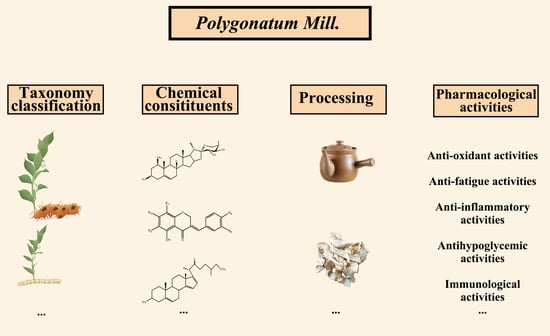
A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes
Abstract
:1. Introduction
2. Classification of Polygonatum Mill.
3. Chemical Constituents of Polygonatum
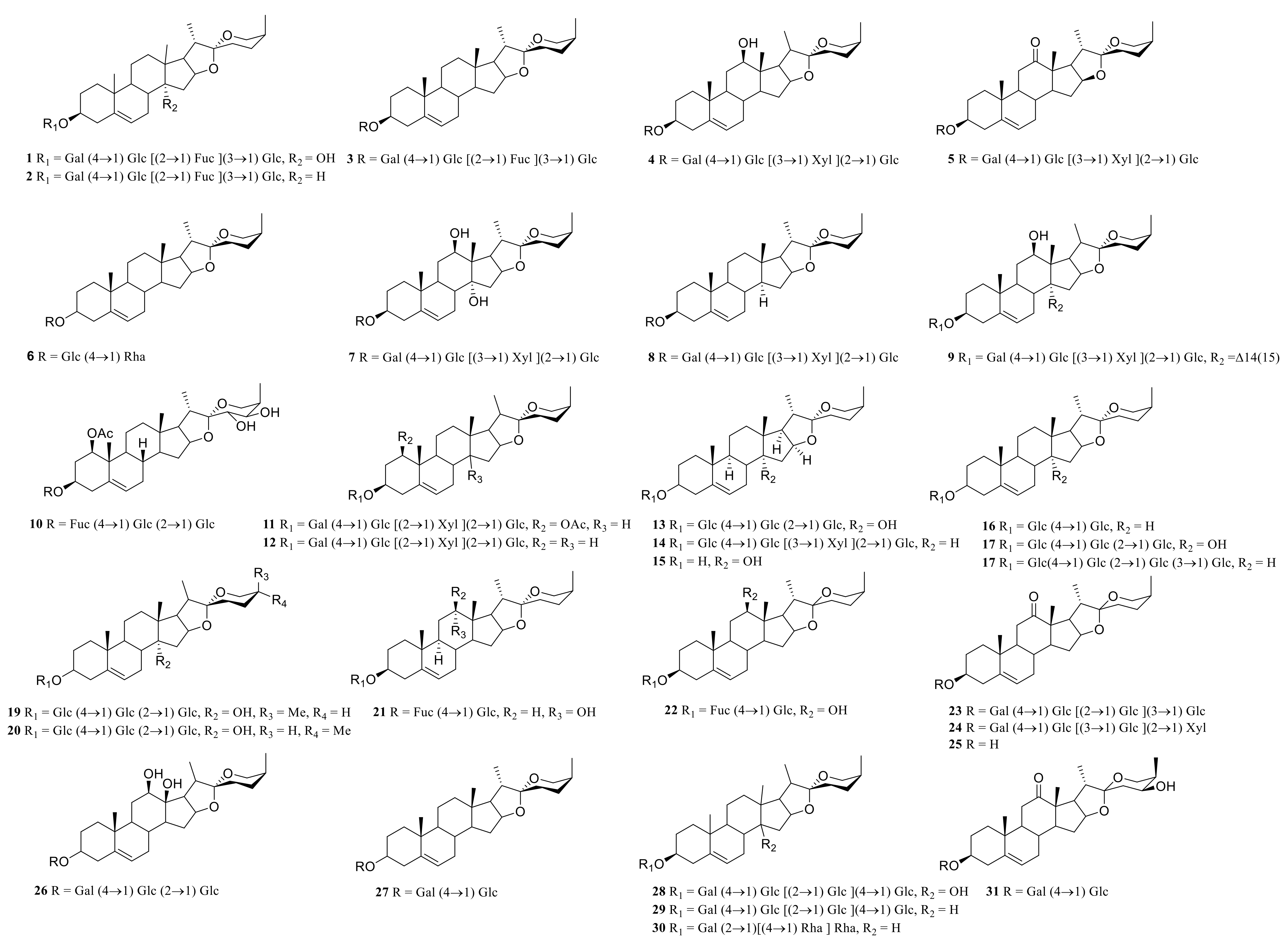
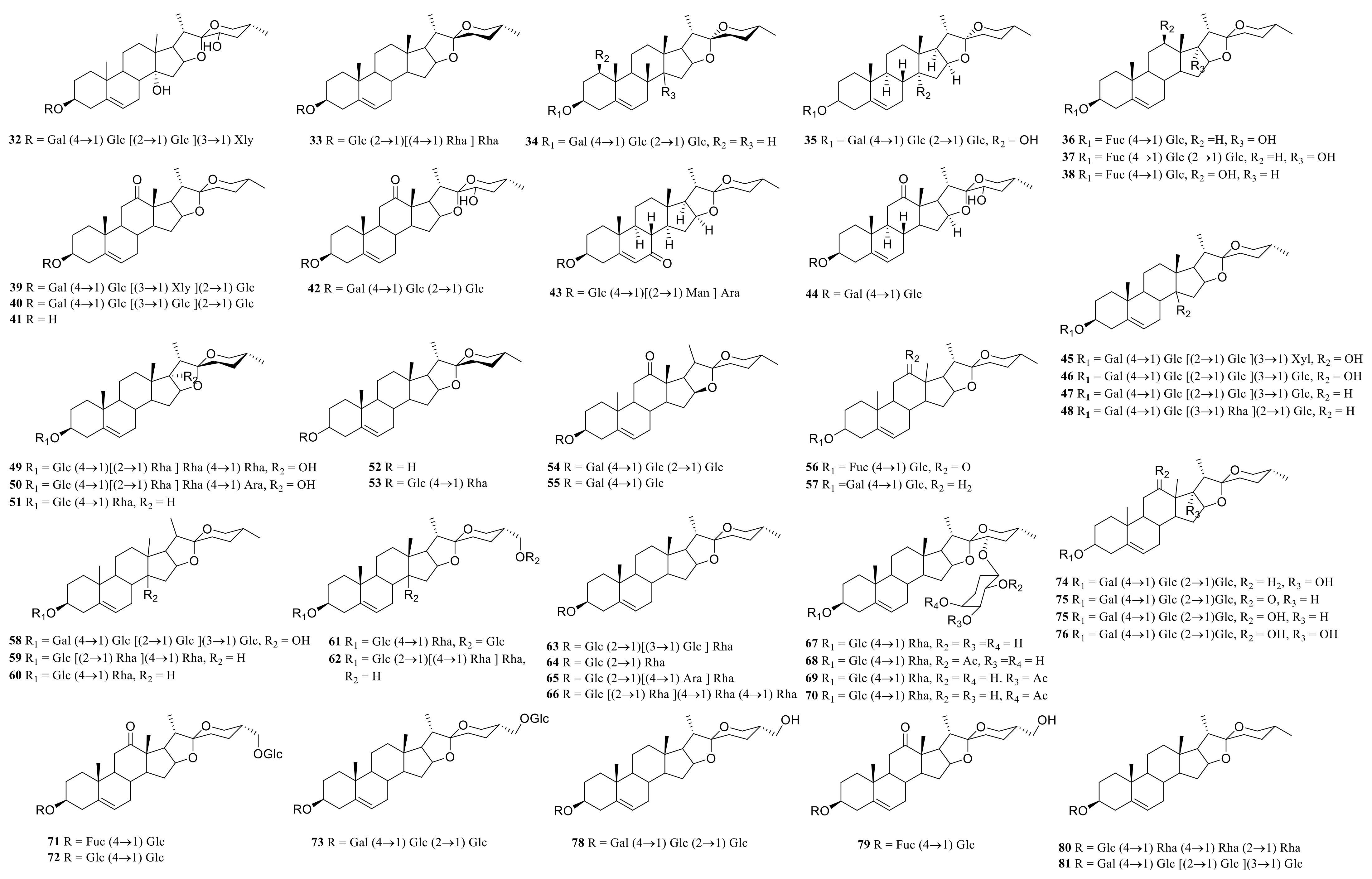
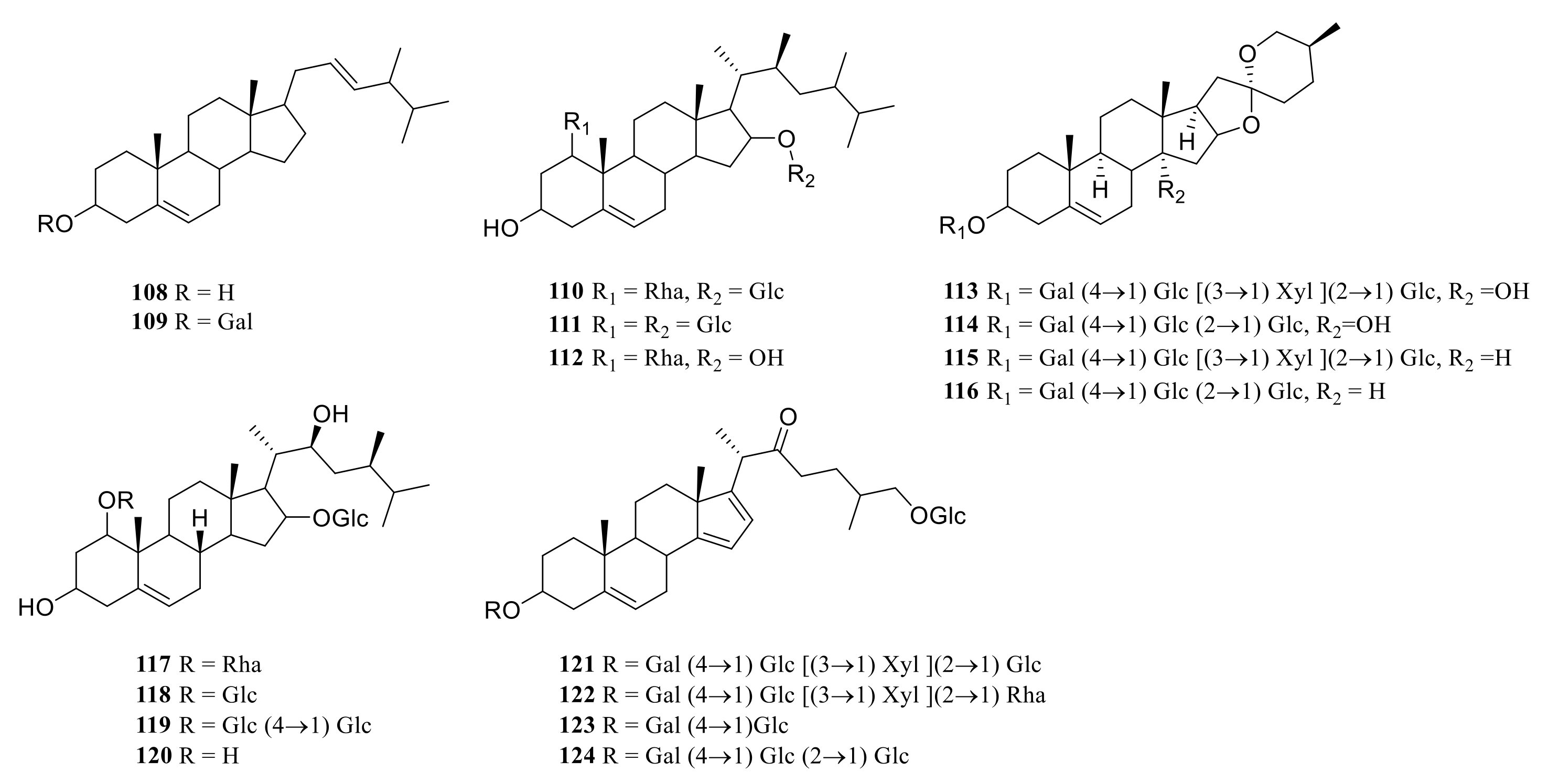
3.1. Steroidal Saponins
3.2. Flavonoids
3.3. Triterpenoid Saponins
3.4. Alkaloids
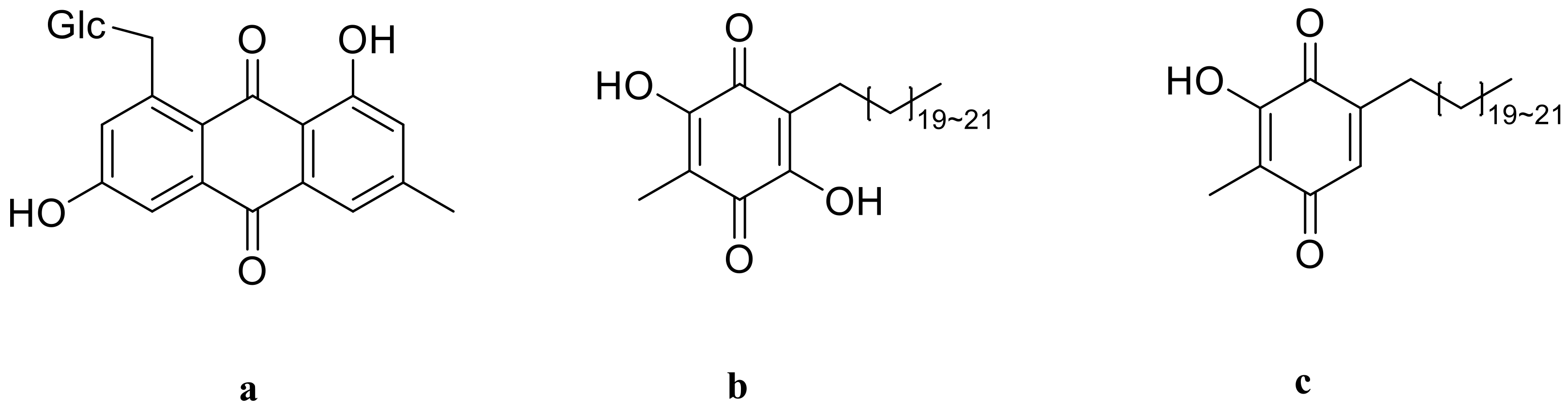
3.5. Quinones
3.6. Lignans
3.7. Polysaccharides
4. Pharmacological Activities
4.1. Antioxidant Activities
4.2. Anti-Fatigue Activities
4.3. Anti-Inflammatory Activities
4.4. Antihypoglycemic Activities
4.5. Immunological Activities
4.6. Other Activities of Polygonatum Mill.
5. Processing of Polygonatum Mill.
5.1. Processing Methods of Polygonatum Mill.
5.2. Effect of Processing on the Chemical Composition
5.3. Influence of Processing on Pharmacological Effects
5.3.1. Antioxidant Activities after Processing
5.3.2. Anti-Fatigue Activities after Processing
5.3.3. Anti-Inflammatory Activities after Processing
5.3.4. Anti-Hypoglycemic Activities after Processing
5.3.5. Immunological Activities after Processing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institutional Repository of the Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1978; Volume 15, p. 52. [Google Scholar]
- Chinese Materia Medica Editorial Committee of the State Administration of Traditional Chinese Medicine. Chinese Materia Medica; CRC Press: Boca Raton, FL, USA, 1999; Volume 5, pp. 134–151. [Google Scholar]
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. One; China Medical Science and Technology Press: Beijing, China, 2020; Volume 4, pp. 387–388. [Google Scholar]
- Qiang, L.; Wei, L.; Nagata, K.; Hongwei, F.; Kazuo, K. Separation from Polygonatum odoratum, structure Elucidation and analysis of steroidal glycosides by liquid chromatography-mass spectrometry. Acta Agri-Food Chem. 2018, 66, 521–531. [Google Scholar]
- Bai, J.B.; Ge, J.C.; Zhang, W.J.; Liu, W.; Luo, J.P.; Xu, F.Q.; Wu, D.L.; Xie, S.Z. Physicochemical, morpho-structural, and biological characterization of polysaccharides from three Polygonatum spp. RSC Adv. 2021, 11, 37952–37965. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, P.; Wu, W.; Li, D.; Shang, E.-X.; Guo, S.; Qian, D.; Yan, H.; Wang, W.; Duan, J.-A. Multi-constituents variation in medicinal crops processing: Investigation of nine cycles of steam-sun drying as the processing method for the rhizome of Polygonatum cyrtonema. J. Pharm. Biomed. Anal. 2022, 209, 114497. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Do, J.C.; Kang, S.S. Steroidal Saponins from the Rhizomes of Polygonatum sibiricum. J. Nat. Prod. 1990, 53, 333–339. [Google Scholar] [CrossRef]
- Lin, H.; Han, G.; Liao, S. Study on the effective ingredients of Chinese medicine Polygonatum odoratum. Acta Pharm. Sci. 1994, 29, 215. [Google Scholar]
- Gvazava, L.N.; Skhirtladze, A.V. Steroidal saponin from Polygonatum verticillatum. Chem. Nat. Compd. 2016, 52, 1052–1055. [Google Scholar] [CrossRef]
- Dongmei, W.; Jingfang, Z.; Xiaoming, L.; Chen, G.X.; Zhao, T.Z. The chemical constituents of steroidal saponins and their antibacterial activities in the root of Polygonatum cirrhifolium. For. Sci. 2007, 8, 91–95. [Google Scholar]
- Wang, D.; Li, D.; Zhu, W.; Zhang, J.; Peng, P. Steroidal saponins from the rhizomes of Polygonatum odoratum. Nat. Prod. Res. 2009, 23, 940. [Google Scholar] [CrossRef]
- Ahn, M.; Kim, C.Y.; Yoon, K.; Ryu, M.Y.; Cheong, J.H.; Chin, Y.W.; Kim, J. Steroidal saponins from the rhizomes of Polygonatum sibiricum. Nat. Prod. 2006, 69, 360. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, J.-Y.; Wang, Y.-J.J.; Zheng, W.; Zhang, J.; Chen, X.-J.; Chen, S.; Yu, L.-Y.; Ma, B.-P. Steroidal glycosides, homoisoflavanones and cinnamic acid derivatives from Polygonatum odoratum and their inhibitory effects against influenza A virus. Fitoterapia 2020, 146, 104689. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Nagata, K.; Zheng, W.; Ma, B.P. Isolation, structural elucidation, and liquid chromatography-mass spectrometry analysis of steroidal glycosides from Polygonatum odoratum. Agric. Food Chem. 2018, 66, 521. [Google Scholar] [CrossRef]
- Bai, H.; Li, W.; Zhao, H.; Anzai, Y.; Li, H.; Guo, H.; Kato, F.; Koike, K. Isolation and structural elucidation of novel cholestane glycosides and spirostane saponins from Polygonatum odoratum. Steroids 2014, 80, 7–14. [Google Scholar] [CrossRef]
- Tang, C.; Yu, Y.; Qi, Q.; Wu, X.-D.; Wang, J.; Tang, S.-A. Steroidal saponins from the rhizome of Polygonatum sibiricum. Asian Nat. Prod. Res. 2018, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wu, Q.; Ping, Y.; Yuan, M.; Zhou, Z.; Qian, Y.; Xu, Y.; Ning, H. Simultaneous determination of 5-Hydroxy Maltol, 5-Hydroxymethyl Furfural and Polygonatine A in Polygonatum Cyrtonema by HPLC. Chin. J. Exp. Formulas 2018, 21, 1683–1686. [Google Scholar]
- Ma, K.; Huang, X.; Kong, L. Steroidal saponins from Polygonatum cyrtonema. Chem. Nat. Compd. 2013, 49, 888. [Google Scholar] [CrossRef]
- Ning, H.H.; Yuan, M.; Wu, Q.-P.; Ping, Y.-H.; Zhou, Z.-Q.; Xu, Y.; Wu, Y.; Yin, H.-X. Identification of Chemical Constituents from Polygonatum cyrtonema. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 77–82. [Google Scholar]
- Gao, S.; Yuan, M.; Ning, H.; Kan, R.; Huang, L.; Fu, X.; Wu, Y. Chemical Consitiuetns of Ethly Acetate Extract from Stewed Polygonatum Ehizoma. J. Chin. Med. Mater. 2021, 44, 2332–2336. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Ju, Y.; Zhang, H.; Liu, M.; Li, X. Effects of two saponins extracted from the Polygonatum zanlanscianense pamp on the human leukemia (HL-60) cells. Biol. Pharm. Bull. 2001, 24, 159. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.S.; Ma, B.P.; Kang, L.P.; Zhang, T.; Jiang, F.J.; Zhang, J.; Zou, P.; Zhao, Y.; Xiong, C.Q.; Tan, D.W.; et al. Saponins from the processed rhizomes of Polygonatum kingianum. Chem. Pharm. Bull. 2009, 57, 1011–1014. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, J.; Kang, L.; Han, L.F.; Zou, P.; Zhao, Y.; Xiong, C.Q.; Tan, D.W.; Song, X.B.; Yu, K.; et al. Three new saponins from the fresh rhizomes of Polygonatum kingianum. Pharm. Soc. Jpn. 2009, 57, 1. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.S.; Ma, B.P.; Song, X.B.; Kang, L.P.; Zhang, T.; Fu, J.; Zhou, Y.; Xiong, C.Q.; Tan, D.W.; Zhang, L.J.; et al. Two new steroidal saponins from the processed Polygonatum kingianum. Helv. Chim. Acta 2010, 93, 1086. [Google Scholar] [CrossRef]
