rep. 2009, 21, 1525–1528. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Feng, F.; Wu, J.; Li, L.; Xu, H.; Yang, L.; Xu, K.; Wang, C. Diosmetin inhibits cell growth and proliferation by regulating the cell cycle and lipid metabolism pathway in hepatocellular carcinoma. Food Funct. 2021, 12, 12036–12046. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, X.-B.; Huang, L.-G.; Wang, Z.-X.; Wan, R.-Z.; Zhang, P.; Zhang, Q.-Y.; Chen, Z.; Zhang, B.-S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723–30733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; Yang, C.; Zhu, Y.; Chen, Q.; Zhang, B. Diosmetin Ameliorates Nonalcoholic Steatohepatitis through Modulating Lipogenesis and Inflammatory Response in a STAT1/CXCL10-Dependent Manner. J. Agric. Food Chem. 2021, 69, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bong, S.-K.; Lee, J.W.; Park, N.-J.; Choi, Y.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.-N. Diosmetin and Its Glycoside, Diosmin, Improve Atopic Dermatitis-Like Lesions in 2,4-Dinitrochlorobenzene-Induced Murine Models. Biomol. Ther. 2020, 28, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F.J. Antiinflammatory and Immunomodulating Properties of Fungal Metabolites. Mediat. Inflamm. 2005, 2005, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Qing, C.; Liu, M.-H.; Yangi, W.-M.; Zhang, Y.; Wang, L.; Liu, J.-K. Effects of Albaconol from the Basidiomycete Albatrellus confluens on DNA Topoisomerase II-Mediated DNA Cleavage and Relaxation. Planta Med. 2004, 70, 792–796. [Google Scholar] [CrossRef]
- Hirata, Y.; Nakanishi, K. Grifolin, an antibiotic from a basidiomycete. J. Biol. Chem. 1950, 184, 135–143. [Google Scholar] [CrossRef]
- Nukata, M.; Hashimoto, T.; Yamamoto, I.; Iwasaki, N.; Tanaka, M.; Asakawa, Y. Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochemistry 2002, 59, 731–737. [Google Scholar] [CrossRef]
- Liu, Q.; Shu, X.; Wang, L.; Sun, A.; Liu, J.; Cao, X. Albaconol, a Plant-Derived Small Molecule, Inhibits Macrophage Function by Suppressing NF-κB Activation and Enhancing SOCS1 Expression. Cell. Mol. Immunol. 2008, 5, 271–278. [Google Scholar] [CrossRef]
- Liu, Q.; Shu, X.; Sun, A.; Sun, Q.; Zhang, C.; An, H.; Liu, J.; Cao, X. Plant-derived small molecule albaconol suppresses LPS-triggered proinflammatory cytokine production and antigen presentation of dendritic cells by impairing NF-κB activation. Int. Immunopharmacol. 2008, 8, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Qing, C.; Liu, J.K.; Zhang, Y.L.; Wang, L.J.S. Study on the antitumor activity of albaconol and its effect on DNA topoisomerase II. Chin. Pharmacol. Bull. 2004, 20, 1224–1228. [Google Scholar]
- Crespo, A.; Filla, M.B.; Russell, S.W.; Murphy, W.J. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: Partial role of autocrine/paracrine interferon-α/β. Biochem. J. 2000, 349, 99. [Google Scholar] [CrossRef]
- Kinjyo, I.; Hanada, T.; Inagaki-Ohara, K.; Mori, H.; Aki, D.; Ohishi, M.; Yoshida, H.; Kubo, M.; Yoshimura, A. SOCS1/JAB Is a Negative Regulator of LPS-Induced Macrophage Activation. Immunity 2002, 17, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Mirshafiey, A.; Taeb, M.; Mortazavi-Jahromi, S.S.; Jafarnezhad-Ansariha, F.; Rehm, B.H.A.; Esposito, E.; Cuzzocrea, S.; Matsuo, H. Introduction of β-d-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property based on COX-1/COX-2 activity and gene expression. Pharmacol. Rep. 2017, 69, 1067–1072. [Google Scholar] [CrossRef]
