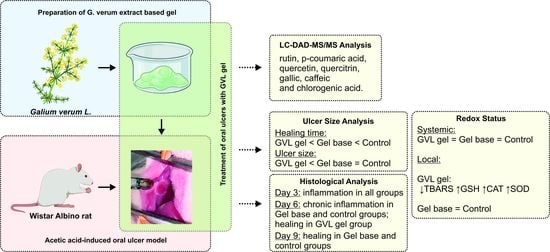
The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Analysis of G. verum Extract
2.3. Preparation of G. verum Extract-Based Mucoadhesive Oral Gel
2.4. In Vivo Oral Ulcer Healing Examinations
2.4.1. Animals
2.4.2. Induction of RAS Model
2.4.3. Experimental Animals
2.4.4. Estimation of Oral Buccal Ulcer Healing
2.5. Biochemical Analysis
2.5.1. Evaluation of Systemic Redox State
Determination of the Values of Pro-Oxidants in Plasma Samples
Determination of the Values of Antioxidants in Erythrocyte Samples
2.5.2. Evaluation of Redox State in Aphthous Lesions
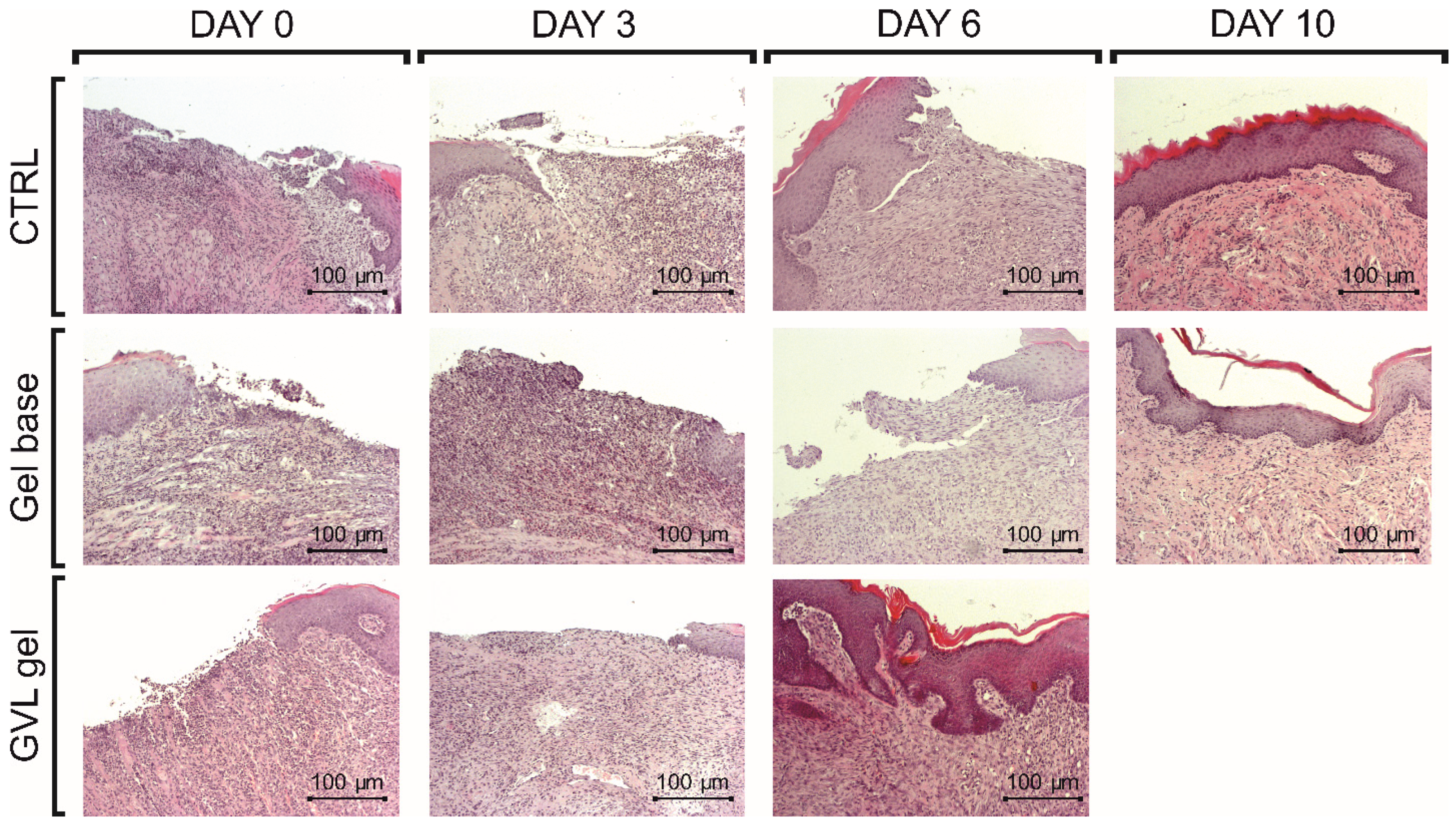
2.6. Histologic Analysis
- Presence of epithelial necrosis, no signs of inflammation;
- The inflammatory reaction has started, with no new capillary proliferation;
- The inflammatory reaction is prominent with few capillary proliferations on the basis of the ulcer, but no epithelization at the surface;
- The inflammatory reaction is decreased, new capillary proliferation has reached the surface, and epithelization has started at the surface;
- The epithelization is complete.