- Dongmei, W.; Wei, Z.; Juanli, L. Chemical constituents and antibacterial activity of Polygonatum cirrhifolium rhizome research. J. Sichuan Univ. Nat. Sci. Ed. 2007, 44, 918. [Google Scholar]
- Li, X.C.; Yang, C.R.; Ichikawa, M.; Matsuura, H.; Kasai, R.; Yamasaki, K. Steroid saponins from Polygonatum kingianum. Phytochemistry 1992, 31, 3559. [Google Scholar]
- Yang, Q.X.; Yang, C.R. Cytotoxic steroidal saponins from Polygonatum punctatum. Chem. Biodivers. 2006, 3, 1349. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, W.; Ye, S.; Wang, H.; Wang, T.; Su, Y.; Wu, L.; Wang, Y.; Xu, Q.; Xu, C.; et al. Protective Effects of an Ancient Chinese Kidney-Tonifying Formula against H2O2-Induced Oxidative Damage to MES23.5 Cells. Parkinsons Dis. 2017, 2017, 2879495. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhang, Y.; Li, H.; Yang, C.R. Cytotoxic steroidal saponins from Polygonatum zanlanscianense. J. Nat. Prod. 2004, 67, 1992. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Li, X.; Li, J.; Zhu, W. The chemical formation of steroidal saponins in the root of Polygonatum cirrhifolium and its antibacterial activity. For. Sci. 2007, 8, 91. [Google Scholar]
- Li, X.C.; Yang, C.R.; Matsuura, H.; Kasai, R.; Yamasaki, K. Steroid glycosides from Polygonatum prattii. Phytochemistry 1993, 33, 465. [Google Scholar]
- Janeczko, Z.; Jansson, P.E.; Sendra, J. A new stereoidal saponin from Polygonatum Officinale. Planta Med. 1987, 53, 52. [Google Scholar] [CrossRef]
- Xu, D.-P.; Hu, C.-Y.; Zhang, Y. Two new steroidal saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2009, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Hu, W.-C.; Ma, G.-X.; Zhu, N.L.; Sun, X.-B.; Wu, H.F.; Xu, X.D. A new steroidal saponin from Polygonatum sibiricum. Asian Nat. Prod. Res. 2018, 20, 586. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, Z.; Wang, P. New secondary metabolites in the traditional Chinese medicine Yuzhu. Chin. J. Tradit. Chin. Med. 2004, 29, 42. [Google Scholar]
- Zhang, J.; Ma, B.P.; Kang, L.P.; Yu, H.S.; Yang, Y.; Yan, X.Z.; Dong, F.T. Furostanol saponins from the fresh rhizomes of Polygonatum kingianum. Chem. Pharm. Bull. 2006, 54, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Patil, S.; Qian, A.; Zhao, C. Bioactive Compounds of Polygonatum sibiricum - Therapeutic Effect and Biological Activity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Virk, J.K.; Kumar, S.; Singh, R.; Tripathi, A.C.; Saraf, S.K.; Gupta, V.; Bansal, P. Isolation and characterization of quinine from Polygonatum verticillatum: A new marker approach to identify substitution and adulteration. J. Adv. Pharm. Technol. Res. 2016, 7, 153–158. [Google Scholar]
- Ying, G.; Chulu, Q.; Lei, Z. The chemical constituents of Polygonatum fresh medicinal materials. Pharm. Clin. Res. 2015, 23, 365. [Google Scholar]
- Li, L.; Ren, F.; Chen, S.; Zhang, Q. The new dihydro high isoflavones in Polygonatum odoratum. Med. J. Sci. 2009, 44, 764. [Google Scholar]
- Li, X.; Lai, G.; Wang, Y.; Zhang, G.B.; Luo, S.D. Study on the chemical constituents of Polygonatum yunnanensis (II). Chin. Herb. Med. 2008, 39, 825. [Google Scholar]
- Wang, W.; Dabu, X.; He, J.; Yang, H.; Yang, S.; Chen, J.; Fan, W.; Zhang, G.; Cai, J.; Ai, H.; et al. Polygonatone H, a new homoisoflavanone with cytotoxicity from Polygonatum cyrtonema Hua. Nat. Prod. Res. 2019, 33, 1727–1733. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, T.; Chen, J. Study on the chemical constituents of Polygonatum kingianum. China Pharm. J. 2003, 28, 524. [Google Scholar]
- Zhang, H.; Yang, F.; Qi, J.; Song, X.C.; Hu, Z.F.; Zhu, D.N.; Yu, B.Y. Homoisoflavonoids from the fibrous roots of Polygonatum odoratum with glucose uptake-stimulatory activity in 3T3-L1 adipocytes. J. Nat. Prod. 2010, 73, 548. [Google Scholar] [CrossRef]
- Huang, P.-L.; Gan, K.-H.; Wu, R.-R.; Lin, C.-N. Benzoquinones, a homoisoflavanone and other constituents from Polygonatum alte-lobatum. Phytochemistry 1997, 44, 1369. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lu, C.-H.; Lai, G.-F.; Cao, J.X.; Luo, S.D. A new indolizinone from Polygonatum kingianum. Planta Med. 2003, 69, 1066. [Google Scholar]
- Zhou, X.L.; Yuping, Z.; Zhao, H.D. Antioxidant homoisoflavonoids from Polygonatum odoratum. Food Chem. 2015, 186, 63. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Eiko, K.; Mimura, N.; Kondo, Y.; Arihara, S. Hovetrichosides C-G, five new glycosides of two auronols, two neolignans, and a phenylpropanoid from the bark of Hovenia trichocarea. J. Nat. Prod. 1998, 61, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J.; Li, D. High Isoflavanization of Polygonatum Odoratum Rhizome in Qinling Mountains. For. Sci. 2008, 44, 125. [Google Scholar]
- Qian, Y.; Liang, J.Y.; Qu, W.; Che, Y. Two new homoisoflavanones from Polygonatum odoratum (Mill.) Druce. Chin. Chem. Lett. 2010, 21, 706. [Google Scholar] [CrossRef]
- Dong, W.; Shi, H.B.; Ma, H.; Miao, Y.B.; Liu, T.J.; Wang, W. Homoisoflavanones from Polygonatum odoratum rhizomes inhibit advanced glycation end product formation. Arch. Pharm. Res. 2010, 33, 669. [Google Scholar] [CrossRef]
- Che, Y.-Y.; Qian, Y.; Wu, Y.; Qu, W.; Liang, J.Y. Two new homoisoflavanones from the rhizome of Polygonatum odoratum. Chem. Nat. Compd. 2015, 51, 54. [Google Scholar] [CrossRef]
- Xu, D.; Sun, J.; Qi, B. Extraction, Separation and Structure of Triterpene Saponins from Polygonatum Identification. Chin. Herb. Med. 2006, 37, 1470. [Google Scholar]
- Hu, C.Y.; Xu, D.P.; Wu, Y.M. Triterpenoid saponins from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2010, 12, 801. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, J.; Kang, L. Research on the NMR of a triterpene saponin in Polygonatum yunnanensis (English). Nat. Prod. Res. Dev. 2007, 19, 7. [Google Scholar]
- Sun, L.; Wang, S.; Li, X. New alkaloids in the traditional Chinese medicine Polygonatum. Chin. Med. J. Chem. 1997, 7, 54. [Google Scholar]
- Sun, L.R.; Li, X.; Wang, S.X. Two new alkaloids from the rhizome of Polygonatum sibiricum. J. Asian Nat. Prod. Res. 2005, 7, 127. [Google Scholar] [CrossRef]
- Lin, C.N.; Huang, P.L.; Lu, C.M.; Wu, R.R.; Hu, W.P.; Wang, J.J. Polygonapholine, an alkaloid with a novel skeleton, isolated from Polygonatum alte-lobatum. Tetrahedron 1997, 53, 2025. [Google Scholar] [CrossRef]
- Kun, H.S.; Jae, C.D.; Sam, S.K. Isolation of adenosine from the rhizomes of Polygonatum sibidcum. Arch. Pharm. Res. 1991, 14, 193. [Google Scholar] [CrossRef]