- Barati, A.; Jamshidi, A.-R.; Ahmadi, H.; Aghazadeh, Z.; Mirshafiey, A. Effects of β-d-mannuronic acid, as a novel non-steroidal anti-inflammatory medication within immunosuppressive properties, on IL17, RORγt, IL4 and GATA3 gene expressions in rheumatoid arthritis patients. Drug Des. Dev. Ther. 2017, 11, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, L.; Moshiri, M.; Dallal, M.M.S.; Asgardoon, M.H.; Nourizadeh, M.; Bokaie, S.; Mirshafiey, A. The Inhibitory Role of M2000 (β-d-Mannuronic Acid) on Expression of Toll-like Receptor 2 and 4 in HT29 Cell Line. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 57–65. [Google Scholar] [CrossRef]
- Hosseini, S.; Abdollahi, M.; Azizi, G.; Fattahi, M.J.; Rastkari, N.; Zavareh, F.T.; Aghazadeh, Z.; Mirshafiey, A. Anti-aging effects of M2000 (β-d-mannuronic acid) as a novel immunosuppressive drug on the enzymatic and non-enzymatic oxidative stress parameters in an experimental model. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 249–255. [Google Scholar] [CrossRef]
- Gaafar, N.A.G.; Razavi, A.; Mirshafiey, A. β-d-Mannuronic Acid (M2000) as a Landmark in Pharmacology. Curr. Drug Discov. Technol. 2021, 18, 47–57. [Google Scholar] [CrossRef]
- Khalatbari, A.; Mahdavi, M.; Jafarnezhad, F.; Afraei, S.; Zavareh, F.T.; Aghazadeh, Z.; Ghaderi, A.; Mirshafiey, A. Efficacy of β-d-Mannuronic Acid [M2000] on the Pro-Apoptotic Process and Inflammatory-Related Molecules NFκB, IL-8 and Cd49d using Healthy Donor PBMC. Curr. Drug Discov. Technol. 2020, 17, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Taeb, M.; Jafarzadeh, A.; Mortazavi-Jahromi, S.S.; Zainodini, N.; Mirzaei, M.R.; Jafarnezhad-Ansariha, F.; Aghazadeh, Z.; Mirshafiey, A. Effect of β-d-Mannuronic Acid (M2000) on Oxidative Stress Enzymes’ Gene Using Healthy Donor Peripheral Blood Mononuclear Cells for Evaluating the Anti-Aging Property. Curr. Drug Discov. Technol. 2019, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Athari Nik Azm, S.; Vafa, M.; Sharifzadeh, M.; Safa, M.; Barati, A.; Mirshafiey, A. Effects of M2000 (d-Mannuronic Acid) on Learning, Memory Retrieval, and Associated Determinants in a Rat Model of Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2017, 32, 12–21. [Google Scholar] [CrossRef]
- Mortazavi-Jahromi, S.S.; Alizadeh, S.; Javanbakht, M.H.; Mirshafiey, A. Anti-diabetic effect of β-d-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property on insulin production, blood glucose, and inflammatory markers in the experimental diabetes model. Arch. Physiol. Biochem. 2019, 125, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Jahromi, S.S.; Alizadeh, S.; Javanbakht, M.H.; Mirshafiey, A. Cardioprotective effect of β-d-mannuronic acid (M2000) as a novel NSAID on gene expression of oxLDL scavenger receptors in the experimental diabetic model. Immunopharmacol. Immunotoxicol. 2018, 40, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Rehm, B.; Abhari, R.S.; Borzooy, Z.; Sotoude, M.; Razavi, A. Production of M2000 (β-d-mannuronic acid) and its therapeutic effect on experimental nephritis. Environ. Toxicol. Pharmacol. 2007, 24, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Cuzzocrea, S.; Rehm, B.H.A.; Matsuo, H. M2000: A revolution in pharmacology. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2005, 11, PI53–PI63. [Google Scholar]
- Fattahi, M.J.; Abdollahi, M.; Agha Mohammadi, A.; Rastkari, N.; Khorasani, R.; Ahmadi, H.; Tofighi Zavareh, F.; Sedaghat, R.; Tabrizian, N.; Mirshafiey, A. Preclinical assessment of β-d-mannuronic acid (M2000) as a non-steroidal anti-inflammatory drug. Immunopharmacol. Immunotoxicol. 2015, 37, 535–540. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Chen, C.-C.; Huynh, P.; Chang, J.-S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Kim, W.-S.; Lee, T.-K.; Kim, Y.-T.; Jeon, Y.-J. Alginic Acid from Padina boryana Abate Particulate Matter-Induced Inflammatory Responses in Keratinocytes and Dermal Fibroblasts. Molecules 2020, 25, 5746. [Google Scholar] [CrossRef]