2.7. Statistical Analysis
3. Results
3.1. HPLC-DAD Analysis of G. verum Ethanol Extract
3.2. Ulcer Healing Rate
3.3. Redox Status
3.3.1. Systemic Redox Status
3.3.2. Redox Status in Oral Buccal Ulcer Lesion
3.4. Histological Analysis of Oral Buccal Ulcer Lesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Akintoye, S.O.; Greenberg, M.S. Recurrent aphthous stomatitis. Dent Clin N. Am. 2014, 58, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.K.; Saikaly, T.S.; Saikaly, L.E. Recurrent aphthous ulceration: A review of potential causes and novel treatments. J. Dermatol. Treat. 2018, 29, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, E.A.; Lalla, R.V. Recurrent oral ulceration: Etiology, classification, management and diagnostic algorithm. Periodontology 2019, 80, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Tugrul, S.; Koçyiğit, A.; Doğan, R.; Eren, S.B.; Şentürk, E.; Ozturan, O.; Ozar, O.F. Total antioxidant status and oxidative stress in recurrent aphthous stomatitis. Int. J. Dermatol. 2016, 55, e130–e135. [Google Scholar] [CrossRef]
- Fitzpatrick, S.G.; Cohen, D.M.; Clark, A.N. Ulcerated Lesions of the Oral Mucosa: Clinical and Histologic Review. Head Neck Pathol. 2019, 13, 91–102. [Google Scholar] [CrossRef]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphtous Stomatitis: A Review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36. [Google Scholar]
- Ghalayani, P.; Zolfaghary, B.; Farhad, A.R.; Tavangar, A.; Soleymani, B. The efficacy of Punica granatum extract in the menagement of recurrent aphtous stomatitis. J. Res. Pharm. Pract. 2013, 2, 88–92. [Google Scholar]
- Manfredini, M.; Guida, S.; Giovani, M.; Lippolis, N.; Spinas, E.; Farnetani, F.; Dattola, A.; Di Matteo, E.; Pellacani, G.; Giannetti, L. Recurrent Aphthous Stomatitis: Treatment and Management. Dermatol. Pract. Concept. 2021, 11, e2021099. [Google Scholar] [CrossRef]
- Shavakhi, M.; Sahebkar, A.; Shirban, F.; Bagherniya, M. The efficacy of herbal medicine in the treatment of recurrent aphthous stomatitis: A systematic review of randomized clinical trials. Phytother. Res. 2022, 36, 672–685. [Google Scholar] [CrossRef]
- Li, C.L.; Huang, H.L.; Wang, W.C.; Hua, H. Efficacy and safety of topical herbal medicine treatment on recurrent aphtous stomatitis: A systematic review. Drug Des. Dev. Ther. 2016, 10, 107–115. [Google Scholar]
- Bradic, J.; Jeremic, N.; Petkovic, A.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Sretenovic, J.; Matic, S.; Jakovljevic, V.; Tomovic, M. Cardioprotective effects of Galium verum L. extract against myocardial ischemia-reperfusion injury. Arch. Physiol. Biochem. 2020, 126, 408–415. [Google Scholar] [CrossRef]
- Bradic, J.; Petkovic, A.; Tomovic, M. Phytochemical and Pharmacological Properties of Some Species of the Genus Galium, L. (Galium verum and mollugo). Ser. J. Exp. Clin. Res. 2021, 22, 187–193. [Google Scholar] [CrossRef]
- Bradic, J.; Zivkovic, V.; Srejovic, I.; Jakovljevic, V.; Petkovic, A.; Turnic, T.N.; Jeremic, J.; Jeremic, N.; Mitrovic, S.; Sobot, T.; et al. Protective Effects of Galium verum L. Extract against Cardiac Ischemia/Reperfusion Injury in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2019, 2019, 4235405. [Google Scholar] [CrossRef]