- Chen, H.; Gu, N.; Hao, Z. Chemical Constituents of Ethyl Acetate Extract from the Rhizomes of Polygonatum sibiricum. J. Chin. Med. Mater. 2017, 40, 1345–1347. [Google Scholar]
- Liu, L.; Dong, Q.; Dong, X.T.; Fang, J.N.; Ding, K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohydr. Polym. 2007, 70, 304–309. [Google Scholar] [CrossRef]
- Wu, W.; Huang, N.; Huang, J.; Wang, L.; Wu, L.; Wang, Q.; Zhao, H. Effects of the steaming process on the structural properties and immunological activities of polysaccharides from Polygonatum cyrtonema. J. Funct. Foods 2022, 88, 104866. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Guo, K.; Jia, A.; Shi, Y.; Gao, G.; Sun, Z.; Liu, C. Cellulase-assisted extraction, characterization, and bioactivity of polysaccharides from Polygonatum odoratum. Int. J. Biol. Macromol. 2015, 75, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Wang, Y.; Yan, Y.; Guo, L.; Huang, L.; Gao, W. Characterization and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S. Protective effect of Polygonatum sibiricum Polysaccharide on D-galactose-induced aging rats model. Sci. Rep. 2020, 10, 2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.-D.; Li, X.-Y.; Deng, Y.-Y.; Zha, X.-Q.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P. Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 2021, 175, 235–241. [Google Scholar] [CrossRef]
- Ma, W.; Wei, S.; Peng, W.; Sun, T.; Huang, J.; Yu, R.; Zhang, B.; Li, W. Antioxidant Effect of Polygonatum sibiricum Polysaccharides in D-Galactose-Induced Heart Aging Mice. BioMed Res. Int. 2021, 2021, 6688855. [Google Scholar] [CrossRef]
- Horng, C.-T.; Huang, J.-K.; Wang, H.Y.; Huang, C.C.; Chen, F.A. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients 2014, 6, 5327–5337. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Zhang, X.; Wang, Y.; Qiang, Y.; Wang, B.; Zou, J.; Niu, J.; Wang, Z. Structural characterization and antioxidant activity of Polygonatum sibiricum polysaccharides. Carbohydr. Polym. 2022, 291, 119524. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, H.; Xia, W. Construction of Polygonatum sibiricum Polysaccharide Functionalized Selenium Nanoparticles for the Enhancement of Stability and Antioxidant Activity. Antioxidants 2022, 11, 240. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, R.; Kumar, D. Quantitative analysis of flavonols, flavonol glycoside and homoisoflavonoids in Polygonatum verticillatum using UHPLC-DAD-QTOF-IMS and evaluation of their antioxidant potential. Phytochem. Anal. 2020, 31, 333–339. [Google Scholar] [CrossRef]
- Wei, W.; Li, Z.P.; Zhu, T.; Fung, H.Y.; Wong, T.L.; Wen, X.; Ma, D.L.; Leung, C.H.; Han, Q.B. Anti-Fatigue Effects of the Unique Polysaccharide Marker of Dendrobium officinale on BALB/c Mice. Molecules 2017, 22, 155. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wang, Q.; Hou, S.; Chen, G. Chemical constituents from the rhizomes of Polygonatum sibiricum Red. and anti-inflammatory activity in RAW264.7 macrophage cells. Nat. Prod. Res. 2019, 33, 2359–2362. [Google Scholar] [CrossRef]
- Kumar Singh, S.; Patra, A. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). J. Integr. Med. 2018, 16, 273–282. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Y.; Song, Z.; Ge, J. Polygonatum sibiricum F. Delaroche polysaccharide ameliorates HFD-induced mouse obesity via regulation of lipid metabolism and inflammatory response. Mol. Med. Rep. 2021, 24, 501. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Jin, S.; Wang, D.; Wu, K.; Yang, Q.; Han, R.; Chen, S.; Liang, Z.; Jia, Q. Structure characterization and bioactivity of neutral polysaccharides from different sources of Polygonatum Mill. Biopolymers 2022, 23, e23490. [Google Scholar] [CrossRef]
- Luo, J.; Chai, Y.; Zhao, M.; Guo, Q.; Bao, Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020, 11, 4327–4338. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Sun, C.; Sun, D.; Wang, Y. Polysaccharides from Polygonatum sibiricum Delar. ex Redoute induce an immune response in the RAW264.7 cell line via an NF-κB/MAPK pathway. RSC Adv. 2019, 9, 17988–17994. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Jiang, P.; Xu, G.; Sun, T. Immunological regulation of the active fraction from Polygonatum sibiricum F. Delaroche based on improvement of intestinal microflora and activation of RAW264.7 cells. J. Ethnopharmacol. 2022, 293, 115240. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Kong, X.; Li, H. Characterization and Immunological Activities of Polysaccharides from Polygonatum sibiricum. Biol. Pharm. Bull. 2020, 43, 959–967. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, H.; Zhao, C.; Li, X.; Wang, Y.; Huang, L.; Gao, W. Purification, characterization and immunomodulatory activity of fructans from Polygonatum odoratum and P. cyrtonema. Carbohydr. Polym. 2019, 214, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, L.; Fu, B.; Chu, J.; Chen, C.; Li, X.; Ma, J.; Tang, W. Protective effects of Polygonatum kingianum polysaccharides and aqueous extract on uranium-induced toxicity in human kidney (HK-2) cells. Int. J. Biol. Macromol. 2022, 202, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Xie, P.; Li, C.; Bian, Z.; Wang, X.; Peng, D.; Zhu, G. Polysaccharides from Polygonatum cyrtonema Hua Reduce Depression-Like Behavior in Mice by Inhibiting Oxidative Stress-Calpain-1-NLRP3 Signaling Axis. Oxid. Med. Cell Longev. 2022, 2022, 2566917. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pi, X.; Zheng, W.; Cen, Y.; Ni, J.; Xu, L.; Wu, K.; Liu, W.; Li, L. The Methanol Extract of Polygonatum odoratum Ameliorates Colitis by Improving Intestinal Short-Chain Fatty Acids and Gas Production to Regulate Microbiota Dysbiosis in Mice. Front. Nutr. 2022, 9, 899421. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fang, Q.; Lai, Y.; Lei, H.; Zhang, D.; Niu, H.; Wang, R.; Song, C. Polysaccharides from the leaves of Polygonatum sibiricum Red. regulate the gut microbiota and affect the production of short-chain fatty acids in mice. AMB Express. 2022, 12, 35. [Google Scholar] [CrossRef]
- Li, W.; Fu, B.; Yan, C.; Xie, H.; Zhang, X.; Xiang, L.; Hu, J.; Ma, J.; Tan, C.; Yuan, H. Analysis on the times of Polygonati Rhizoma steamed by multiple times based on entropy weight and gray relative analysis method. China J. Tradit. Chin. Med. Pharm. 2021, 36, 6764–6769. [Google Scholar]