- Gaafar, N.A.G.; Aslani, M.; Aghazadeh, Z.; Mortazavi-Jahromi, S.S.; Razavi, A.; Mirshafiey, A. Effects of mannuronic acid (M2000) on gene expression profile of signal transducer and activator of transcription proteins (STATs) in rheumatoid arthritis patients. Reumatismo 2020, 72, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pourgholi, F.; Hajivalili, M.; Razavi, R.; Esmaeili, S.; Baradaran, B.; Movasaghpour, A.A.; Sadreddini, S.; Goodarzynejad, H.; Mirshafiey, A.; Yousefi, M. The Role of M2000 as an Anti-inflammatory Agent in Toll-Like Receptor 2/microRNA-155 Pathway. Avicenna J. Med. Biotechnol. 2017, 9, 8–12. [Google Scholar] [PubMed]
- Mortazavi-Jahromi, S.S.; Aslani, M.; Omidian, S.; Ahmadzadeh, A.; Rezaieyazdi, Z.; Mirshafiey, A. Immunopharmacological effect of β-d-mannuronic acid (M2000), as a new immunosuppressive drug, on gene expression of miR-155 and its target molecules (SOCS1, SHIP1) in a clinical trial on rheumatoid arthritis patients. Drug Dev. Res. 2020, 81, 295–304. [Google Scholar] [CrossRef]
- Najafi, S.; Saadat, P.; Beladi Moghadam, N.; Manoucherinia, A.; Aghazadeh, Z.; Vali Mohammadi, A.; Pashaiefar, H.; Hosseini, M.; Mirshafiey, A. Evaluation of the Effect of Mannuronic Acid as a Novel NSAID with Immunosuppressive Properties on Expression of SOCS1, SOCS3, SHIP1, and TRAF6 Genes and Serum Levels of IL-6 and TNF-α in Patients with Multiple Sclerosis. J. Clin. Pharmacol. 2021, 61, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Saadat, P.; Beladi Moghadam, N.; Manoucherinia, A.; Aghazadeh, Z.; Vali Mohammadi, A.; Noorbakhsh, S.M.; Movahedi, M.; Nikouei Moghaddam, M.R.; Pashaiefar, H.; et al. The Effects of Mannuronic Acid on IL-1β, IL-17A, STAT1, and STAT3 Gene Expressions and TLR2 and TLR4 Molecules in Multiple Sclerosis. J. Clin. Pharmacol. 2022, 62, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C. Anti-Oxidant, Anti-Inflammatory and Anti-Allergic Activities of Luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Kao, T.-K.; Ou, Y.-C.; Lin, S.-Y.; Pan, H.-C.; Song, P.-J.; Raung, S.-L.; Lai, C.-Y.; Liao, S.-L.; Lu, H.-C.; Chen, C.-J. Luteolin inhibits cytokine expression in endotoxin/cytokine-stimulated microglia. J. Nutr. Biochem. 2011, 22, 612–624. [Google Scholar] [CrossRef]
- Xia, N.; Chen, G.; Liu, M.; Ye, X.; Pan, Y.; Ge, J.; Mao, Y.; Wang, H.; Wang, J.; Xie, S. Anti-inflammatory effects of luteolin on experimental autoimmune thyroiditis in mice. Exp. Ther. Med. 2016, 12, 4049–4054. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Lin, H.-W.; Yang, D.-J.; Chen, S.-Y.; Tseng, J.-K.; Chang, T.-J.; Chang, Y.-Y. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free. Radic. Biol. Med. 2016, 95, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Liu, Y.; Zhou, J.; Wei, Y.; Deng, J.; Dong, B.; Chai, L. Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1β-induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 2015, 37, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Baolin, L.; Weiwei, W.; Ning, T. Topical Application of Luteolin Inhibits Scratching Behavior Associated with Allergic Cutaneous Reaction in Mice. Planta Med. 2005, 71, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kimata, M.; Inagaki, N.; Nagai, H. Effects of Luteolin and Other Flavonoids on IgE-Mediated Allergic Reactions. Planta Med. 2000, 66, 25–29. [Google Scholar] [CrossRef]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Palma, E.; Cordaro, M.; D’Amico, R.; Peritore, A.F.; Licata, P.; Crupi, R. Canine atopic dermatitis: Role of luteolin as new natural treatment. Vet. Med. Sci. 2020, 6, 926–932. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Zhang, H.; Xie, Y. Protective effect of Luteolin on atopic dermatitis murine model via IgE mediated immune response. Isr. J. Plant Sci. 2021, 68, 99–106. [Google Scholar] [CrossRef]