- Hijazi, A.; Al Masri, D.S.; Farhan, H.; Nasser, M.; Rammal, H.; Annan, H. Effect of different ethanol concentrations, using different extraction techniques, on the antioxidant capacity of Lebanese Eryngium creticum. J. Pharm. Chem. Biol. Sci. 2015, 3, 262–271. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 17, 13–20. [Google Scholar] [CrossRef]
- Salaj, N.; Kladar, N.; Conic, B.S.; Jeremic, K.; Barjaktarovic, J.; Hitl, M.; Gavaric, N.; Bozin, B. Stabilization of sunflower and olive oils with savory (Satureja kitaibelii, Lamiaceae). J. Food Nutr. Res. 2020, 59, 259–271. [Google Scholar]
- Andjic, M.; Bozin, B.; Draginic, N.; Kočović, A.; Jeremić, J.N.; Tomović, M.; Milojević Šamanović, A.; Kladar, N.; Čapo, I.; Jakovljević, V.; et al. Formulation and Evaluation of Helichrysum italicum Essential Oil-Based Topical Formulations for Wound Healing in Diabetic Rats. Pharmaceuticals 2021, 14, 813. [Google Scholar] [CrossRef]
- Aslani, A.; Zolfaghari, B.; Davoodvandi, F. Design, Formulation and Evaluation of an Oral Gel from Punica Granatum Flower Extract for the Treatment of Recurrent Aphthous Stomatitis. Adv. Pharm. Bull. 2016, 6, 391–398. [Google Scholar] [CrossRef]
- Ayoub, N.; Badr, N.; Al-Ghamdi, S.S.; Alsanosi, S.; Alzahrani, A.R.; Abdel-Naim, A.B.; Nematallah, K.A.; Swilam, N. HPLC/MSn Profiling and Healing Activity of a Muco-Adhesive Formula of Salvadora persica against Acetic Acid-Induced Oral Ulcer in Rats. Nutrients 2021, 14, 28. [Google Scholar] [CrossRef]
- Chen, P.; Yao, H.; Su, W.; Zheng, Y.; Fan, W.; Zhang, L.; Chen, T.; Wu, S.; Zhang, W.; He, Y.; et al. Pharmacodynamic and Metabolomics Studies on the Effect of Kouyanqing Granule in the Treatment of Phenol-Induced Oral Ulcer Worsened by Sleep Deprivation. Front. Pharmacol. 2020, 11, 824. [Google Scholar] [CrossRef]
- Karavana Hizarcioğlu, S.Y.; Sezer, B.; Güneri, P.; Veral, A.; Boyacioğlu, H.; Ertan, G.; Epstein, J.B. Efficacy of topical benzydamine hydrochloride gel on oral mucosal ulcers: An in vivo animal study. Int. J. Oral. Maxillofac. Surg. 2011, 40, 973–978. [Google Scholar] [CrossRef]
- Sretenovic, J.; Joksimovic Jovic, J.; Srejovic, I.; Zivkovic, V.; Mihajlovic, K.; Labudovic-Borovic, M.; Trifunovic, S.; Milosevic, V.; Lazic, D.; Bolevich, S.; et al. Morphometric analysis and redox state of the testicles in nandrolone decanoate and swimming treated adult male rats. Basic Clin. Androl. 2021, 31, 17. [Google Scholar] [CrossRef]
- Sretenovic, J.; Ajdzanovic, V.; Zivkovic, V.; Srejovic, I.; Corbic, M.; Milosevic, V.; Jakovljevic, V.; Milosavljevic, Z. Nandrolone decanoate and physical activity affect quadriceps in peripubertal rats. Acta Histochem. 2018, 120, 429–437. [Google Scholar] [CrossRef]