- Wang, J.; Yue, Y.; Tang, F.; Tao, W. Comparative analysis of volatile fractions in Polygonati Rhizoma and its processed products by GC-MS. China J. Chin. Materia Medica 2011, 36, 2187–2191. [Google Scholar]
- Li, R.; Tao, A.; Yang, R.; Fan, M.; Zhang, X.; Du, Z.; Shang, F.; Xia, C.; Duan, B. Structural characterization, hypoglycemic effects and antidiabetic mechanism of a novel polysaccharides from Polygonatum kingianum Coll. et Hemsl. Biomed. Pharmacother. 2020, 131, 110687. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, J.; Deng, Y.; Ye, X.; Xia, L.; Liu, M.; Liu, Y.; Chen, Y.; Zhang, Q.; Wang, T. Analysis of Chemical Constitutions of Polygonatum cyrtonema Dried Rhizomes Before and After Processing with Wine Based on UPLC-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Formulae 2021, 4, 110–121. [Google Scholar]
- Sirong, Y.; Jian, Q.; Pinming, L. Research progress in the preparation of Polygonatum. Chin. J. Tradit. Chin. Med. 2017, 32, 4575–4578. [Google Scholar]
- Wang, X.; Ye, D. A Collection of Traditional Chinese Medicine Processing Methods in Past Dynasties (Ancient Part); Jiangxi Science and Technology Press: Nanchang, China, 1998; p. 136. [Google Scholar]
- Dong, L.; Li, S.; Wang, C.; Zhang, H.; Liu, Y.; Zhang, S. Brief on the Academic Thought of Zhang Shanlei’s Rectification of the Meaning of Materia Medica. World J. Integr. Tradit. West. Med. 2014, 6, 573–575. [Google Scholar] [CrossRef]
- Yu, J.; Yu, B.; Qian, Z. Accelerate the compilation of the “National Standards for the Processing of Chinese Herbal Medicines” and standardize the unified national standards for the processing of Chinese herbal medicines. Chin. J. Chin. Materia Medica 2011, 36, 4. [Google Scholar]
- Tao, C. Huizhitang Experience Prescription; Chinese Medicine Ancient Books Publishing House: Beijing, China, 1994. [Google Scholar]
- Fan, B.; Wei, G.; Gan, X.; Li, T.; Qu, Y.; Xu, S.; Liu, C.; Qian, C. Study on the varied content of Polygonatum cyrtonema polysaccharides in the processing of steaming and shining for nine times based on HPLC-MS/MS and chemometrics. Microchem. J. 2020, 159, 105352. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, H.; Li, Y.; Liu, Y.; Dai, W.; Fang, J.; Cao, C.; Die, Y.; Liu, Q.; Wang, C.; et al. Physicochemical properties and immunological activities of polysaccharides from both crude and wine-processed Polygonatum sibiricum. Int. J. Biol. Macromol. 2020, 143, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lao, J.; Zhou, R.; He, W.; Qin, Y.; Zhong, C.; Xie, J.; Liu, H.; Wan, D.; Zhang, S. Simultaneous Identification and Dynamic Analysis of Saccharides during Steam Processing of Rhizomes of Polygonatum cyrtonema by HPLC–QTOF–MS/MS. Molecules 2018, 23, 2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zeng, J.; Gong, P.; Wu, Y.; Li, H. Effect of steaming process on the structural characteristics and antioxidant activities of polysaccharides from Polygonatum sibiricum rhizomes. Glycoconj. J. 2021, 38, 561–572. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Z.; Chen, Y.; Huang, Y.F.; He, L.H.; Zhang, Z.F. Changes in the content of polysaccharides and saponins during the processing of Huangjing “Nine Steaming and Nine Preparations”. J. Hunan Normal Univ. Med. Ed. 2015, 5, 141–144. [Google Scholar]
- Xia, G.; Li, X.; Zhang, Z.; Jiang, Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021, 16, 92–101. [Google Scholar] [CrossRef]
- Xia, G.H.; Li, X.H.; Zhang, Z.; Jiang, Y.H. Effects of fermentation treatments on Polygonatum odoratum flavones’ antioxidant activities. Saudi J. Biol. Sci. 2021, 28, 5011–5016. [Google Scholar] [CrossRef]
- Teng, H.; Wang, R.; Wu, D.; Liu, C.; Tang, X.; Huang, S.; Xu, F. Antioxidant and hypoglycemic activities of different polar extracts from crude and steam-processed Polygonatum cyrtonema Hua. Food Ferment. Ind. 2022, 48, 70–75. [Google Scholar] [CrossRef]
- Du, X.; Liang, Z.; Xia, Y.; Zhang, X.; Zhang, W.; Han, J.; Wu, Z. Comparative study on anti-exercise fatigue effects of different extracts from raw materials of Polygonatum cyrtonema Hua and its processed products. J. Anhui Agric. Univ. 2021, 48, 26–30. [Google Scholar]
- Chen, Y.; Hu, H.; Feng, G.; Wei, T.; Li, X.; Yu, L.; He, X. Anti-Fatigue and Anti-Oxidant Effects of Crude and Processed Polygonatum Cyrtonema on Exhaustive Swimming Mice. Tradit. Chin. Med. Pharmacol. Clin. 2021, 2, 92–96. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, X.; Cao, M.; Zheng, S.; Ma, Y.; Huang, Q. NF-κB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2022, 291, 115153. [Google Scholar] [CrossRef]
- Wang, Q. Study on the Anti-Inflammatory and Hypoglycemic Effects Produced by Crude Polysaccharides Between Raw Materials and Nine-Steam-Nine-Bask Processing, P. cyrtonema Hua. Master’s Thesis, Anhui University of Traditional Chinese Medicine, Hefei, China, 2020. [Google Scholar]
- Huanhuan, T.; Renzhong, W.; Deling, W. Antioxidative and hypoglycemic activity of different polar parts of Polygonatum polyflora before and after processing. Food Ferment. Ind. 2022, 48, 70–75. [Google Scholar]
- Li, C.; Li, J.; Shang, Y.; Wang, Y.; Gao, J.; Xue, N.; Huang, C.; Li, F.; Li, J. Hypoglycemic and Hypolipidemic Activity of Polygonatum sibiricum Fermented with Lactobacillus brevis YM 1301 in Diabetic C57BL/6 Mice. J. Med. Food. 2021, 2, 720–731. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhang, X.; Dabu, X.; Juan, H.; Yang, S.C.; Chen, J.W.; Wei, F.; Zhang, G.H.; Ai, H.L.; Meirong, H. Analysis of chemical constituents from Polygonatum cyrtonema after “Nine-Steam-Nine-Bask” processing. Phytochem. Lett. 2019, 29, 35–40. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, B.; Huang, J.; Li, W.; Yi, P.; Yi, M.; Peng, W. Identification of the protective effect of Polygonatum sibiricum polysaccharide on d-galactose-induced brain ageing in mice by the systematic characterization of a circular RNA-associated ceRNA network. Pharm. Biol. 2021, 59, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Yelithao, K.; Surayot, U.; Park, W.; Lee, S.; Lee, D.H.; You, S. Effect of sulfation and partial hydrolysis of polysaccharides from Polygonatum sibiricum on immune-enhancement. Int. J. Biol. Macromol. 2019, 122, 10–18. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yuan, L.; Ruan, H.; Zhu, Z.; Fan, X.; Zhu, L.; Peng, X. Blood-Enriching Effects and Immune-Regulation Mechanism of Steam-Processed Polygonatum Sibiricum Polysaccharide in Blood Deficiency Syndrome Mice. Front. Immunol. 2022, 13, 813676. [Google Scholar] [CrossRef] [PubMed]

| Number | Species | Distribution | First Recorded Time |