- Merfort, I.; Heilmann, J.; Hagedorn-Leweke, U.; Lippold, B.C. In vivo skin penetration studies of camomile flavones. Pharmazie 1994, 49, 509–511. [Google Scholar]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Heng, M. Topical Curcumin: A Review of Mechanisms and uses in Dermatology. Int. J. Dermatol. Clin. Res. 2017, 3, 010–017. [Google Scholar] [CrossRef] [Green Version]
- Slika, L.; Patra, D. Traditional Uses, Therapeutic Effects and Recent Advances of Curcumin: A Mini-Review. Mini-Rev. Med. Chem. 2020, 20, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Friedman, A.J. Curcumin: A novel treatment for skin-related disorders. J. Drugs Dermatol. JDD 2013, 12, 1131–1137. [Google Scholar] [PubMed]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial Role of Curcumin in Skin Diseases. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; pp. 343–357. [Google Scholar]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. BioFactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. BioFactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Rajanikant, G. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of γ-radiation. J. Surg. Res. 2004, 120, 127–138. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Yu, K.; Yan, Q.; Xing, C.; Chen, Y.; Yan, Z.; Shi, Y.; Zhao, K.-W.; Gao, S. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class Ι histone deacetylases. Carcinogenesis 2013, 34, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Chen, C.; Wang, L.; Hong, L.; Wu, J.; Dong, P.; Yu, F. Histone deacetylases inhibitor sodium butyrate inhibits JAK2/STAT signaling through upregulation of SOCS1 and SOCS3 mediated by HDAC8 inhibition in myeloproliferative neoplasms. Exp. Hematol. 2013, 41, 261–270.e4. [Google Scholar] [CrossRef]
- Xiong, H.; Du, W.; Zhang, Y.-J.; Hong, J.; Su, W.-Y.; Tang, J.-T.; Wang, Y.-C.; Lu, R.; Fang, J.-Y. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol. Carcinog. 2012, 51, 174–184. [Google Scholar] [CrossRef]
- Guimarães, M.R.; Leite, F.R.M.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch. Oral Biol. 2013, 58, 1309–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin Regulates Anti-Inflammatory Responses by JAK/STAT/SOCS Signaling Pathway in BV-2 Microglial Cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.-M.; Xu, R.; Huang, X.-Y.; Cheng, S.-M.; Huang, M.-F.; Yue, H.-Y.; Wang, X.; Zou, Y.; Lu, A.-P.; Liu, D.-Y. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front. Pharmacol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, J.; Ye, B.; Wang, Q.; Xie, X.; Shen, H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement. Altern. Med. 2016, 16, 299. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.K.; Ha, S.J.; Jung, C.H.; Kim, Y.T.; Lee, H.; Kim, M.O.; Lee, M.; Mottamal, M.; Bode, A.M.; Lee, K.W.; et al. Naringenin targets ERK 2 and suppresses UVB-induced photoaging. J. Cell. Mol. Med. 2016, 20, 909–919. [Google Scholar] [CrossRef]
- Seyedrezazadeh, E.; Kolahian, S.; Shahbazfar, A.-A.; Ansarin, K.; Pour Moghaddam, M.; Sakhinia, M.; Sakhinia, E.; Vafa, M. Effects of the Flavanone combination Hesperetin-Naringenin, and Orange and Grapefruit Juices, on Airway inflammation and Remodeling in a murine asthma model. Phytother. Res. 2015, 29, 591–598. [Google Scholar] [CrossRef]