- Femiano, F.; Lanza, A.; Buonaiuto, C.; Gombos, F.; Nunziata, M.; Piccolo, S.; Cirillo, N. Guidelines for Diagnosis and Management of Aphthous Stomatitis. Pediatr. Infect. Dis. J. 2007, 26, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Natah, S.; Konttinen, Y.; Enattah, N.; Ashammakhi, N.; Sharkey, K.; Häyrinen-Immonen, R. Recurrent aphthous ulcers today: A review of the growing knowledge. Int. J. Oral Maxillofac. Surg. 2004, 33, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.P.; Pai, A. Is Optimal Management of Recurrent Aphthous Stomatitis Possible? A Reality Check. J. Clin. Diagn. Res. 2016, 10, ZE08–ZE13. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Lozada-Nur, F.; Migliorati, C. Clinical efficacy of prednisone in the treatment of patients with oral inflammatory ulcerative diseases: A study of fifty-five patients. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 360–363. [Google Scholar] [CrossRef]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Schmidt, M.; Polednik, C.; Roller, J.; Hagen, R. Galium verum aqueous extract strongly inhibits the motility of head and neck cancer cell lines and protects mucosal keratinocytes against toxic DNA damage. Oncol. Rep. 2014, 32, 1296–1302. [Google Scholar] [CrossRef]
- Vlase, L.; Mocan, A.; Hanganu, D.; Benedec, D.; Gheldiu, A.M.; Crisan, G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four galium species (Rubiaceae). Dig. J. Nanomater. Biostruct. 2014, 9, 1085–1109. [Google Scholar]
- Cavalcante, G.M.; Sousa de Paula, R.J.; Souza, L.P.; Sousa, F.B.; Mota, M.R.; Alves, A.P. Experimental model of traumatic ulcer in the cheek mucosa of rats. Acta Circ. Bras. 2011, 26, 227–234. [Google Scholar] [CrossRef][Green Version]
- Mansour, G.; Ouda, S.; Shaker, A.; Abdallah, H.M. Clinical efficacy of new aloe vera- and myrrh-based oral mucoadhesive gels in the management of minor recurrent aphthous stomatitis: A randomized, double-blind, vehicle-controlled study. J. Oral Pathol. Med. 2014, 43, 405–409. [Google Scholar] [CrossRef]
- Amanlou, M.; Babaee, N.; Saheb-Jamee, M.; Salehnia, A.; Farsam, H.; Tohidast Akrad, Z. Efficacy of Satureja khuzistanica extract and its essential oil preparations in the management of recurrent aphthous stomatitis. DARU 2007, 15, 231–235. [Google Scholar]
- Salehi, B.; Selamoglu, Z.; Sevindik, M.; Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Csupor-Löffler, B.; Csupor, D.; Yazdi, S.E.; Sharifi-Rad, J.; et al. Achillea spp.: A comprehensive review on its ethnobotany, phytochemistry, phytopharmacology and industrial applications. Cell. Mol. Biol. 2020, 66, 78–103. [Google Scholar] [CrossRef]
- Arafa, M.G.; Ghalwash, D.; El-Kersh, D.M.; Elmazar, M.M. Propolis-based niosomes as oromuco-adhesive flms: A randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers. Sci. Rep. 2018, 8, 18056. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Fang, H. Enzymatic antioxidants status in patients with recurrent aphthous stomatitis. J. Oral. Pathol. Med. 2017, 46, 817–820. [Google Scholar] [CrossRef]
- Bagan, J.; Saez, G.; Tormos, C.; Gavalda, C.; Sanchis, J.M.; Bagan, L.; Scully, C. Oxidative stress and recurrent aphthous stomatitis. Clin. Oral Investig. 2014, 18, 1919–1923. [Google Scholar] [CrossRef]
- Costa, P.; Boeing, T.; Somensi, L.B.; Cury, B.J.; Espíndola, V.L.; França, T.C.S.; de Almeida, M.O.; Arruda, C.; Bastos, J.K.; da Silva, L.M.; et al. Hydroalcoholic extract from Baccharis dracunculifolia recovers the gastric ulcerated tissue, and p-coumaric acid is a pivotal bioactive compound to this action. Biofactors 2019, 45, 479–489. [Google Scholar] [CrossRef]