|---|---|---|---|
| 1 | P. acuminatifolium Kom | Russian, China | 1916 |
| 2 | P. adnatum | China | 1987 |
| 3 | P. amabile | Japan | 1892 |
| 4 | P. angelicum | Arunachal Pradesh, Tibet | 2015 |
| 5 | P. arisanense | China (Taiwan) | 1920 |
| 6 | P. autumnale | Arunachal Pradesh | 2015 |
| 7 | P. annamense | Vietnam | 2015 |
| 8 | P. azegamii | Japan | 2008 |
| 9 | P. biflorum | Canada, United Mexican States | 1817 |
| 10 | P. brevistylum | Nepal, Darjiling | 1875 |
| 11 | P. buschianum | Krym | 1979 |
| 12 | P. campanulatum | China | 2015 |
| 13 | P. cathcartii | Nepal, China | 1875 |
| 14 | P. cirrhifolium | Himalaya, China | 1839 |
| 15 | P. costatum | Thailand | 2015 |
| 16 | P. cryptanthum | Korea, Japan | 1908 |
| 17 | P. curvistylum | Nepal, China | 1892 |
| 18 | P. cyrtonema | China | 1892 |
| 19 | P. daminense | China | 2020 |
| 20 | P. desoulavyi | Korea, Japan | 1931 |
| 21 | P. domonense | Japan | 1970 |
| 22 | P. falcatum | Korea, Japan | 1859 |
| 23 | P. falcatum var. hyugaense | Japan | 1957 |
| 24 | P. falcatum var. trichosanthum | Japan | 2008 |
| 25 | P. filipes | China | 1980 |
| 26 | P. franchetii | China | 1892 |
| 27 | P. geminiflorum | Pakistan, Himalaya | 1844 |
| 28 | P. glaberrimum | Turkey, Iran | 1849 |
| 29 | P. gongshanense | China, Myanmar | 2014 |
| 30 | P. govanianum | Pakistan, Himalaya | 1839 |
| 31 | P. graminifolium | Himalaya | 1851 |
| 32 | P. grandicaule | Korea | 1998 |
| 33 | P. griffithii | Arunachal Pradesh, Tibet | 1875 |
| 34 | P. hirtellum | China | 1936 |
| 35 | P. hookeri | Himalaya, China | 1875 |
| 36 | P. humile | Kazakhstan, Japan | 1859 |
| 37 | P. inflatum | Korea, Japan | 1901 |
| 38 | P. infundiflorum | Korea | 1998 |
| 39 | P. involucratum | Russian, Korea, Japan | 1883 |
| 40 | P. jinzhaiense | China | 2000 |
| 41 | P. kingianum | China | 1890 |
| 42 | P. lasianthum | Korea, Japan | 1883 |
| 43 | P. latifolium | Europe, Turkey | 1807 |
| 44 | P. leiboense | China | 1984 |
| 45 | P. longistylum | China | 1990 |
| 46 | P. luteoverrucosum | Arunachal Pradesh, Tibet | 2015 |
| 47 | P. macranthum | Japan | 1919 |
| 48 | P. macropodum | China | 1832 |
| 49 | P. megaphyllum | China | 1966 |
| 50 | P. mengtzense | China, Vietnam | 1936 |
| 51 | P. multiflorum | Europe, Caucasus | 1785 |
| 52 | P. nervulosum | Himalaya | 1875 |
| 53 | P. nodosum | China | 1892 |
| 54 | P. odoratum | China, Europe, Japan | 1906 |
| 55 | P. omeiense | China | 1992 |
| 56 | P. oppositifolium | Nepal, Assam | 1839 |
| 57 | P. orientale | Krym, Turkey, Iran | 1807 |
| 58 | P. prattii | China | 1892 |
| 59 | P. pseudopolyanthemum | Caucasus | 1928 |
| 60 | P. pubescens | Canada, American | 1813 |
| 61 | P. punctatum | Nepal, China | 1850 |
| 62 | P. qinghaiense | China (Qinghai) | 2005 |
| 63 | P. robustum | Korea | 1917 |
| 64 | P. roseum | Asia, China (Xinjiang) | 1850 |
| 65 | P. sewerzowii | Iran, Asia | 1868 |
| 66 | P. sibiricum | Siberia, Korea, Bhutan | 1811 |
| 67 | P. singalilense | Nepal, Bhutan | 1965 |
| 68 | P. sparsifolium | China | 2002 |
| 69 | P. stenophyllum | Russian, Korea | 1859 |
| 70 | P. stewartianum | China | 1912 |
| 71 | P. tessellatum | Assam, China | 1936 |
| 72 | P. tsinlingense | China | 1949 |
| 73 | P. undulatifolium | Arunachal Pradesh, Tibet | 2018 |
| 74 | P. urceolatum | China, Vietnam | 2014 |
| 75 | P. verticillatum | Europe, China | 1785 |
| 76 | P. wardii | Assam, Tibet | 1937 |
| 77 | P. yunnanense | China | 1916 |
| 78 | P. zanlanscianense | China | 1915 |
| 79 | P. zhejiangensis | China (Zhejiang) | 1994 |
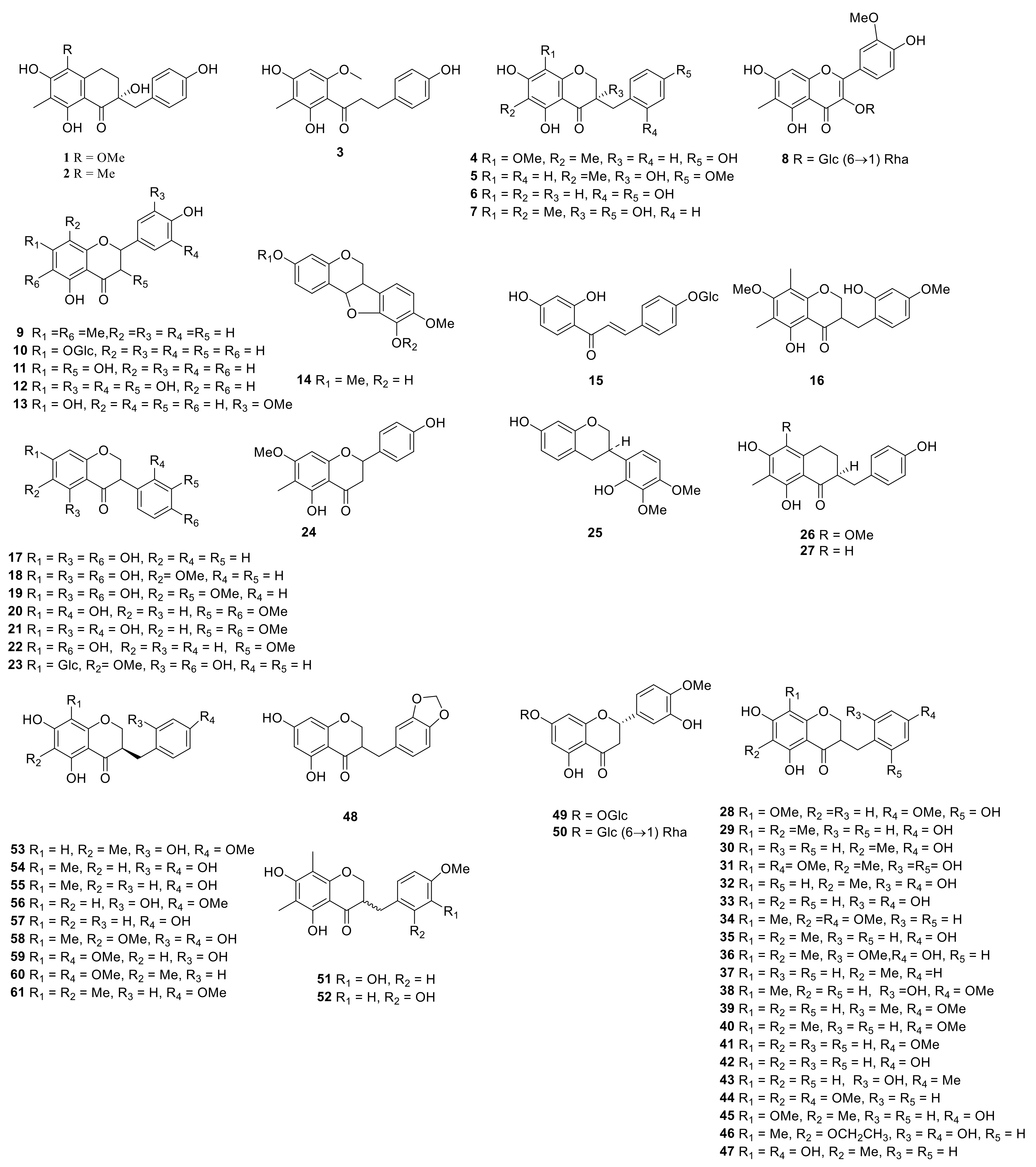
| No. | Compounds | Species | Parts | References |
|---|---|---|---|---|
| 1 | neoprazerigeninA-3-O-β-D-lycotetraosid | P. sibiricum | rhizome | [7] |
| 2 | (25R)-spirost-5-ene-3β,14α-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-Β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [8] |
| 3 | (25S)-spirost-5-en-3-ol-3-O-β-D-glucopyranosyl-(1→3)-[β-D-fucopyranosyl-(1→2)]- β-D-glucopyranosyl-(1→4) -β-D-galactopyranoside | P. verticillatum | rhizome | [9] |
| 4 | (25S)-spirost-5-ene-3β,12β-diol-3-O-{β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)} -β-D-galactopyranoside | P. cirrhifolium | rhizome | [10] |
| 5 | (25S)-Spirosta-5,14-diene-3β-ol-3-O-{β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)} -β-D-galactopyranoside | P. odoratum P. cirrhifolium | rhizome | [10] |
| 6 | (25S)-spirost-5-en-3β-ol-3-O-α-L-rhamnose (1→2)-[α-L-rhamnose (1→4)]-β-D-Glucoside | P. cirrhifolium | rhizome | [10] |
| 7 | (25S)-spirost-5-ene-3β,14α-diol-3-O-{β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3) ]-β-D-glucopyranosyl-(1→4)}-β-D-galactopyranoside | P. odoratum P. cirrhifolium | rhizome | [10,11] |
| 8 | 3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-galactopyranoside-25(S)-spirost-5(6) -en-3-ol | P. odoratum | rhizome | [11] |
| 9 | 3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-galactopyranoside-25(S)-spirost-5(6) -en-3β, 14α-diol | P. odoratum | rhizome | [11] |
| 10 | neosibiricoside A | P. sibiricum | rhizome | [12] |
| 11 | neosibiricoside B | P. sibiricum | rhizome | [12] |
| 12 | neosibiricoside C | P. sibiricum | rhizome | [12] |
| 13 | polygoside A | P. odoratum | rhizome | [13] |
| 14 | (3β,14α)-3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside- yamogenin | P. odoratum | rhizome | [13] |
| 15 | (25S)-spirost-5-ene-3β, 14α-dihydroxy | P. odoratum | rhizome | [13] |
| 16 | (25S)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [13] |
| 17 | (25S)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [13] |
| 18 | polygonatumoside F | P. odoratum | rhizome | [14] |
| 19 | polygonatumoside D | P. odoratum | rhizome | [15] |
| 20 | polygonatumoside E | P. odoratum | rhizome | [15] |
| 21 | (25S)-spirost-5-en-3-O-β-D-glucopyranosyl-(1→4)-β-D-fucopyranosyl-3β, 17α-diol | P. sibiricum | rhizome | [16] |
| 22 | (25S)-spirost-5-en-3β,12β-diol-3-O-β-D-glucopyranosyl-(1→4) -β-D-fucopyranosyl | P. sibiricum | rhizome | [16] |
| 23 | (25S)-spiroster-5-en-12-one-3-OD-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. cyrtonema | rhizome | [17] |
| 24 | (25S)-spirost-5-en-12-one-3-O-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. cyrtonema | rhizome | [18] |
| 25 | (25S) -3-β-hydroxy-spirost-5-en-12-one | P. cyrtonema | rhizome | [18] |
| 26 | 25S-pratioside D1 | P. kingianum | rhizome | [19] |
| 27 | 25S-Yunnan Polygonatum A | P. kingianum | rhizome | [19] |