- Lim, K.H.; Kim, G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed. Dermatol. 2018, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Ma, J.; Fan, Y.; Wang, X.; Zheng, S.; Feng, J.; Li, J.; Fan, Z.; Li, G.; Ye, Q. Naringenin: A Promising Therapeutic Agent against Organ Fibrosis. Oxidative Med. Cell. Longev. 2021, 2021, 1210675. [Google Scholar] [CrossRef] [PubMed]
- Al-Roujayee, A.S. Naringenin improves the healing process of thermally-induced skin damage in rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Wiejak, J.; Dunlop, J.; Mackay, S.P.; Yarwood, S.J. Flavanoids induce expression of the suppressor of cytokine signalling 3 (SOCS3) gene and suppress IL-6-activated signal transducer and activator of transcription 3 (STAT3) activation in vascular endothelial cells. Biochem. J. 2013, 454, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-H.; Kim, G.-D.; Ahn, H.-J.; Cho, J.-J.; Park, Y.S.; Park, C.-S. The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice. Life Sci. 2013, 93, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Pitchaimani, V.; Afrin, R.; Harima, M.; Krishnamurthy, P.; Suzuki, K.; et al. Naringenin ameliorates skin inflammation and accelerates phenotypic reprogramming from M1 to M2 macrophage polarization in atopic dermatitis NC/Nga mouse model. Exp. Dermatol. 2016, 25, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Matsushima, M.; Takagi, K.; Ogawa, M.; Hirose, E.; Ota, Y.; Abe, F.; Baba, K.; Hasegawa, T.; Hasegawa, Y.; Kawabe, T. Heme oxygenase-1 mediates the anti-allergic actions of quercetin in rodent mast cells. Inflamm. Res. 2009, 58, 705–715. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Hur, D.Y.; Song, S.B.; Park, Y.; Kim, T.S.; Bang, S.I.; Kim, S.; Song, H.K.; Park, H.; Cho, D.H. Tannic Acid and Quercetin Display a Therapeutic Effect in Atopic Dermatitis via Suppression of Angiogenesis and TARC Expression in Nc/Nga Mice. J. Investig. Dermatol. 2010, 130, 1459–1463. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical Anti-Inflammatory Potential of Quercetin in Lipid-Based Nanosystems: In Vivo and In Vitro Evaluation. Pharm. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igbe, I.; Shen, X.-F.; Jiao, W.; Qiang, Z.; Deng, T.; Li, S.; Liu, W.-L.; Liu, H.-W.; Zhang, G.-L.; Wang, F. Dietary quercetin potentiates the antiproliferative effect of interferon-α in hepatocellular carcinoma cells through activation of JAK/STAT pathway signaling by inhibition of SHP2 phosphatase. Oncotarget 2017, 8, 113734–113748. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Yanagihara, S.; Otsuka, A. Innovation in the treatment of atopic dermatitis: Emerging topical and oral Janus kinase inhibitors. Allergol. Int. 2022, 71, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Szalus, K.; Trzeciak, M.; Nowicki, R.J. JAK-STAT Inhibitors in Atopic Dermatitis from Pathogenesis to Clinical Trials Results. Microorganisms 2020, 8, 1743. [Google Scholar] [CrossRef]
- Gündüz, Ö. JAK/STAT pathway modulation: Does it work in dermatology? Dermatol. Ther. 2019, 32, e12903. [Google Scholar] [CrossRef]
- Sakamoto, T.; Miyazaki, E.; Aramaki, Y.; Arima, H.; Takahashi, M.; Kato, Y.; Koga, M.; Tsuchiya, S. Improvement of dermatitis by iontophoretically delivered antisense oligonucleotides for interleukin-10 in NC/Nga mice. Gene Ther. 2004, 11, 317–324. [Google Scholar] [CrossRef]