- Ahmad, A.M.; Ibrahim, K.Y.; Mohammad, A.M. Efficacy of extract from Ononis spinosa L. on ethanol-induced gastric ulcer in rats. J. Tradit. Chin. Med. 2021, 41, 270–275. [Google Scholar]
- Shi, J.; Chen, Q. COX-2 inhibitors: A novel way to prevent and treat the carcinogenesis of oral mucosa. Zhonghua Kou Qiang Yi Xue Za Zhi 2005, 40, 262–264. [Google Scholar]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; El-Ghffar, E.A.A. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019, 109, 314–321. [Google Scholar] [CrossRef]
- Martins, M.D.; Marques, M.M.; Bussadori, S.K.; Martins, M.A.; Pavesi, V.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P. Comparative Analysis between Chamomilla recutita and Corticosteroids on Wound Healing. An In Vitro and In Vivo Study. Phytother. Res. 2009, 23, 274–278. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Shenoy, R.; Udupa, A.L.; Udupa, S.L.; Shetty, S. Wound healing property of ethanolic extract of leaves of Hyptis suaveolens with supportive role of antioxidant enzymes. Indian J. Exp. Biol. 2003, 41, 238–241. [Google Scholar]
- Mandawgade, S.D.; Patil, K.S. Wound healing potential of some active principles of Lawsoina alba Lamp leaves. Indian J. Pharm. Sci. 2003, 65, 390–394. [Google Scholar]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]









| Name of Compound | G. verum Ethanol Extract |
|---|---|
| Rutin | 23.81 ± 1.90 |
| p-Coumaric acid | 9.35 ± 0.93 |
| Quercetin | 0.76 ± 0.05 |
| Quercitrin | 0.72 ± 0.04 |
| Gallic acid | 0.53 ± 0.08 |
| Caffeic acid | 0.12 ± 0.01 |
| Chlorogenic acid | 0.16 ± 0.01 |
| Ferulic acid | <0.1 |
| trans-Cinnamic acid | <0.03 |
| Rosmarinic acid | <0.02 |
| Day 0 | Day 3 | Day 6 | Day 10 | |
|---|---|---|---|---|
| Control | 2 ± 0.00 | 3 ± 0.00 | 4.16 ± 0.41 | 4.83 ± 0.41 |
| GEL base | 2.33 ± 0.82 | 3.16 ± 0.41 | 4.33 ± 0.52 | 5 ± 0.00 |
| GVL gel | 2.67 ± 0.51 | 4 ± 0.00 | 5 ± 0.00 * | 5 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuletic, M.; Jakovljevic, V.; Zivanovic, S.; Papic, M.; Papic, M.; Mladenovic, R.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Andjic, M.; et al. The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules 2022, 27, 4680. https://doi.org/10.3390/molecules27154680
Vuletic M, Jakovljevic V, Zivanovic S, Papic M, Papic M, Mladenovic R, Zivkovic V, Srejovic I, Jeremic J, Andjic M, et al. The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules. 2022; 27(15):4680. https://doi.org/10.3390/molecules27154680
Chicago/Turabian StyleVuletic, Miona, Vladimir Jakovljevic, Suzana Zivanovic, Milos Papic, Mirjana Papic, Rasa Mladenovic, Vladimir Zivkovic, Ivan Srejovic, Jovana Jeremic, Marijana Andjic, and et al. 2022. "The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats" Molecules 27, no. 15: 4680. https://doi.org/10.3390/molecules27154680
APA StyleVuletic, M., Jakovljevic, V., Zivanovic, S., Papic, M., Papic, M., Mladenovic, R., Zivkovic, V., Srejovic, I., Jeremic, J., Andjic, M., Kocovic, A., Sretenovic, J., Mitrovic, S., Božin, B., Kladar, N., Bolevich, S., & Bradic, J. (2022). The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules, 27(15), 4680. https://doi.org/10.3390/molecules27154680