| 28 | (25S)-spirost-5-ene-3β,14α-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl- (1→3)]-β-D-glucopyranosyl-(1→4) -β-D-galactopyranoside | P. odoratum | rhizome | [7] |
| 29 | (25S)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [7] |
| 30 | (25S)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(l→2) -[β-D-xylopyranosyl-(l→3) ]-β-D-glucopyranosyl-(l→4)-β-D-galactopyranoside | P. odoratum | Fresh rhizome | [20] |
| 31 | kingianoside H | P. kingianum | rhizome processed | [21] |
| 32 | sibiricoside B | P. sibiricum | rhizome | [7] |
| 33 | (25R)-spirost-5-en-3β-ol-3-O-α-L-rhamnose (1→2)-[α-L-rhamnose (1→4)]-β-D- Glucoside | P. cirrhifolium | rhizome | [10] |
| 34 | neosibiricoside D | P. sibiricum | rhizome | [12] |
| 35 | polygoside B | P. odoratum | rhizome | [13] |
| 36 | (25R)-spirost-5-en-3β,17α-diol-3-O-β-D-glucopyranosyl-(1→4)-β-D-fucopyranosyl | P. sibiricum | rhizome | [17] |
| 37 | (25r)-spirost-5-en-3β,17α-diol-3-O-β-D-glucopyranosyl-(1→4)-β-D-fucopyranosyl | P. sibiricum | rhizome | [17] |
| 38 | (25R)-spirost-5-en-3β,12β-diol-3-O-β-D-glucopyranosyl-(1→4)-β-D-fucopyranosyl | P. sibiricum | rhizome | [18] |
| 39 | (25R) Spiroster-5-en-12-one-3-OD-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4) -β-D-galactopyranoside | P. cyrtonema | rhizome | [19] |
| 40 | cyrtonemoside A | P. cyrtonema | rhizome | [22] |
| 41 | (25r)-3-β-hydroxy-spirost-5-en-12-one | P. cyrtonema | rhizome | [23] |
| 42 | (25r) -kingianoside G | P. kingianum | rhizome | [24] |
| 43 | kingianoside K | P. kingianum | rhizome processed | [25] |
| 44 | kingianoside I | P. kingianum | rhizome processed | [25] |
| 45 | (25R)-spirost-5-ene-3β,14α-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [9] |
| 46 | (25R)-spirost-5-ene-3β,14α-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-Β-D-glucopyranosyl-(1→4) -β-D-galactopyranoside | P. odoratum | rhizome | [9] |
| 47 | (25R)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→ 3)]- β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. odoratum | rhizome | [9] |
| 48 | (25R)-spirost-5-en-3β-ol-3-O-β-D-glucopyranosyl-(l→2)-[β-D-xylopyranosyl-(l→3)] -β-D-glucopyranosyl-(l→4) -β-D-galactopyranoside | P. odoratum | Fresh rhizome | [21] |
| 49 | saponin Tg | P. kingianum | rhizome processed | [23] |
| 50 | polygonatoside C1 | P. kingianum | rhizome processed | [23] |
| 51 | ophiopogonin C’ | P. kingianum | rhizome processed | [23] |
| 52 | Diosgenin | P. cirrhifolium | rhizome | [10] |
| 53 | (25R)-spirost-5-en-3β-ol-3-O-α-L-rhamnose (1→4)-β-D-glucoside | P. cirrhifolium | rhizome | [25] |
| 54 | pratioside D1 | P. prattii P. kingianum | rhizome | [23] |
| 55 | kingianoside A | P. kingianum | rhizome | [24,26] |
| 56 | kingianoside B | P. kingianum | rhizome | [26] |
| 57 | funkioside C | P. kingianum | rhizome | [26] |
| 58 | (25R)-spirost-5-ene-3β,14α-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-Β-D-glucopyranosyl-(1→4) -β-D-galactopyranoside | P. odoratum | rhizome | [6] |
| 59 | Dioscin | P. kingianum P. punctatum P. cirrhifolium P. zanlanscianense | rhizome, rhizome processed | [24,25,26,27,28,29] |
| 60 | Dracaenoside F | P. cirrhifolium | roots and rhizomes | [28,30] |
| 61 | polygonatoside D | P. zanlanscianense | rhizome | [29] |
| 62 | Isobalanin-3-O-α-L-rhamnopyranosyl-(1→2)- [α-L-rhamnopyranosyl-(1→4)]-β-D-pyranosyl Glucopyranoside | P. zanlanscianense | rhizome | [29] |
| 63 | saponin Pa | P. kingianum | rhizome processed | [24] |
| 64 | prosapogenin A of dioscin | P.punctatum | rhizome | [27] |
| 65 | gracillin | P. zanlanscianense | rhizome | [30] |
| 66 | parissaponin Pb | P. zanlanscianense | rhizome processed | [29] |
| 67 | polypunctoside A | P. punctatum | rhizome | [27] |
| 68 | polypunctoside B | P. punctatum | rhizome | [27] |
| 69 | polypunctoside C | P. punctatum | rhizome | [27] |
| 70 | polypunctoside D | P. punctatum | rhizome | [27] |
| 71 | polygonatoside A | P. zanlanscianense | rhizome | [29] |
| 72 | polygonatoside B | P. zanlanscianense | rhizome | [29] |
| 73 | pratioside C | P. prattii | root | [31] |
| 74 | pratioside A | P. prattii | root | [31] |
| 75 | pratioside D1 | P. prattii | root | [31] |
| 76 | pratiosides E1 | P. prattii | root | [31] |
| 77 | pratiosides F1 | P. prattii | root | [31] |
| 78 | isonarthogenin-3-O-β-D-glucopyranosyl-(1→2)-β-D- glucopyranosyl-(1→4) -β-D-galactopyranoside | P. zanlanscianense | rhizome | [32] |
| 79 | polygonatoside C | P. zanlanscianense | rhizome | [32] |
| 80 | saponin Tb | P. kingianum | rhizome processed | [33] |
| 81 | odospiroside | P. odoratum | rhizome | [34] |
| 82 | sibiricoside A | P. sibiricum | rhizome | [7] |
| 83 | sibiricogenin-3-O-β-lycotetraoside | P. sibiricum | rhizome | [7] |
| 84 | polygonatumoside G | P. odoratum | rhizom | [15] |
| 85 | timosaponin H1 | P. odoratum | rhizom | [15] |
| 86 | (25S) -funkioside B | P. odoratum | rhizom | [15] |
| 87 | 25R-22 Hydroxy-curvetoxin C | P. kingianum | rhizom | [20] |
| 88 | 22-Hydroxy-curvetoxin C | P. kingianum | rhizom | [20] |
| 89 | kingianoside Z | P. sibiricum | rhizome | [35] |
| 90 | 22-Hydroxy-25(R)-furost-5-en-12-one-3β,22,26-triol-26-O-β-D-glucopyranoside | P. odoratum | rhizome | [36] |
| 91 | kinginaoside E | P. kingianum | rhizome | [20] |
| 92 | 25S-kinginaoside E | P. kingianum | rhizome | [20] |
| 93 | 25S-kinginaoside C | P. kingianum | rhizome | [20] |
| 94 | 25S-kinginaoside D | P. kingianum | rhizome | [20] |
| 95 | kingianoside C | P. kingianum | rhizome | [20] |
| 96 | kingianoside D | P. kingianum | rhizome | [20] |
| 97 | saponin Pb | P. kingianum | rhizome processed | [25] |
| 98 | 25S-kinginaoside F | P. kingianum | rhizome | [20] |
| 99 | 3β,26-diol-25(R)-Δ5,20(22)-diene-furosta-26-O-β- D-glucopyranoside | P. odoratum | fresh rhizome | [22] |
| 100 | (3β,23ξ, 25R)-3-{[2-O-(6-deoxy-α-L-mannopyranosyl) -β-D-glucopyranosyl]-oxy}-22-hydroxy-furost-5-en-26-yl-β-D- glucopyranoside | P. punctatum | rhizome | [28] |
| 101 | protodioscin | P. punctatum | rhizome | [28] |
| 102 | 26-β-D-glucopyranosyl-22-methoxy-(25R) -furost-5-en-3β, 26-diol-3-O-[α-L-rhamnopyranosyl-(1→2)][α-L- rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | P. zanlanscianense | root | [22] |
| 103 | pratioside B | P. prattii | roots | [31] |
| 104 | polygonoide A | P. sibiricum | rhizome | [33] |
| 105 | polygonoide B | P. sibiricum | rhizome | [33] |
| 106 | 22-hydroxy-25(S)-furost-5-en-12-one-3β,22,26-triol-26-O-β-D -glucopyranoside | P. odoratum | rhizome | [35] |
| 107 | kingianoside F | P. kingianum | rhizome | [36] |