- Rożalski, M.; Rudnicka, L.; Samochocki, Z. MiRNA in atopic dermatitis. Adv. Dermatol. Allergol. 2016, 3, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.-B. Discovery and Development of Janus Kinase (JAK) Inhibitors for Inflammatory Diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]

| Natural Compound | Source | Experimental Evidence | Dosage | Target Action | Ref. |
|---|---|---|---|---|---|
| Resveratrol | Resveratrol-enriched rice | DNCB-induced AD NC/Nga mice | 2.5% w/v | ↓ scratching behavior, ↓ dermatitis, ↑ skin hydration, ↓ IL-6, IL-1β, IgE, IL-31 | [93,109] |
| TNF-α/IFN-γ-stimulated HaCaT cells | 1–50 µg/mL | ↓ IL-6, IL-31, IgE | |||
| Grapes, Berries, Polygonum cuspidatum | LPS-stimulated RAW 264.7 cells | 0–20 µM | ↓ inflammatory cytokines, ↓ STAT1/STAT3, ↑ SOCS1 | [107] | |
| Leonurine | Herba leonuri | CML cells | 0.4 and 0.6 mM | ↑ SOCS5, ↓ JAK2/STAT3 pathway | [119] |
| Xenograft BALB/c animal model | 150 mg/kg/4 weeks | ||||
| Astragalin | Persimmon, Rosa agrestis leaves | OVA-induced BALB/c asthma animal model | 1 mg/kg/day | ↓ IL-4, IL-5, IL-13, IgE, ↑ SOCS5, ┴ Th2 differentiation | [129] |
| Diosmetin | Citrus fruits | RBL-2H3 cells | 5, 10, 25 mg/kg/day | ↓ IL-4 | [136] |
| DNCB-induced AD mice | 50 mg/kg | ↓ IL-4 and IgE, ┴ Th2 differentiation | |||
| Albaconol | Albatrellus confluens | LPS-activated RAW 264.7 cells | 5 µg/mL | ↑ SOCS1, ┴ IL-6, IL-1β, NF-κB | [141] |
| β-d-Mannuronic acid | Marine brown algae | RA patients | 500 mg/day | ↑ SOCS1, ┴ IL-6 and TNF-α | [164] |
| MC patients | - | ↑ SOCS1 and SOCS3, ↓ IL-1β, IL-17A, STAT1, and STAT3 | [166] | ||
| Luteolin | Reseda luteola, Apple skin, Rosemary, Oregano, Peppermint | LPS & IFNγ-stimulated BV-2 cells | 20 µM | ↑ SOCS3, ┴ STAT1 | [170] |
| In vitro canine AD model | 1–8 µM | ↓ IL-33, IL 1β, IL-6, IL-8 | [178] | ||
| DNCB-induced murine AD model | 10, 20, and 30 mg/kg | ↓ IgE, IL-1β, IL-6, TNF-α, IFNγ, IL-4, IL-17A, NF-κB | [179] | ||
| Curcumin | Curcuma longa | LPS-stimulated BV-2 | 10, 30, and 50 μM | ↑ SOCS1, ↓ JAK2/STAT3 | [195] |
| TNBS-induced colitis mice | 100 mg/kg/wk | ↑ SOCS1,SOCS3, ┴ JAK2/STAT3, STAT6 | [196,197] | ||
| OVA-induced murine AD model | 20 mg/kg/wk | ↓ Th2 expression, ↓ IL-4/IL-5/IL-13/IL-31, ↓ STAT6 | [198] | ||
| Naringenin | Tomato and grapes | DNFB-induced NC/Nga mouse AD model | 50 and 100 mg/kg | ↓ IgE, IFN-γ, ↓ Infiltration of mast and T-cells | [207] |
| IL-6-induced HUVEC cells | 100 µM | ↑ SOCS3, ↓ STAT3 | [206] | ||
| Quercetin | All fruits and vegetables | House-dust-mite-allergens-induced AD in Nc/Nga mice | 2.5 mM | ↓ neoangiogenesis | [214] |
| TNF-α-stimulated HaCaT cells | 1, 5 and 10 µM | ↓ Th2 cytokine expression, TSLP, TARC | |||
| IFNα-induced HepG2 and Huh7 cells | 1–10 μM | ┴ SHP2, ↓ JAK1/STAT3, no effect on SOCS1/SOCS3 | [216] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopalli, S.R.; Annamneedi, V.P.; Koppula, S. Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis. Molecules 2022, 27, 4660. https://doi.org/10.3390/molecules27144660
Kopalli SR, Annamneedi VP, Koppula S. Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis. Molecules. 2022; 27(14):4660. https://doi.org/10.3390/molecules27144660
Chicago/Turabian StyleKopalli, Spandana Rajendra, Venkata Prakash Annamneedi, and Sushruta Koppula. 2022. "Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis" Molecules 27, no. 14: 4660. https://doi.org/10.3390/molecules27144660
APA StyleKopalli, S. R., Annamneedi, V. P., & Koppula, S. (2022). Potential Natural Biomolecules Targeting JAK/STAT/SOCS Signaling in the Management of Atopic Dermatitis. Molecules, 27(14), 4660. https://doi.org/10.3390/molecules27144660