| 108 | ergosta-7, 22-diene-3β, 5α, 6β-triol | P. odoratum | rhizome | [14] |
| 109 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol-1-O-α-L- rhamnopyranosyl-16-O-β-D-glucopyranoside | P. odoratum | rhizome | [14] |
| 110 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol-1,16-di-O-β-D- glucopyranoside | P. odoratum | rhizome | [15] |
| 111 | (25S)-3β,14α-dihydroxy-spirost-5-ene-3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | P. odoratum | rhizome | [15] |
| 112 | (25S)3β,14α-dihydroxy-spirost-5-ene-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | P. odoratum | rhizome | [15] |
| 113 | 3-O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside-yamogenin | P. odoratum | rhizome | [15] |
| 114 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol-1-O-α-L-rhamnopyranosyl-16-O-β-D-glucopyranoside | P. odoratum | rhizome | [15] |
| 115 | polygonatumoside A | P. odoratum | rhizome | [16] |
| 116 | polygonatumoside B | P. odoratum | rhizome | [16] |
| 117 | polygonatumoside C | P. odoratum | rhizome | [16] |
| 118 | 3-O-β-D-glucopyranosyl(1→4)-β-D-fucopyranosyl-(25R)-spirost-5-en-3β,17α-diol | P. sibiricum | rhizome | [37] |
| 119 | 3-O-β-D glucopyranosyl (1→4)-β-D-fucopyranosyl-(25S)-spirost-5-en-3β | P. sibiricum | rhizome | [37] |
| 120 | 17α-diol (2), 3-O-β-D-glucopyranosyl(1→2)-β-D-glucopyranosyl (1→4)-β-D- fucopyranosyl-(25R)-spirost-5-en-3β,17α-diol | P. sibiricum | rhizome | [37] |
| 121 | 3-O-β-D glucopyranosyl(1→4)-β-D-fucopyranosyl-(25R/S)-spirost-5-en- 3β,12β-diol | P. sibiricum | rhizome | [37] |
| 122 | (25S)-spirost-5-en-3 -ol 3-O-β-D-glucopyranosyl-(1→3)- [β-D fucopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | P. verticillatum | rhizome | [38] |
| 123 | 26-O-β-D-glucopyranosyl-22ξ-hydroxy-(25R)-furost-5-en-3β, 26-diol, 3-O-β [xylopyranosyl (1→3) α-L-rhamnopyranosyl (1→2) β-D-glucopyranoside] | P. verticillatum | rhizome | [39] |
| 124 | 3-O-β-D-xylopyranosyl (1→3) α-L-rhamnopyranosyl (1→3) β-D-glucopyranoside diosgenin | P. verticillatum | rhizome | [39] |
| No. | Compounds | Species | Parts | References |
|---|---|---|---|---|
| 1 | polygonatone B | P. odoratum | rhizome | [13] |
| 2 | polygonatone C | P. odoratum | rhizome | [13] |
| 3 | polygonatone D | P. odoratum | rhizome | [13] |
| 4 | (25S)-spirost-5-ene-3β,12β-diol-3-O-{β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)}-β-D-galactopyranoside | P. odoratum | rhizome | [13] |
| 5 | (3S)-3, 5, 7-trihydroxy-6-methyl-3-(4’-methoxybenzyl) -chroma-4-one | P. odoratum | rhizome | [14] |
| 6 | 5, 7-dihydroxy-3-(2’, 4’-dihydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [14] |
| 7 | (3S)-3, 5, 7-trihydroxy-6, 8-dimethyl-3-(4’-hydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [14] |
| 8 | isorhamnetin-3-O-(6″-O-α-L-rhamnopyransoyl) -β-D-glucopyranoside | P. odoratum | rhizome | [14] |
| 9 | 5,4’-Dihydroxy-7-methoxy-6-methylflavonoid | P. odoratum | rhizome | [13] |
| 10 | Apigenin-7-O-β-D-glucoside | P. sibiricum | fresh rhizome | [39] |
| 11 | kaempferol | P. sibiricumP. cyrtonema | fresh rhizome | [39] |
| 12 | myricetin | P. sibiricum | fresh rhizome | [39] |
| 13 | chrysoeriol | P. odoratum | rhizome | [40] |
| 14 | (6aR, 1laR)-10-hydroxy-3,9-dimethoxy pterostane | P. kingianum | rhizome | [41] |
| 15 | neoisoliquiritin | P. kingianum | rhizome | [41] |
| 16 | 5-hydroxy-7-methoxy-6, 8-dimethyl-3-(2’-hydroxy-4’-methoxybenzyl) -chroma-4-one | P.cyrtonema | rhizome | [42] |
| 17 | 5, 7, 4’-trihydroxy isoflavone | P. odoratum | rhizome | [14] |
| 18 | 5, 7, 4’-trihydroxy-6-methoxy isoflavone | P. odoratum | rhizome | [14] |
| 19 | 5, 7, 4’-trihydroxy-6, 3’-dimethoxy isoflavone | P. odoratum | rhizome | [14] |
| 20 | 2’, 7-Dihydroxy-3’, 4’-Dimethoxyisoflavan | P. kingianum | rhizome | [41] |
| 21 | isoliquiritin | P. kingianumP. alternicirrhosum | Rhizome | [41] [43] |
| 22 | 4’,7-Dihydroxy-3’-Methoxy Isoflavone | P. kingianum | rhizome | [43] |
| 23 | tectoridin | P. odoratum | root | [44] |
| 24 | liquiritigenin | P. kingianumP. alte-lobatum P. odoratum | rhizome | [41,43] [45] |
| 25 | isomucronulatol | P. kingianum | rhizome | [46] |
| 26 | (3R)-5, 7-dihydroxy-6-methyl-3-(4’-hydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [47] |
| 27 | (3R)-5,7-Dihydroxy-6-methyl-8-methoxy-3-(4’-hydroxybenzyl)-chroman-4-one | P. odoratum | rhizome | [47] |
| 28 | polygonatone A | P. odoratum | rhizome | [13] |
| 29 | (3R)-5,7-Dihydroxy-6,8-dimethyl-3-(4’-hydroxybenzyl)-chroman-4-one | P. odoratum | rhizome | [13] |
| 30 | (3R)-5,7-Dihydroxy-6-methyl-3-(4’-hydroxybenzyl)-chroman-4-one | P. odoratum | rhizome | [13] |
| 31 | 5,7-Dihydroxy-6-methyl-8-methoxy-3-(4’-methoxybenzyl)-chroman-4-one | P. odoratum | root | [13] |
| 32 | 5,7-Dihydroxy-6-methyl-3-(2’,4’-dihydroxybenzyl)-chroman-4-one | P.cyrtonema | rhizome | [39] |
| 33 | disporopsin | P. odoratum | rhizome | [40] |
| 34 | 5,7-Dihydroxy-6-methoxy-8-methyl-3-(4’-methylbenzyl)-chroman-4-one | P. odoratum | rhizome | [40] |
| 35 | 5,7-Dihydroxy-6,8-dimethyl-3-(4’-hydroxybenzyl)-chroman-4-one | P. cyrtonemaP. alte-lobatum P. odoratum | rhizome rhizome rhizome | [42] [48] [49] |
| 36 | 5, 7-dihydroxy-6, 8-dimethyl-3-(2’-methoxy-4’-hydroxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 37 | 5, 7-dihydroxy-6-methyl-3-(4’-hydroxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 38 | 5, 7-dihydroxy-8-methyl-3-(4’-hydroxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 39 | 5, 7-dihydroxy-6-methyl-3-(4’-methoxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 40 | 5, 7-dihydroxy-6, 8-dimethyl-3-(4’-methoxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 41 | 5, 7-dihydroxy-3-(4’-methoxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 42 | 5, 7-dihydroxy-3-(4’-hydroxybenzyl) -chroma-4-one | P. kingianum P. cyrtonema | rhizome | [12] [42] |
| 43 | 5, 7-dihydroxy-3-(2’-hydroxy-4’-methoxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 44 | methylophiopogonanone B | P. odoratum | root | [44] |
| 45 | 5,7-Dihydroxy-6-methyl-8-methoxy-3-(4’-hydroxybenzyl)-chroman-4-one | P. odoratum | root | [44] |
| 46 | ophiopogonanone E | P. odoratum | root | [44] |
| 47 | (3R)-5,7-dihydroxy-8-methoxy-3-(4-methoxybenzyl)-6-methylchrom-an-4-one | P. odoratum | rhizome | [50] |
| 48 | 6-Methyl-4’,5,7-trihydroxy homoisoflavanone | P. odoratum | rhizome | [49] |
| 49 | 5,7-Dihydroxy-6-methoxy-8-methyl-3-(2’,4’-dihydroxybenzyl)-chroman-4-one | P. odoratum | rhizome | [50] |
| 50 | (3R)-5,7,8-trihydroxy-3-(4-hydroxybenzyl) -6-methyl-chroma-4-one | P. odoratum | rhizome | [50] |
| 51 | 5,7-Hydroxy-8-methoxy-3-(3’,4’-methylenedioxybenzyl)-chroman-4-one (Methyl Ophiopogon flavanone A) | P. cyrtonema | aboveground | [49] |
| 52 | neoliquiritin | P. kingianum | rhizome | [41] |
| 53 | hesperidin | P. odoratum | root | [44] |
| 54 | (±) 5, 7-dihydroxy-6, 8-dimethyl-3-(3’-hydroxy-4’-methoxybenzyl) -chroma-4-one | P. odoratum | root | [44] |
| 55 | (±) 5, 7-dihydroxy-6, 8-dimethyl-3-(2′-hydroxy-4′-methoxybenzyl) -chroma-4-one | P. odoratum | root | [44] |
| 56 | (3R)-5, 7-dihydroxy-6-methyl-3-(2′-hydroxy-4′-methoxybenzyl) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 57 | (3R)-5, 7-dihydroxy-8-methyl-3-(2′, 4′-dihydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 58 | (3R)-5, 7-dihydroxy-8-methyl-3-(4′-hydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 59 | (3R)-5, 7-dihydroxy-3-(2′-hydroxy-4′-methoxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 60 | (3R)-5, 7-dihydroxy-3-(4′-hydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 61 | (3R)-5, 7-dihydroxy-6-methoxy-8-methyl-3-(2′, 4′-dihydroxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 62 | (3R)-5, 7-dihydroxy-8-methoxy-3-(2′-hydroxy-4′-methoxybenzyl) -chroma-4-one | P. odoratum | rhizome | [46] |
| 63 | (3R)-5, 7-dihydroxy-6-methyl-8-methoxy-3-(4′-methoxybenzyl)-chroma-4-one | P. odoratum | rhizome | [46] |
| 64 | 6, 8-dimethyl-5, 7-dihydroxy-3-(4′-methoxybenzyl) | P. odoratum | rhizome | [51] |
| 65 | 5, 7-dihydroxy-3-(4′-hydroxybenzylidene) -chroma-4-one | P. cyrtonema | rhizome | [42] |
| 66 | € 5, 7-dihydroxy-6, 8-dimethyl-3-(3, 4-dihydroxybenzylidene) -chroma-4-one | P. odoratum | rhizome | [44] |
| 67 | € -7-O-β-D-glucopyranoside-5-hydroxy-3-(4′-hydroxybenzylidene) -chroma-4-one | P. odoratum | root | [44] |
| 68 | € 5, 7-dihydroxy-8-methoxy-6-methyl-3-(3, 4-dihydroxybenzylidene) -chroma-4-one | P. odoratum | rhizome | [52] |
| No. | Compounds | Species | Parts | References |
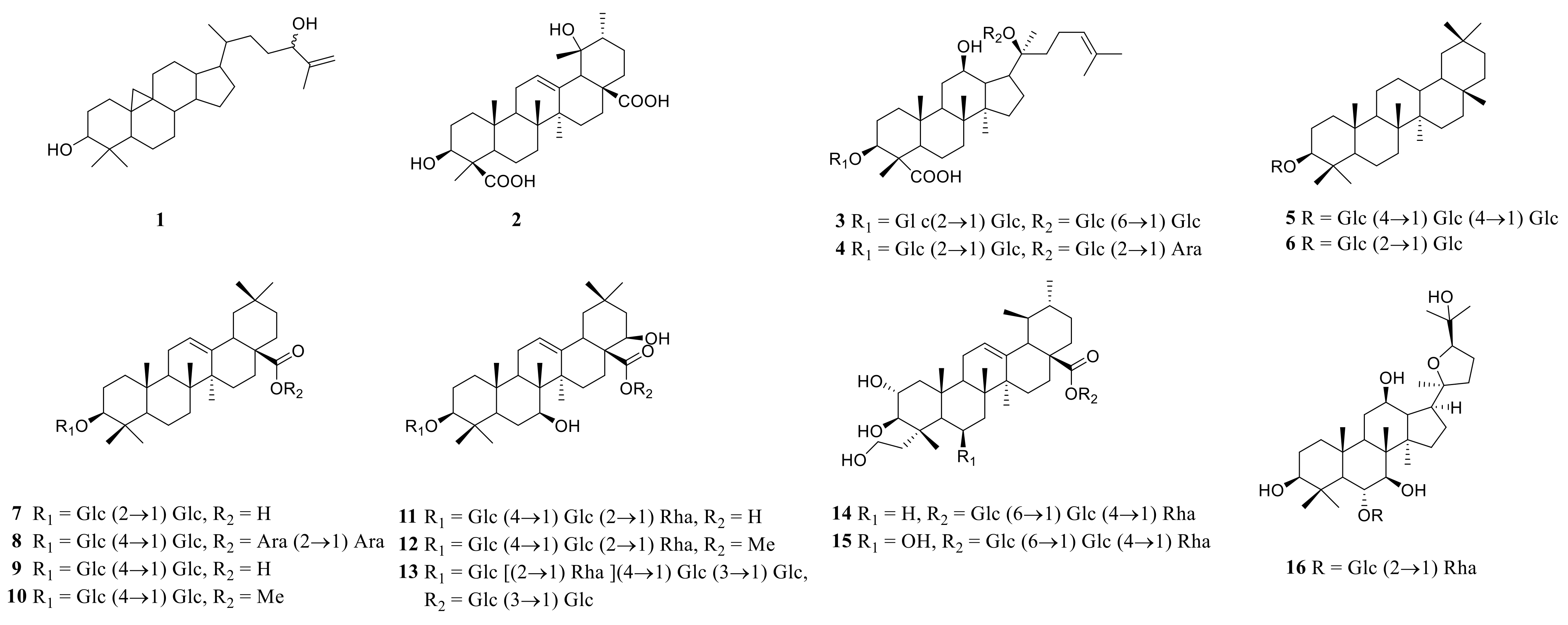
|---|---|---|---|---|
| 1 | (24R/S)-9,19-CycloAltin-25-ene-3β,24-diol | P. odoratum | rhizome | [10] |
| 2 | 3β, 19α-dihydroxy-12-en-24, 28-dioic acid | P. odoratum | rhizome | [14] |
| 3 | ginsenoside Rb1 | P. kingianum | rhizome processed | [23] |
| 4 | ginsenoside Rc | P. kingianum | rhizome processed | [25] |
| 5 | β(OH)-(3→1) glucose-(4→1) glucose-(4→1) glucose-oleanane | P. sibiricum | rhizome | [53] |
| 6 | 3β(OH)-(3→1) glucose-(2→1) glucose-oleanolic acid | P. sibiricum | rhizome | [53] |
| 7 | 3β(OH)-(3→1) glucose-(4→1) glucose-(28→1) arabinose-(2→1) arabinose-oleanolic acid | P. sibiricum | rhizome | [53] |
| 8 | β, 30β(OH) 2-(3→1) glucose-(2→1) glucose-oleanane | P. sibiricum | rhizome | [53] |
| 9 | polygonoide C | P. sibiricum | rhizome | [54] |
| 10 | polygonoide D | P. sibiricum | rhizome | [54] |
| 11 | polygonoides C | P. sibiricum | rhizome | [54] |
| 12 | polygonoides D | P. sibiricum | rhizome | [54] |
| 13 | polygonoides E | P. sibiricum | rhizome | [54] |
| 14 | 2β, 3β, (OH) 2-(28→1) glucose-(6→1) glucose-(4→1) rhamnose-ursic acid (asiaticoside) | P. sibiricum | rhizome | [53,55] |
| 15 | 2β, 3β, 6β, (OH) 3-(28→1) glucose-(6→1) glucose-(4→1) rhamnose-ursic acid Oxalin) | P. sibiricum | rhizome | [53] |
| 16 | Pseudoginsenoside F11 | P. kingianum | rhizome | [55] |
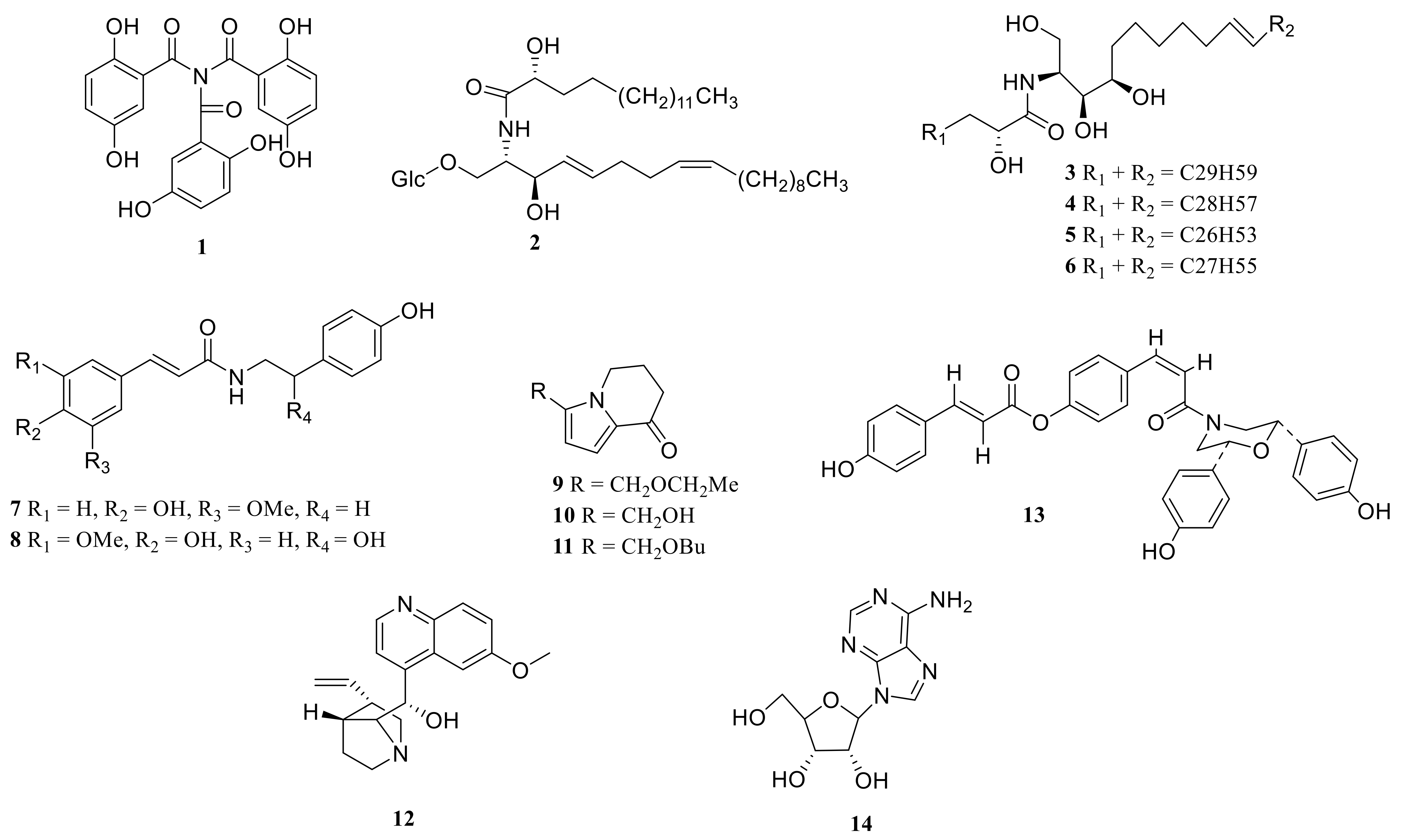
| No. | Compounds | Species | Parts | References |
|---|---|---|---|---|
| 1 | N, N-bis(2,5-dihydroxybenzoyl)-2,5-dihydroxybenzamide | P. cirrhifolium | rhizome | [10] |
| 2 | soyacerebroside II | P. odoratum | rhizome | [38] |
| 3 | Polygonatum sphingolipid A | P. kingianum | rhizome | [38] |
| 4 | Polygonatum sphingolipid B | P. kingianum | rhizome | [38] |
| 5 | Polygonatum sphingolipid C | P. kingianum | rhizome | [38] |
| 6 | Polygonatum sphingolipid D | P. kingianum | rhizome | [38] |
| 7 | N-trans-feruloyltyramine | P. odoratum | rhizome | [40] |
| 8 | N-trans-feruloyloctopamine | P. odoratum | rhizome | [40] |
| 9 | 3-methoxyethyl-5,6,7,8-tetrahydro-8-indolinone | P. sibiricum P. kingianum | rhizome rhizome | [56] [46] |
| 10 | 3-ethoxymethyl-5,6,7,8-tetrahydroindolizin-8-one | P. sibiricum | rhizome | [57] |
| 11 | kinganone | P. kingianum | rhizome | [46] |
| 12 | quinine | P. verticillatum | rhizome | [38] |
| 13 | polygonapholine | P. alte-lobatum | rhizome | [58] |
| 14 | adenosine | P. sibiricum | rhizome | [59] |
| Processing Method | Auxiliary Dosage | Bibliography Source | References |
|---|---|---|---|
| If you take it alone, first use boiling water to remove the bitter juice, then steam and dry nine times. | - | Ming Dynasty “Introduction to Medicine” | [91] |
| Excellently steamed and ready to eat. | - | Qing Dynasty “Materia Medica Justice” | [92] |
| Remove impurities, wash, and remove; thoroughly moisten for 1 day, steam for 8 h, simmer for 12 h, take it out, sun until semi-dry, steam again for 8 h, simmer for 12 h until black, simmering, and oily, cut into thick slices, and dry. | - | “Guangdong Province Traditional Chinese Medicine Processing Regulations” 1984 | [93] |
| Wash, stew thoroughly, or steam with wine, cut into thick slices, and dry. | For every 100 kg of Polygonatum, use 20 kg of Huangjiu | “Chinese Pharmacopoeia” 2020 | [94] |
| A total of 400 g of Polygonatum and 2 L of black beans, cooked at the same time to remove the beans; avoid ironware. | - | Ming Dynasty “Forbidden Prescriptions in Lu Mansion” | [91] |
| Polygonatum Mill. is boiled until it is thin; squeeze the juice to remove the residue, and add honey. | herb: honey = 7:3/4:6 | Qing Dynasty “Huizhitang Experience Prescription” | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Qiu, Y.; Gong, L.; Wang, W.; Wen, R. A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes. Molecules 2022, 27, 4821. https://doi.org/10.3390/molecules27154821
Luo L, Qiu Y, Gong L, Wang W, Wen R. A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes. Molecules. 2022; 27(15):4821. https://doi.org/10.3390/molecules27154821
Chicago/Turabian StyleLuo, Lu, Yixing Qiu, Limin Gong, Wei Wang, and Ruiding Wen. 2022. "A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes" Molecules 27, no. 15: 4821. https://doi.org/10.3390/molecules27154821
APA StyleLuo, L., Qiu, Y., Gong, L., Wang, W., & Wen, R. (2022). A Review of Polygonatum Mill. Genus: Its Taxonomy, Chemical Constituents, and Pharmacological Effect Due to Processing Changes. Molecules, 27(15), 4821. https://doi.org/10.3390/molecules27154821